Abstract
Objective:
It is unknown whether racial differences in exposure to acute precipitants of stroke, specifically infection, contribute to racial disparities in stroke mortality.
Methods:
Among participants in the nationally representative Health and Retirement Study with linked Medicare data (1991–2007), we conducted a case-crossover study employing within-person comparisons to study racial/ethnic differences in the risks of death and hospitalization from ischemic stroke following acute infection.
Results:
There were 964 adults hospitalized for ischemic stroke. Acute infection increased the 30-day risks of ischemic stroke death (5.82-fold) and ischemic stroke hospitalization (1.87-fold). Acute infection was a more potent trigger of acute ischemic stroke death in non-Hispanic blacks (odds ratio [OR] 39.21; 95% confidence interval [CI] 9.26–166.00) than in non-Hispanic whites (OR 4.50; 95% CI 3.14–6.44) or Hispanics (OR 5.18; 95% CI 1.34–19.95) (race-by-stroke interaction, p = 0.005). When adjusted for atrial fibrillation, infection remained more strongly associated with stroke mortality in blacks (OR 34.85) than in whites (OR 3.58) and Hispanics (OR 3.53). Acute infection increased the short-term risk of incident stroke similarly across racial/ethnic groups. Infection occurred often before stroke death in non-Hispanic blacks, with 70% experiencing an infection in the 30 days before stroke death compared to a background frequency of 15%.
Conclusions:
Acute infection disproportionately increases the risk of stroke death for non-Hispanic blacks, independently of atrial fibrillation. Stroke incidence did not explain this finding. Acute infection appears to be one factor that contributes to the black–white disparity in stroke mortality.
Significant racial disparities in stroke mortality persist in the United States and are widening.1–3 Non-Hispanic blacks have a greater risk of dying from stroke than non-Hispanic whites, and the excess risk of stroke death increased 55% for men and 26% for women from 1979 to 2006.2 While much attention has focused on the role of differences in vascular risk factors or health behaviors in these racial disparities,2,4 less attention has focused on whether racial differences in exposure to acute precipitants of stroke, so-called triggers, might contribute to racial differences in stroke mortality.
Acute infection may precipitate stroke.5 However, confounding by vascular risk factors or social class may bias estimates, leading to false-positive associations between infection and stroke.6 Given that randomized controlled trials of infection precipitating stroke are unfeasible, observational study designs that minimize confounding, such as a case-crossover study in which each person serves as his or her own control, can be used.7–9 Two case-crossover studies10,11 suggest that acute infection is a trigger of incident stroke, yet another does not.12
Racial differences in acute infection may contribute to the black–white disparity in stroke mortality. Compared to whites, blacks appear to have higher risks of acute infection, invasive infectious disease, death from infection, and conditions associated with immunosuppression such as diabetes.1,13–17 It is unknown whether infection is a more potent trigger of stroke death in blacks than in whites.
Therefore, we undertook a case-crossover study in a nationally representative population to determine whether acute infection contributes to racial disparities in the incidence of18,19 and mortality from ischemic stroke. We hypothesized that infection is a more potent trigger of ischemic stroke incidence and mortality in blacks than in whites.
METHODS
Data source and study population.
Subjects were participants in the Health and Retirement Study (HRS), who constitute a nationally representative prospective cohort study of older Americans.20 Every 2 years since 1992, HRS participants have been interviewed regarding physical health and functioning, health insurance, and other factors. Data regarding medical services received—both inpatient and outpatient—were available from the Centers for Medicare & Medicaid Services (CMS) for participants who were enrolled in Medicare fee-for-service from 1991 to 2007 and agreed to the linkage. This included dates of services including hospitalizations, emergency room visits, physician visits, other outpatient visits, skilled nursing facility stays, and home health visits. Mortality data were available from the National Death Index (NDI) and linked to HRS data. Using the linked HRS-CMS-NDI files, we conducted a case-crossover study in which each person served as his or her own control.
Study design.
A case-crossover design was used. The frequency of infection before stroke hospitalization was compared to the frequency of infection at other time periods. We considered the “at-risk” time period to be a 2-week window before the hospitalization for stroke. Therefore, the frequency of infections during this at-risk period was compared to the frequency of infections during 4 comparison periods within the same person, each period being 14 days with a 30-day washout period between each period (figure 1).
Figure 1. Design of case-crossover study of infection and hospitalization for ischemic stroke.
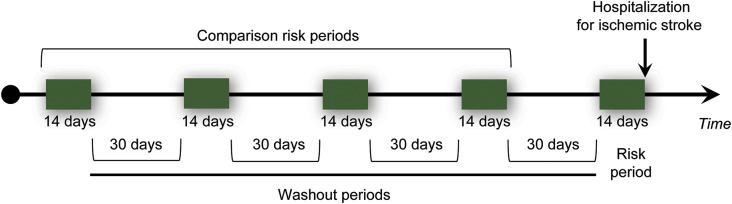
Since the exact length of the at-risk time period was unknown, we also assessed the risk associated with infection during the 30-day period before ischemic stroke hospitalization. Infections that occurred during this period were compared with 4 30-day comparison periods, each with a 30-day washout period in between.
Measurements.
Co-primary outcomes were ischemic stroke hospitalization and ischemic stroke death. Subjects hospitalized for ischemic stroke were identified using valid ICD-9 codes (ICD-9-CM codes 433.x1, 434.xx, 436.xx as the primary diagnosis).21,22 For those with multiple hospitalizations for ischemic stroke, the first hospitalization was used. To capture incident stroke events, individuals who had a diagnosis of ischemic stroke during a medical visit/stay before the index hospitalization were excluded (n = 19). To ensure that data regarding infections could be captured in the medical files during the relevant time periods, individuals who did not have continuous fee-for-service Medicare coverage during the time period before the outcomes were excluded (n = 49).
Information regarding infection before the ischemic stroke hospitalization was collected from the CMS Inpatient, Skilled Nursing Facility, Outpatient, Home Health Agency, and Carrier (provider) files for the risk and comparison periods. That is, infections during a clinic or other outpatient visit, home health visit, emergency room visit, hospital stay, or nursing home stay were identified within each risk and comparison period. Therefore, for purposes of this study, infection had to be serious enough to warrant medical attention. Presence of infection was determined using ICD-9-CM primary and secondary diagnosis codes that explicitly stated infection (for example, 0xx.xx) or provided evidence of infection (purulent, suppurative, septic, pyogenic, or abscess) (table e-3 on the Neurology® Web site at Neurology.org). The type of infection (genitourinary tract, respiratory tract, skin, septicemia, digestive tract, other) was also extracted.
The time between an infection-related visit/stay and hospitalization for ischemic stroke was also examined. The frequency distribution for these times was plotted on a 60-day histogram, since exact dates of services were known.
Using a similar case-crossover design, mortality information was obtained from NDI files and linked to HRS-CMS data. Subjects who died with ischemic stroke listed as the immediate or underlying cause of death (ICD-9-CM codes 433.x1, 434.xx, or 436.xx or ICD-10 codes I63 or I64) were identified. Information regarding infection-related visits/stays within the 30-day time window before death was compared to 4 30-day comparison periods with a 30-day washout period in between.
Since infection is associated with atrial fibrillation, a known risk factor for stroke,23 we identified those patients with atrial fibrillation (ICD-9-CM code 427.3x) in the CMS Inpatient, Skilled Nursing Facility, Outpatient, Home Health Agency, and Carrier (provider) files. Data were extracted from the HRS regarding age, sex, race, and ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other), body mass index (BMI) (at the time of the first interview for hospitalization as the outcome and at the time of the last interview for death as the outcome), diabetes, and smoking status (ever, never).
Statistical analysis.
Statistical analyses followed recommended procedures for case-crossover studies.7–9 Conditional fixed-effects logit models were used for longitudinal (panel) data, accounting for within-person repeated measures. Odds ratios (ORs) for matched data were calculated with 95% confidence intervals (CIs) for infection and atrial fibrillation occurring in the 14-day and 30-day risk and comparison time periods. There were 3 individuals with missing data for race and 2 individuals with missing data for BMI at the time of the first interview. All missing data were imputed by best-subset regression before the analysis. The α was set at 0.05, 2-tailed. We examined the acute infection-by-race interaction term in the models. We also simultaneously included infection and atrial fibrillation in the models to evaluate their independent effects on ischemic stroke death or hospitalization.
For analyses regarding the entire cohort of participants in the HRS, survey-weighted Poisson regression was utilized, offset by person-years of observation. The incident rate ratio for the association between the number of infection-related visits/stays and the number of ischemic stroke hospitalizations was calculated. To assess whether infection rates differed by race/ethnicity, we assessed race/ethnicity-specific survey-weighted rates of infection and regressed race on number of infection-related visits/stay using a Poisson distribution, offset by the log of person-years under observation. Analyses were conducted in Stata/MP.
Standard protocol approvals, registrations, and patient consents.
This study received human subjects approval from the University of Michigan and the CMS Privacy Board.
RESULTS
Infection before hospitalization for ischemic stroke.
There were 964 individuals hospitalized for ischemic stroke. Their characteristics are shown in table 1. Within 2 weeks of having an infection, participants were 2.11 times more likely to be hospitalized for ischemic stroke than at other time periods (table 1). This elevated risk of stroke hospitalization after infection was evident across age groups, sex, BMI, and smoking status, except for the underweight BMI category, in which the number of patients was small.
Table 1.
Odds ratios for infection in the 14 days and 30 days before hospitalization for ischemic stroke
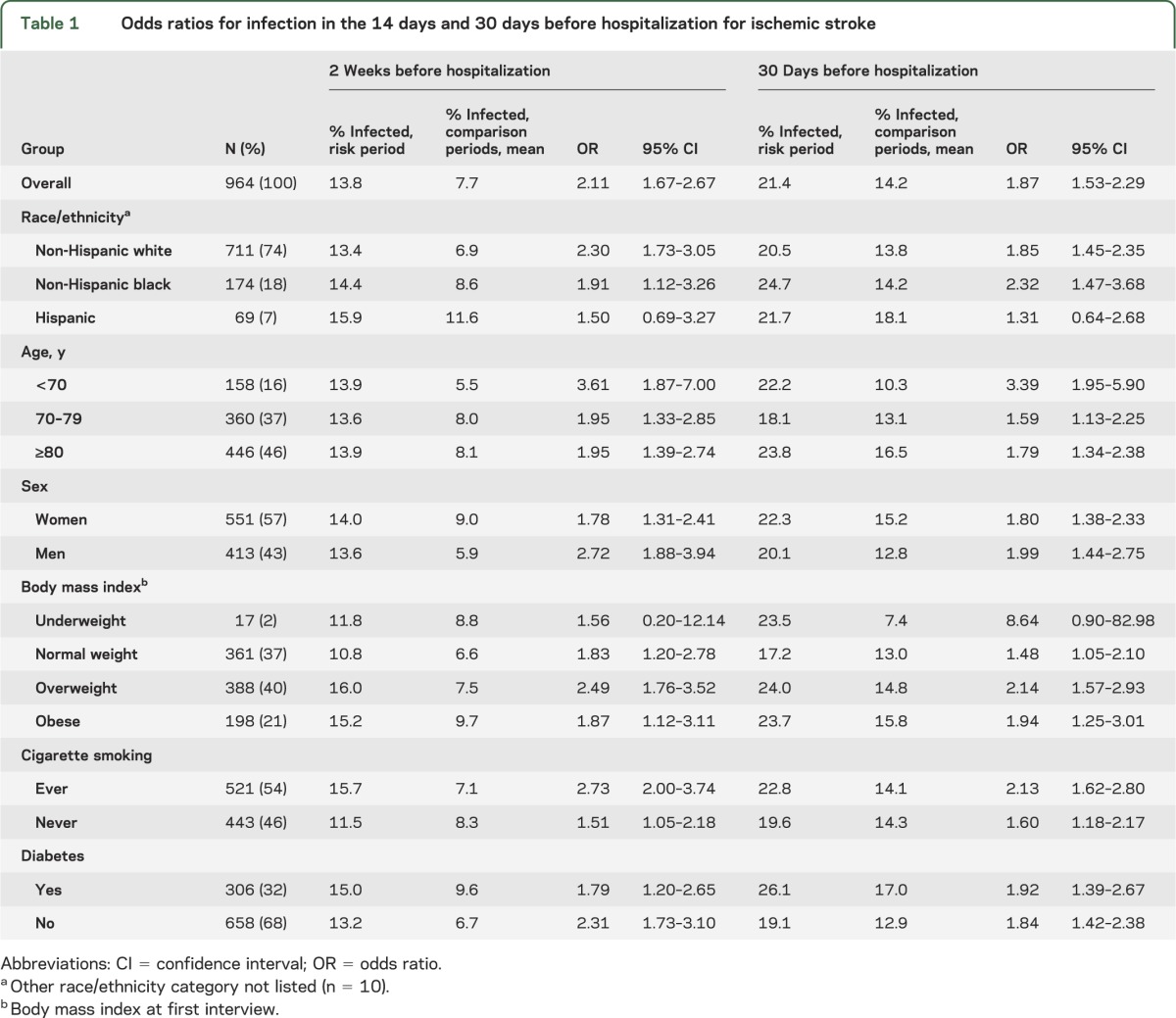
Compared to non-Hispanic whites, non-Hispanic blacks had similar risks of infection within 14 days (whites: OR 2.30; blacks: OR 1.91) or within 30 days (whites: OR 1.85; blacks: OR 2.32) before ischemic stroke hospitalization. Hispanics were not at greater risk of infection but the number of Hispanics hospitalized for ischemic stroke was low. The results were similar for infections occurring within a 30-day period before hospitalization for stroke (table 1).
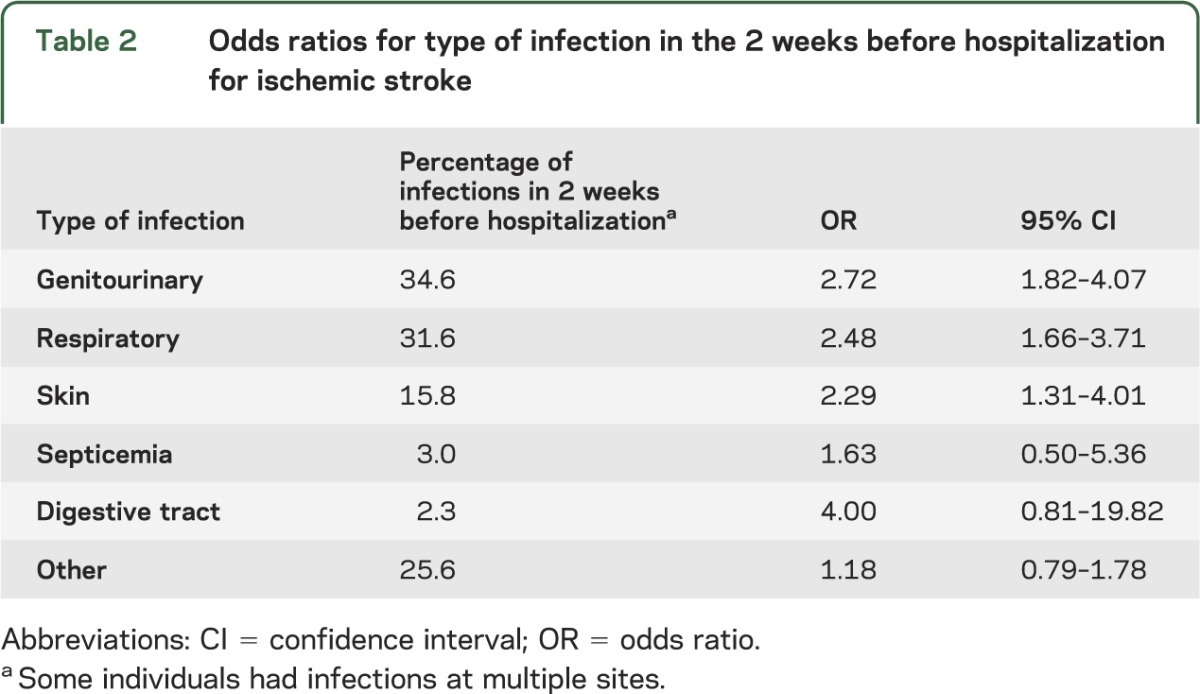
The most frequent sites of infection in the 2 weeks before hospitalization for stroke were the genitourinary tract (34.6%), the respiratory tract (31.6%), and the skin (15.8%) (table 2). Individuals with infections at these 3 sites had greater than a 2-fold increase in the risk of ischemic stroke hospitalization (OR 2.72 for genitourinary, OR 2.48 for respiratory, OR 2.29 for skin).
Table 2.
Odds ratios for type of infection in the 2 weeks before hospitalization for ischemic stroke
Infection was more likely to occur closer to hospitalization for ischemic stroke (figure e-1). Of such stroke hospitalizations, most occurred in December, with March, October, and November being the next most common. Most infections (84.3%) were documented during outpatient visits, compared to hospital or skilled nursing facility stays (15.7%). The association between infection and ischemic stroke hospitalization was evident when the infection occurred in an outpatient setting (OR 2.22; 95% CI 1.72–2.85) and not when the infection occurred in a previous hospitalization or skilled nursing facility stay (OR 1.38; 95% CI 0.78–2.42).
Infection before death from ischemic stroke.
There were 287 individuals who died from ischemic stroke. Table 3 shows their characteristics. Of the 287 stroke deaths, 103 (36%) occurred in a hospital, 34 (12%) in a skilled nursing facility, 52 (18%) under hospice care, 12 (4%) while receiving outpatient services, and 86 (30%) at other locations. Of non-Hispanic blacks, 48% died from stroke in the hospital; this compared with 33% of non-Hispanic whites. Stroke deaths were most common in January, February, and March.
Table 3.
Odds ratios for infection in the 30 days before ischemic stroke death
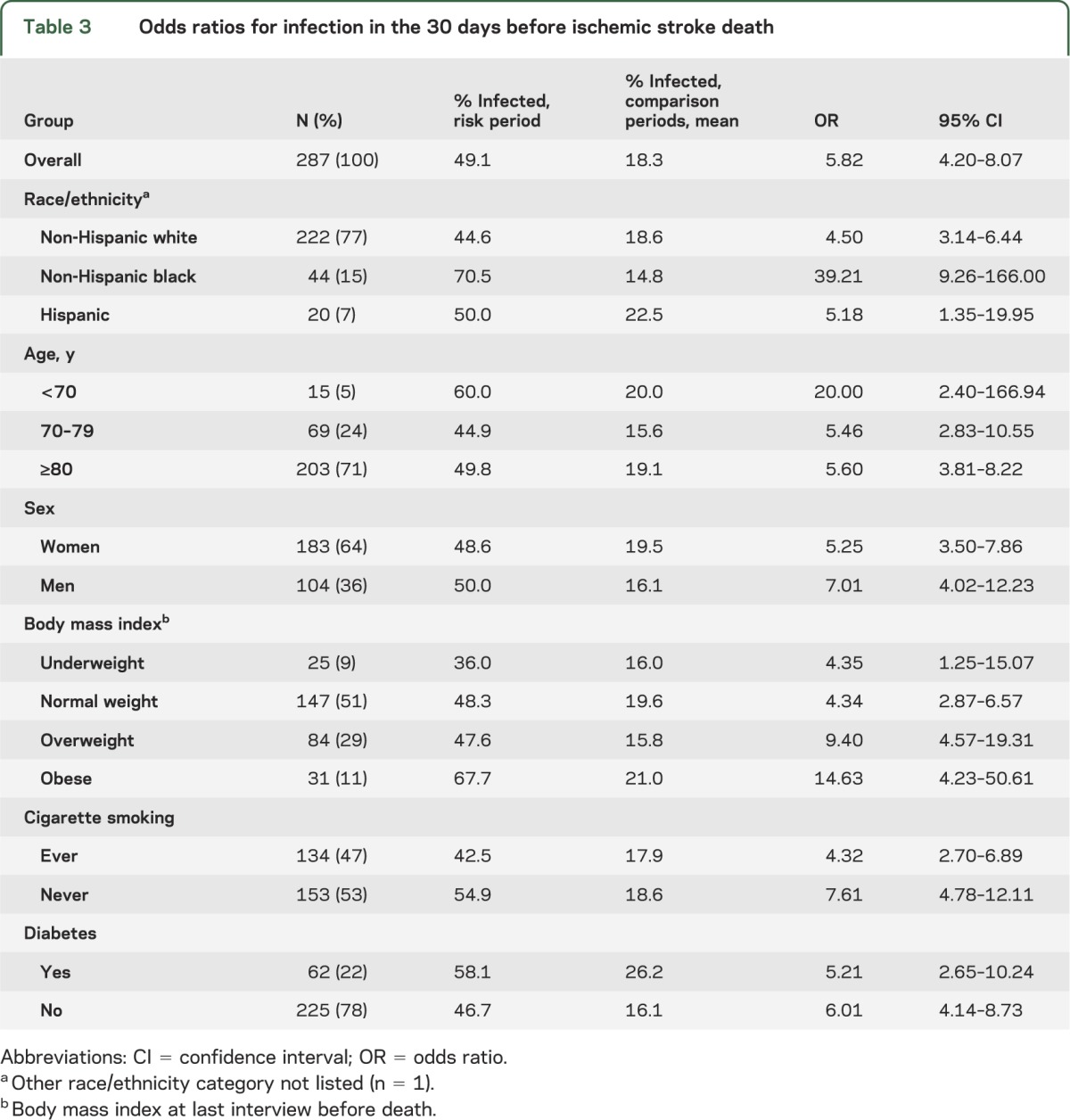
Individuals were 5.82 times more likely to die from ischemic stroke if they had experienced an infection within the previous 30 days (95% CI 4.20–8.07). This elevated risk was evident across age, sex, race, ethnicity, BMI, and smoking status (table 3).
There was effect modification by race (p = 0.005). Non-Hispanic blacks were 39 times more likely to die of a stroke if an infection occurred in the previous 30 days when compared to other time periods (95% CI 9.26–166.0). In non-Hispanic whites, the strength of this association was significantly lower (OR 4.50; 95% CI 3.14–6.44).
Most of the infections (57.4%) preceding ischemic stroke death were in the inpatient setting (either hospital or skilled nursing facility). Ischemic stroke death was associated with inpatient infection (OR 7.73; 95% CI 5.14–11.62) and less so with outpatient infection (OR 2.01; 95% CI 1.40–2.88).
Effect of adjustment for atrial fibrillation.
Atrial fibrillation was more common in the 30 days either before ischemic stroke hospitalization (OR 2.53) or before ischemic stroke death (OR 9.99) than in the comparator time periods (table e-1, table e-2). These associations were evident in each racial/ethnic group.
Infection and atrial fibrillation each were independent predictors of stroke (table 4). After adjustment for atrial fibrillation, infection remained strongly associated with hospitalization (OR 2.00) and death (OR 4.74) due to ischemic stroke. Of note, non-Hispanic blacks had a particularly high probability of death from ischemic stroke after an infection, with a significant OR of 34.85 after adjustment for atrial fibrillation.
Table 4.
Odds ratios for infection and atrial fibrillation in the 30-day periods before hospitalization and before death due to ischemic stroke by race/ethnicity
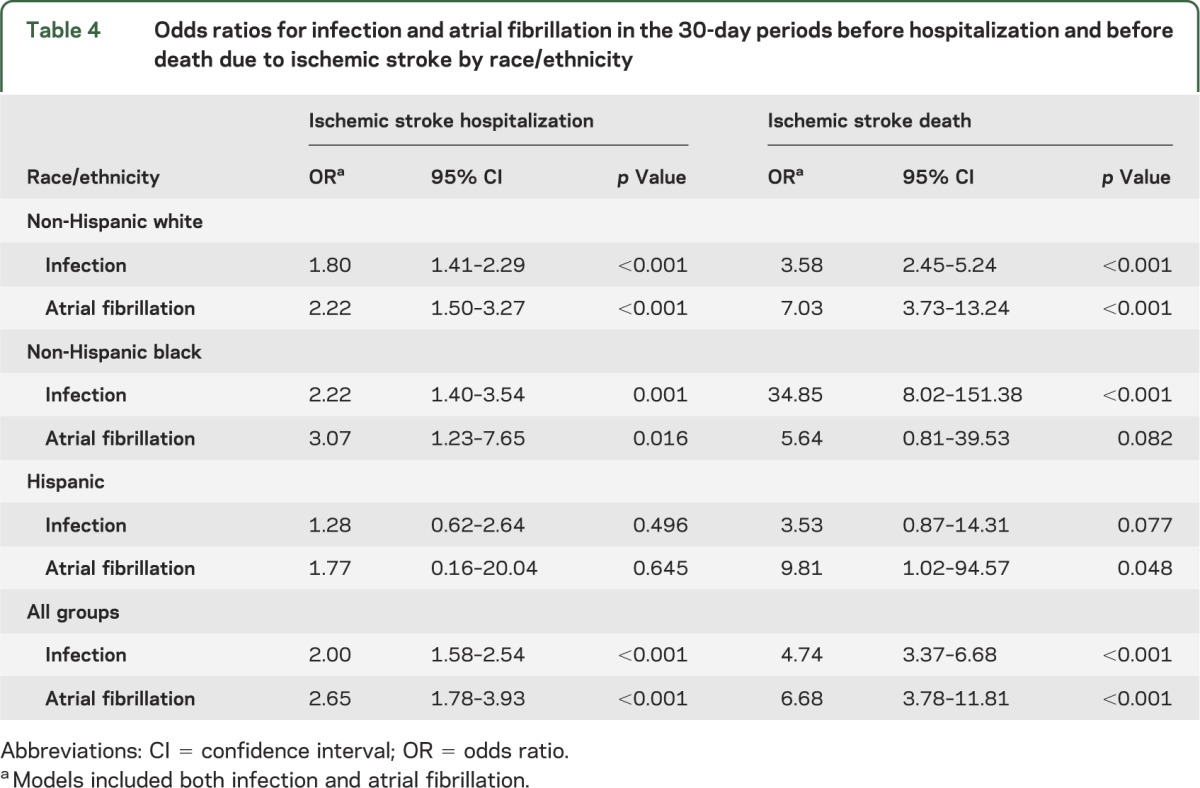
After adjustment for atrial fibrillation, ischemic stroke death remained associated with inpatient infection (OR 5.49; 95% CI 3.59–8.42) and also with outpatient infection (OR 1.96; 95% CI 1.34–2.88).
Overall rates of infection by race.
In the overall population of HRS participants, the likelihood of having an infection-related medical visit or stay was greater among non-Hispanic blacks (survey-weighted mean 151.3 visits/100 person-years; 95% CI 142.3–160.4) and Hispanics (167.6 visits/100 person-years; 95% CI 156.7–178.6) than among non-Hispanic whites (137.0 visits/100 person-years; 95% CI 132.6–141.3). There was a significant association between number of medical visits/stays in which an infection was recorded and the number of hospitalizations for ischemic stroke (p < 0.001). For each infection-related visit, the risk of an ischemic stroke hospitalization increased by 1.6% (95% CI 1.3%–1.8%).
Sensitivity analysis.
We repeated the analyses (n = 235) shown in table 4 after excluding all patients who died under hospice care because treatment potentially was withheld, and results were similar. The overall OR for infection in the 30 days before death from stroke was 6.08 (95% CI 4.23–8.72). In particular, the effect modification by race remained. In non-Hispanic blacks, the OR was 32.17 (95% CI 7.52–137.73), and in non-Hispanic whites, the OR was 4.72 (95% CI 3.17–7.02).
DISCUSSION
In this nationally representative cohort of older Americans, we found that infection is a more potent trigger of acute ischemic stroke death in blacks than in whites. Acute infection increased the short-term risk of stroke death disproportionately for non-Hispanic blacks (a 35-fold increase in stroke death) compared to non-Hispanic whites (a 3.6-fold increase) after adjusting for atrial fibrillation. Infection occurred often before stroke death in non-Hispanic blacks, with 70% experiencing an infection in the 30 days before stroke death compared to a background (or comparison) frequency of 15%. Acute infection increased the short-term risk of incident stroke similarly across racial/ethnic groups, suggesting that stroke incidence does not explain the racial differences in stroke death related to acute infection.
We found a differential effect of acute infection on the transient risk of stroke mortality by race. Stroke incidence did not explain this finding. Potential reasons that acute infection is more common, more lethal, or a more potent trigger of stroke death in blacks are unknown but may include genetic susceptibility, clinical, socioeconomic, environmental factors, or behaviors.15,24–26 Although racial disparities in vaccination rates27 and access to care28 have been reported, we were unable to assess vaccination and access problems in this study. Infections may promote atherosclerosis and thrombosis through several biological mechanisms.29
Our data suggest that atrial fibrillation partially explains the infection–stroke association, consistent with previous reports of an association between acute infection and an increased risk of cardioembolic stroke.12,30 Studies have also shown that acute infection is associated with stroke due to large-vessel atherothromboembolism.12 Interestingly, cardioembolic strokes and large-artery infarcts are ischemic stroke types that are more common in whites compared with blacks.12,30,31 Some reports suggest that the risk of stroke after acute infection appears to be greater in those with less vascular burden.11,12
Previous reports have suggested that respiratory infections are more potent triggers of stroke than other types of infections, with the risk sustained for 90 days but highest in the first 3–7 days.10,12,32 However, we found that genitourinary and skin infections as well as respiratory infections were potent triggers of acute ischemic stroke. We extend previous work by showing that acute infection is a potent trigger of acute ischemic stroke in older adults, even the oldest old, rather than operating primarily in young and middle-aged patients.12,33 We found a trend toward a stronger effect of acute infection on the risks of both incidence of and mortality from ischemic stroke in those less than age 70 than in those aged 70 or older, similar to previous reports.12
Immunization represents one potential strategy to reduce racial disparities in stroke death. Influenza vaccination decreased the risk of stroke in a case-control study34 and the risks of death and ischemic events in patients with coronary artery disease,35 prompting clinical guidelines to recommend annual influenza vaccination for cardiovascular patients.36 Although a recent disappointing study found that pneumococcal vaccination did not reduce the risk of stroke or myocardial infarction,37 the introduction of a pneumococcal conjugate vaccine for children is reducing the racial disparity in pneumococcal disease.38 Whether immunizations for influenza and pneumococcal disease prevent stroke deaths directly by reducing vascular events or indirectly by reducing infections, racial disparities in immunization rates are a major problem. Although the Healthy 2010 initiative sought to eliminate these racial disparities in adult immunization rates, there was no progress in influenza immunization, for which the data showing benefit are stronger.39
Infection was ascertained using diagnosis codes available from hospital stays, skilled nursing facility stays, emergency room visits, physician visits, home health visits, and other outpatient visits. Inaccuracies in the use of such codes would be expected to result in nondifferential misclassification. That is, if the codes were somewhat inaccurate during the at-risk time period, they are also likely to be somewhat inaccurate in the 4 comparison periods. Such nondifferential misclassification drives the OR towards the null and as such, we would be less likely to observe a significant difference and the estimates of effect reported here are likely to be conservative.
There are other limitations of this study. It is possible that factors related to vascular health changed between the risk period and the comparison periods. There was a maximum of 9 months from the beginning of the first comparison period and the end of the risk period. It is possible that new events occurred during this time. Personal data were collected through HRS interviews every 2 years. We considered the possibility of diagnostic bias (e.g., stroke found during a diagnostic workup for infection). However, the date of the visit/stay when the infection was recorded preceded the date of hospitalization for stroke. At the time when the infection was recorded (during a clinic visit, home health visit, or previous hospital stay), stroke was not recorded. This provides evidence that the infection occurred before the stroke. Moreover, because our measure of infection was a health care visit for infection, our results may underestimate the infection rate and magnitude of the infection-triggered stroke risk in those with reduced health care access such as blacks and Hispanics. Although we did not examine season as a confounder or effect modifier, we are not aware of evidence or a biological reason that season would explain or modify whether race modifies the effect of acute infection on ischemic stroke hospitalization or mortality.
Infection is a common potential trigger of ischemic stroke. Infection is more common in non-Hispanic blacks and has a more detrimental impact on their risk of stroke death. These data suggest that acute infection may contribute to the black–white disparity in stroke mortality.
Supplementary Material
GLOSSARY
- BMI
body mass index
- CI
confidence interval
- CMS
Centers for Medicare & Medicaid Services
- HRS
Health and Retirement Study
- ICD-9
International Classification of Diseases, 9th revision
- NDI
National Death Index
- OR
odds ratio
Footnotes
Editorial, page 908
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Literature search: Drs. Levine and Rogers. Figure: Drs. Levine and Rogers. Study design: Drs. Langa, Levine, and Rogers. Data collection: Dr. Rogers. Data analysis: Drs. Levine and Rogers. Data interpretation: Drs. Langa, Levine, and Rogers. Writing: Drs. Langa, Levine, and Rogers.
STUDY FUNDING
Supported by a grant from the NIH National Heart, Lung, and Blood Institute, R21HL093129 (Dr. Rogers, PI). The Health and Retirement Study is sponsored by the National Institute on Aging (U01AG009740) and is conducted by the University of Michigan. Dr. Levine received research support from the NIH (P30DK092926 and K23AG040278). The funding source had no involvement in this study.
DISCLOSURE
The authors report no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Centers for Disease Control and Prevention CDC WONDER. Available at: http://wonder.cdc.gov/. Accessed February 13, 2013
- 2.Howard G, Cushman M, Kissela BM, et al. Traditional risk factors as the underlying cause of racial disparities in stroke: lessons from the half-full (empty?) glass. Stroke 2011;42:3369–3375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleindorfer DO, Khoury J, Moomaw CJ, et al. Stroke incidence is decreasing in whites but not in blacks: a population-based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky Stroke Study. Stroke 2010;41:1326–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howard G, Lackland DT, Kleindorfer DO, et al. Racial differences in the impact of elevated systolic blood pressure on stroke risk. Arch Intern Med 2013;173:46–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guiraud V, Amor MB, Mas JL, Touze E. Triggers of ischemic stroke: a systematic review. Stroke 2010;41:2669–2677 [DOI] [PubMed] [Google Scholar]
- 6.Whincup PH, Mendall MA, Perry IJ, Strachan DP, Walker M. Prospective relations between Helicobacter pylori infection, coronary heart disease, and stroke in middle aged men. Heart 1996;75:568–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol 1991;133:144–153 [DOI] [PubMed] [Google Scholar]
- 8.Maclure M, Mittleman MA. Should we use a case-crossover design? Annu Rev Public Health 2000;21:193–221 [DOI] [PubMed] [Google Scholar]
- 9.Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med 2006;25:1768–1797 [DOI] [PubMed] [Google Scholar]
- 10.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med 2004;351:2611–2618 [DOI] [PubMed] [Google Scholar]
- 11.Elkind MS, Carty CL, O'Meara ES, et al. Hospitalization for infection and risk of acute ischemic stroke: the Cardiovascular Health Study. Stroke 2011;42:1851–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paganini-Hill A, Lozano E, Fischberg G, et al. Infection and risk of ischemic stroke: differences among stroke subtypes. Stroke 2003;34:452–457 [DOI] [PubMed] [Google Scholar]
- 13.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007;298:1763–1771 [DOI] [PubMed] [Google Scholar]
- 14.Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med 2005;352:1436–1444 [DOI] [PubMed] [Google Scholar]
- 15.Kyaw MH, Rose CE, Jr, Fry AM, et al. The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. J Infect Dis 2005;192:377–386 [DOI] [PubMed] [Google Scholar]
- 16.Breiman RF, Spika JS, Navarro VJ, Darden PM, Darby CP. Pneumococcal bacteremia in Charleston County, South Carolina: a decade later. Arch Intern Med 1990;150:1401–1405 [PubMed] [Google Scholar]
- 17.Robinson KA, Baughman W, Rothrock G, et al. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995–1998: opportunities for prevention in the conjugate vaccine era. JAMA 2001;285:1729–1735 [DOI] [PubMed] [Google Scholar]
- 18.Kissela B, Schneider A, Kleindorfer D, et al. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke 2004;35:426–431 [DOI] [PubMed] [Google Scholar]
- 19.Howard VJ, Kleindorfer DO, Judd SE, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol 2011;69:619–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juster FT, Suzman R. An overview of the Health and Retirement Study. J Hum Resour 1995;30:S7–S56 [Google Scholar]
- 21.Reker DM, Hamilton BB, Duncan PW, Yeh SC, Rosen A. Stroke: who's counting what? J Rehabil Res Dev 2001;38:281–289 [PubMed] [Google Scholar]
- 22.Roumie CL, Mitchel E, Gideon PS, Varas-Lorenzo C, Castellsague J, Griffin MR. Validation of ICD-9 codes with a high positive predictive value for incident strokes resulting in hospitalization using Medicaid health data. Pharmacoepidemiol Drug Saf 2008;17:20–26 [DOI] [PubMed] [Google Scholar]
- 23.Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA 2011;306:2248–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagger JP, Zindrou D, Taylor KM. Postoperative infection with methicillin-resistant Staphylococcus aureus and socioeconomic background. Lancet 2004;363:706–708 [DOI] [PubMed] [Google Scholar]
- 25.Wallace TA, Prueitt RL, Yi M, et al. Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res 2008;68:927–936 [DOI] [PubMed] [Google Scholar]
- 26.Koshiol J, Lam TK, Gridley G, Check D, Brown LM, Landgren O. Racial differences in chronic immune stimulatory conditions and risk of non-Hodgkin's lymphoma in veterans from the United States. J Clin Oncol 2011;29:378–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.US Department of Health and Human Services Office of Minority Health. Available at: http://minorityhealth.hhs.gov/templates/content.aspx?ID=3020. Accessed February 14, 2013.
- 28.US Department of Health and Human Services National Healthcare Disparities Report 2008: AHRQ Publication No. 09–0002. Rockville, MD: Agency for Healthcare Research and Quality; 2009. Available at: http://www.ahrq.gov/qual/qrdr08.htm. Accessed February 13, 2013 [Google Scholar]
- 29.Mattila KJ, Valtonen VV, Nieminen MS, Asikainen S. Role of infection as a risk factor for atherosclerosis, myocardial infarction, and stroke. Clin Infect Dis 1998;26:719–734 [DOI] [PubMed] [Google Scholar]
- 30.Grau AJ, Buggle F, Steichen-Wiehn C, et al. Clinical and biochemical analysis in infection-associated stroke. Stroke 1995;26:1520–1526 [DOI] [PubMed] [Google Scholar]
- 31.Testai FD, Gorelick PB. Stroke in black patients. In: Goldstein LB, ed. Primer on Stroke Prevention and Treatment: An Overview Based on AHA/ASA Guidelines. New York: John Wiley & Sons; 2009:129–141 [Google Scholar]
- 32.Macko RF, Ameriso SF, Barndt R, Clough W, Weiner JM, Fisher M. Precipitants of brain infarction: roles of preceding infection/inflammation and recent psychological stress. Stroke 1996;27:1999–2004 [DOI] [PubMed] [Google Scholar]
- 33.Syrjänen J, Valtonen VV, Iivanainen M, Kaste M, Huttunen JK. Preceding infection as an important risk factor for ischaemic brain infarction in young and middle aged patients. BMJ 1988;296:1156–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grau AJ, Fischer B, Barth C, Ling P, Lichy C, Buggle F. Influenza vaccination is associated with a reduced risk of stroke. Stroke 2005;36:1501–1506 [DOI] [PubMed] [Google Scholar]
- 35.Gurfinkel EP, de la Fuente RL, Mendiz O, Mautner B. Influenza vaccine pilot study in acute coronary syndromes and planned percutaneous coronary interventions: the FLU Vaccination Acute Coronary Syndromes (FLUVACS) Study. Circulation 2002;105:2143–2147 [DOI] [PubMed] [Google Scholar]
- 36.Davis MM, Taubert K, Benin AL, et al. Influenza vaccination as secondary prevention for cardiovascular disease: a science advisory from the American Heart Association/American College of Cardiology. Circulation 2006;114:1549–1553 [DOI] [PubMed] [Google Scholar]
- 37.Tseng HF, Slezak JM, Quinn VP, Sy LS, Van den Eeden SK, Jacobsen SJ. Pneumococcal vaccination and risk of acute myocardial infarction and stroke in men. JAMA 2010;303:1699–1706 [DOI] [PubMed] [Google Scholar]
- 38.Flannery B, Schrag S, Bennett NM, et al. Impact of childhood vaccination on racial disparities in invasive Streptococcus pneumoniae infections. JAMA 2004;291:2197–2203 [DOI] [PubMed] [Google Scholar]
- 39.Keppel K, Garcia T, Hallquist S, Ryskulova A, Agress L. Comparing racial and ethnic populations based on Healthy People 2010 objectives. Healthy People Stat Notes 2008:1–16 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


