Abstract
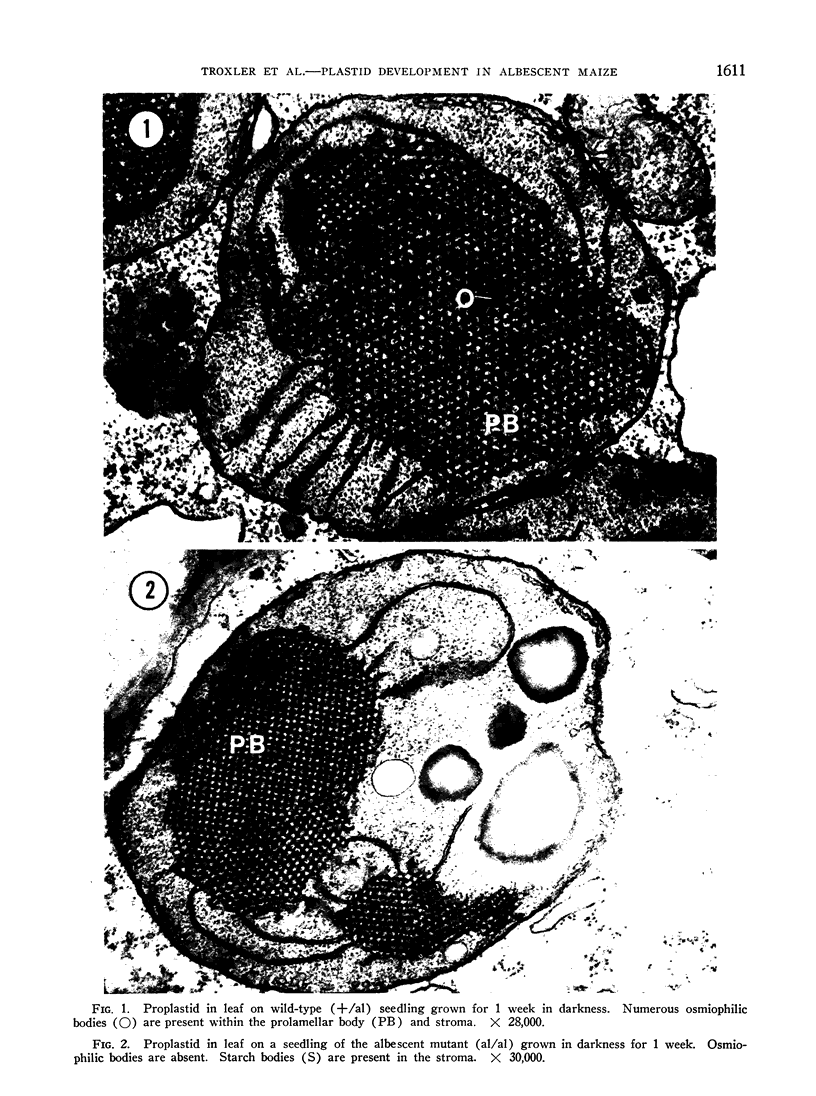
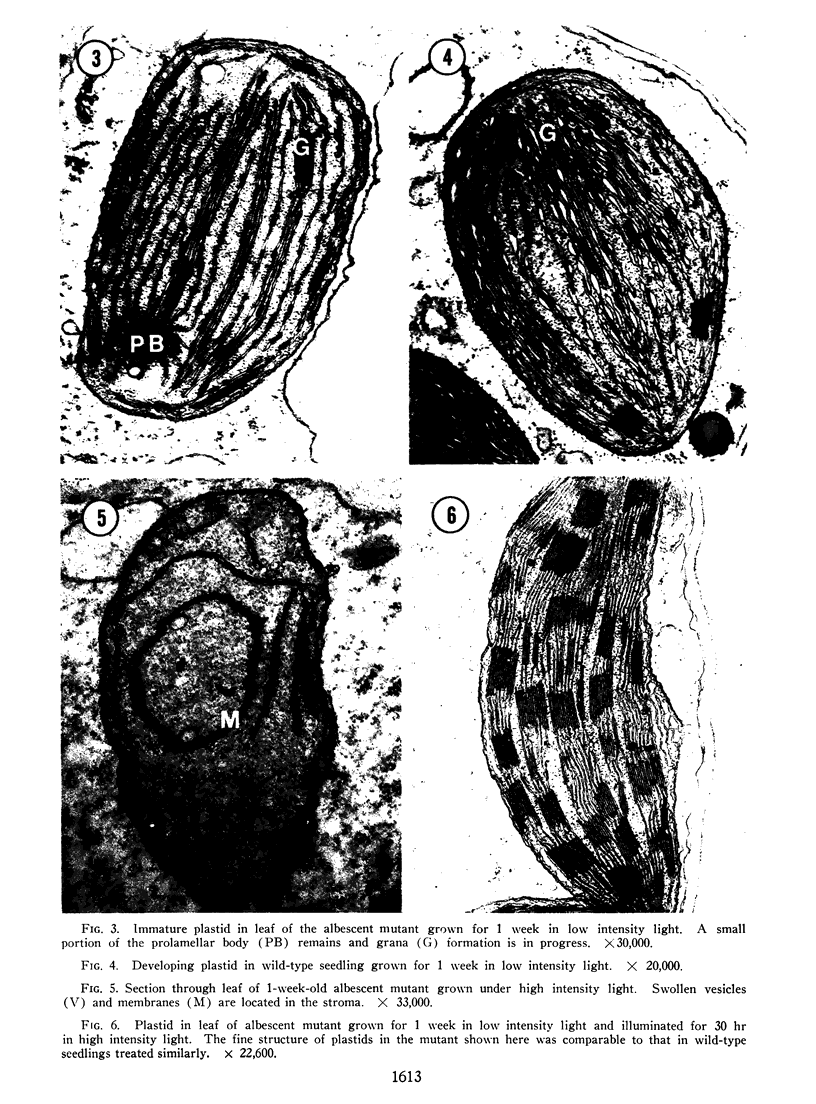
Plastid development in albescent (al/al) and wild-type (+/al) strains of Zea mays has been studied in the electron microscope. Etiolated seedlings of the mutant are severely deficient in colored carotenoid pigments and accumulate carotenoid precursors tentatively identified as phytoene and phytofluene. The fine structure of proplastids in etiolated wild-type and mutant leaves is similar with 1 notable exception. Osmiophilic bodies found in the wild-type were lacking in all sections of albescent proplastids examined suggesting that these structures may be storage centers for carotenoid pigments. Plastid pigments are destroyed, chlorophyll synthesizing potential is lost, and the ultrastructure of plastids is irreversibly altered when mutant seedlings are placed directly in high intensity light. However, synthesis of plastid pigments and development of the photosynthetic apparatus as seen in the electron microscope is normal, and indistinguishable from that in the wild-type, in seedlings of the albescent mutant preilluminated with low intensity light prior to high intensity illumination. During treatment in low intensity light carotenogenesis is initiated in the mutant and proceeds normally thereafter.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson I. C., Robertson D. S. Role of Carotenoids in Protecting Chlorophyll From Photodestruction. Plant Physiol. 1960 Jul;35(4):531–534. doi: 10.1104/pp.35.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M. D., Robertson D. S., Bowen C. C., Anderson I. C. Chloroplast development in pigment deficient mutants of maize. I. Structural anomalies in plastids of allelic mutants at the w3 locus. J Ultrastruct Res. 1967 Nov;21(1):41–60. doi: 10.1016/s0022-5320(67)80005-4. [DOI] [PubMed] [Google Scholar]
- Barr R., Arntzen C. J. The Occurence of delta-Tocopherylquinone in Higher Plants and Its Relation to Senescence. Plant Physiol. 1969 Apr;44(4):591–598. doi: 10.1104/pp.44.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSKI V. M., SMITH J. H. C. Chlorophyll formation in a mutant, white seedling-3. Arch Biochem Biophys. 1951 Nov;34(1):189–195. doi: 10.1016/s0003-9861(51)80024-9. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGER R., ZALOKAR M. Pigments and photosynthesis in a carotenoid-deficient mutant of Chlamydomonas. Nature. 1958 Jul 12;182(4628):98–100. doi: 10.1038/182098a0. [DOI] [PubMed] [Google Scholar]
- Sander C., Laber L. J., Bell W. D., Hamilton R. H. Light sensitivity of plastids and plastid pigments present in the albescent maize mutant. Plant Physiol. 1968 May;43(5):693–697. doi: 10.1104/pp.43.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D. M. A classification of biologic lipids based upon their interaction in aqeous systems. J Am Oil Chem Soc. 1968 Mar;45(3):108–119. doi: 10.1007/BF02915334. [DOI] [PubMed] [Google Scholar]
- Smith J. H., Durham L. J., Wurster C. F. Formation and Bleaching of Chlorophyll in Albino Corn Seedlings. Plant Physiol. 1959 May;34(3):340–345. doi: 10.1104/pp.34.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]