Abstract
The most common mechanism for human exposure to hantaviruses throughout North America is inhalation of virally contaminated particulates. However, risk factors associated with exposure to particulates potentially contaminated with hantaviruses are generally not well understood. In North America, Sin Nombre virus (SNV) is the most common hantavirus that infects humans, causing hantavirus pulmonary syndrome, which has a significant mortality rate (approximately 35%). We investigated human exposure to particulate matter and evaluated the effects of season, location (sylvan and peridomestic environment), and activity (walking and sweeping) on generation of particulates at the breathing zone (1.5 m above the ground). We found greater volumes of small inhalable particulates during the spring and summer compared to the fall and winter seasons and greater volumes of small inhalable particulates produced in peridomestic, compared to sylvan, environments. Also, greater volumes of particulates were generated at the breathing zone while walking compared to sweeping. Results suggest that more aerosolized particles were generated during the spring and summer months. Our findings suggest that simply moving around in buildings is a significant source of human exposure to particulates, potentially contaminated with SNV, during spring and summer seasons. These findings could be advanced by investigation of what particle sizes SNV is most likely to attach to, and where in the respiratory tract humans become infected.
Keywords: Sin Nombre virus, Hantavirus pulmonary syndrome, Exposure risk, Deer mouse, Peromyscus maniculatus, Particulate matter
Introduction
Aerosolized virally contaminated particulates are the major source of human exposure to hantaviruses globally. In North America, Sin Nombre virus (SNV, Bunyaviridae: Hantavirus) is responsible for the majority of hantavirus pulmonary syndrome (HPS) cases, many of which (approximately 35%) are fatal. This virus was first recognized in 1993 in the Four Corners region of the United States (Arizona, New Mexico, Colorado, and Utah) (Nichol et al. 1993) following this outbreak. The reservoir host for SNV was determined to be the widespread, native deer mouse (Peromyscus maniculatus). Since 1993, numerous ecological studies have focused on SNV dynamics in deer mouse populations, but few studies have investigated the mechanisms of human exposure. Here, we investigate human exposure to aerosolized and potentially virally contaminated particulate matter.
The capability of hantaviruses to survive outside the host is critical for the transmission dynamics within rodent populations and from rodents to humans (Schmaljohn et al. 1998; Kallio et al. 2006; Gedeon et al. 2010). Research suggests that humans have the greatest risk of SNV exposure during the early to middle period of the deer mouse breeding season (spring–summer) when SNV transmission among deer mice, and consequently human exposure risk, is likely greatest (Childs et al. 1995a; Zeitz et al. 1995; Mills 2005). Exposure is typically associated with sweeping and cleaning activities within peridomestic settings, but may also occur in sylvan settings (Mills et al. 1999). The number of HPS cases has been greatest in rural peridomestic environments, where SNV transmission among deer mice is greater than adjacent sylvan settings (Armstrong et al. 1995; Childs et al. 1995a; Zeitz et al. 1995). Sweeping or cleaning in peridomestic settings such as barns and outbuildings, could increase human exposure risk to aerosolized particles contaminated with SNV from deer mouse excreta (Armstrong et al. 1995; Childs et al. 1995b; Zeitz et al. 1995). Furthermore, SNV contaminated particulate matter has been hypothesized to survive longer within peridomestic settings because the virus is sheltered from external environmental conditions, such as wind and ultraviolet radiation, that would disperse the contaminated deer mouse excreta and degrade the virus (Carver et al. 2010; Gedeon et al. 2010).
Little is known about how human use of peridomestic settings contributes to potential SNV exposure (Cline et al. 2010). More specifically, human exposure to particulate matter potentially contaminated with SNV has not been studied. Here, we investigated particle exposure while walking through and sweeping in peridomestic and sylvan settings. We hypothesized that there would be a greater concentration of inhalable aerosolized particulate matter generated within peridomestic environments than in sylvan environments due to particulate accumulation within a confined space (buildings). Furthermore, we hypothesized that sweeping or cleaning activities would generate greater concentrations of aerosol particulate due to large amounts of dust and debris being disturbed. Finally, we expected that there would be greater volumes of aerosol particulate in the spring and summer seasons when most cases of HPS are contracted.
Materials and Methods
Study Sites and Sampling
We selected four replicate sampling locations (latitude, longitude) in western Montana: a ranch near Cascade (46.993, −111.577); a ranch near Anaconda (46.038, −112.791); a ranch near Twin Bridges (45.406, −112.448); and a ranch near Glen (45.516, −112.696). At each site, we designated one sampling location as a peridomestic environment (barn) and one as a sylvan environment (field).
The barns all contained corrals, soil, and straw floors and large sliding or swinging doors on each end of the structure. We chose barns because they represent the kind of environments in which humans may become exposed to SNV (Cline et al. 2010). Deer mice utilize a wide variety of sylvan habitat types (i.e., Mills et al. 1998; Douglass et al. 2001) and, here, we focused on nearby (50 m from peridomestic sites) areas containing soil, gravel, and rocks subjected to outdoor elements characteristic of each season in Montana, and representative of sylvan settings frequently used by deer mice (Douglass et al. 2001; Jay et al. 1997; Mills et al. 1998; Otteson et al. 1996). These settings contained comparable substrate (particularly open soil) to periodomestic sites, and a potential source of particles (dust from soil particulates) that could become infected with SNV resulting in human exposure.
We sampled each site once a month during the fall, winter, spring, and summer seasons and under climatic conditions considered “typical” for those seasons. Sampling occurred during the afternoon and consisted of a 30 min particulate matter sample collection while walking, and a 30 min particulate matter sample collection while sweeping with 10 min between periods in both peridomestic and sylvan environments. Both walking and sweeping were performed by a single individual within 3 m of two different air samplers. We placed samplers on stands in the center of the barn lane 1.5 m above ground level. Height of the sampler was intended to reflect an approximate “typical” zone in which individuals inhale and exhale (hereafter referred to as the “breathing zone”). We acknowledge that the height of an individual varies depending on activity, but examination of the elevation profile in particle production was beyond the scope of this study. Walking and sweeping actions were undertaken in circular patterns within 3 m surrounding the sample units. We randomized the order of location and activity during data collection at each site, to avoid potential temporal bias in the data.
We used a Lighthouse model 3016 direct reading laser particle counter and an SKC Airchek® personal sampling pump (model 224-PCXR4) in accordance with occupational safety and health guidelines to sample inhalable particulate matter. The lighthouse particle counter records particle counts at six simultaneous cut points: 0.3, 0.5, 1.0, 5.0, and 10.0 µm. During each sample collection, protection from potential SNV exposure was provided by wearing protective eyewear, a respirator fitted with P100 filters and nitrile gloves, in accordance with institutional OHS guidelines. There were technical challenges encountered while sampling that resulted in occasional sampling efforts being abandoned. In particular, environmental limitations in functioning of the sampler precluded data collection outside during snowfall events. On occasion, equipment malfunctions precluded collection of a small number of samples. However, the majority (75%) of sampling attempts were successful.
Analyses
To examine the effects of season, location (peridomestic vs. sylvan), and activity (sweeping vs. walking) on the amount of particulate matter for each particle size category, we evaluated the data using a one way ANOVA. Prior to analysis, data were evaluated for normality and equality of variances using Shapiro–Wilk’s test of normality and Levene’s test for equality of variances, respectively. Non-normal data were normalized by log transformation. Where significant effects were observed, Tukey’s post-hoc tests were used to evaluate differences among treatment groups.
Results
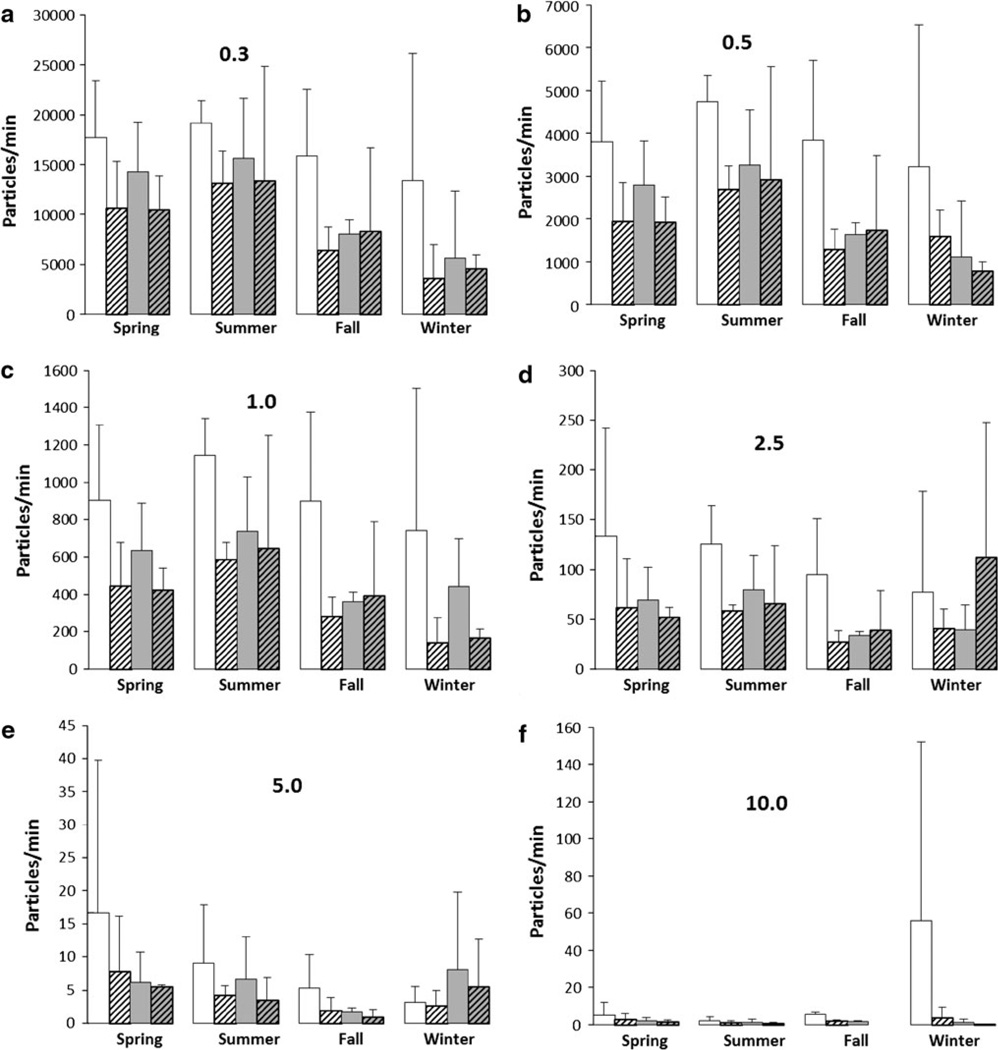
Walking and sweeping produced particles at the breathing zone (1.5 m above the ground) in both peridomestic and sylvan locations and during all seasons (Fig. 1). Across all activities, locations and seasons, large average volumes of particles were produced in the smallest particle size category (0.3 µm, Fig. 1a) and the volume of aerosolized particles produced per minute declined with increasing particle size.
Figure 1.
Particles produced while inside (white) or outside (gray), performing sweeping (hatched) or walking (clear), throughout each season in central and southwest Montana 2011–2012. Numbers represent mean (±SD) number of particles produced per minute for each size category (0.3, 0.5, 1, 2.5, 5 and 10).
We observed greater particle count concentrations in spring and summer relative to fall and winter seasons for particle sizes ≤1 µm (Fig. 1a–c; Table 1). However, this observation did not hold for particle sizes >1 µm (Fig. 1d–f). We also observed a general trend for greater particle production in peridomestic settings, than sylvan (Fig. 1; Table 1). This observation, however, was only significant for the 0.5 µm particle size category. We found that at the height of the sampler, a greater volume of particles was aerosolized per minute from walking than sweeping for particle sizes ≤1 µm (Fig. 1a–c; Table 1). For particle sizes ≤2.5 µm, there was a trend for disproportionately more particles to be produced from walking in peridomestic locations than in sylvan settings, relative to sweeping in either location (Fig. 1d; Table 1).
Table 1.
Effects of Season, Location, Activity and Interactions on the Number of Particles Produced per Minute in Central and Southwest Montana 2011–2012.
| Particle size | Season | Location | Activity | Season × location | Season × activity | Location × activity | Season × location × activity | Error | |
|---|---|---|---|---|---|---|---|---|---|
| 0.3 | F | 4.493 | 2.095 | 10.436 | 0.162 | 0.060 | 2.746 | 0.211 | 32 |
| df | 3 | 1 | 1 | 3 | 3 | 1 | 3 | ||
| P | 0.010* | 0.157 | 0.003 | 0.921 | 0.980 | 0.107 | 0.888 | ||
| 0.5 | F | 3.121 | 4.502 | 11.759 | 0.32 | 0.128 | 3.524 | 0.161 | 32 |
| df | 3 | 1 | 1 | 3 | 3 | 1 | 3 | ||
| P | 0.040# | 0.042 | 0.002 | 0.811 | 0.943 | 0.070 | 0.922 | ||
| 1 | F | 2.796 | 3.205 | 15.717 | 0.154 | 0.028 | 4.06 | 0.169 | 32 |
| df | 3 | 1 | 1 | 3 | 3 | 1 | 3 | ||
| P | 0.056 | 0.083 | <0.001 | 0.927 | 0.993 | 0.052 | 0.916 | ||
| 2.5 | F | 0.780 | 1.392 | 3.714 | 0.328 | 0.839 | 3.919 | 0.133 | 32 |
| df | 3 | 1 | 1 | 3 | 3 | 1 | 3 | ||
| P | 0.514 | 0.247 | 0.063 | 0.805 | 0.483 | 0.056 | 0.940 | ||
| 5 | F | 1.180 | 0.366 | 1.761 | 0.652 | 0.115 | 0.263 | 0.149 | 32 |
| df | 3 | 1 | 1 | 3 | 3 | 1 | 3 | ||
| P | 0.333 | 0.549 | 0.194 | 0.587 | 0.951 | 0.611 | 0.929 | ||
| 10 | F | 1.187 | 1.343 | 0.989 | 1.153 | 0.694 | 0.663 | 0.663 | 32 |
| df | 3 | 1 | 1 | 3 | 3 | 1 | 3 | ||
| P | 0.330 | 0.255 | 0.328 | 0.343 | 0.562 | 0.422 | 0.581 |
Analyses performed using ANOVA. Significant (P ≤ 0.05) effects shown in bold. Where significant differences were observed among seasons, findings from post-hoc comparisons (*summer > fall and winter, spring > winter; #summer > winter).
Discussion
Despite the apparent importance of inhalation of virally contaminated particulates during common human activity in hantavirus exposure, the topic has not been well studied. To address the lack of study, we investigated the effects of season, location (inside vs. outside), and activity (walking vs. sweeping) on particulate exposure at the breathing zone (1.5 m above the ground). Our discoveries about particulate aerosolization and disturbance open a new avenue of research into potential SNV exposure, in relation to when particles may be contaminated with virus. We found significant aerosolization of small (≤1 µm) particulate matter at the breathing zone and, as expected, this declined with increasing particle size. There is currently no information on the binding of SNV shed on to particulates in the environment, but if SNV binds primarily to small particulates sizes or is aerosolized as a small particulate itself, then our results suggest significant exposure risk. We also found a general increase in small particulate matter within the peridomestic environment, as compared to sylvan settings. This effect could be due to the lack of ambient moisture and stifled air flow within peridomestic settings, compared to sylvan settings, resulting in peridomestic environments being more suitable for small particles to linger at the breathing zone. Greater aerosolization of small particles in peridomestic environments is consistent with our initial hypothesis, and reinforces the findings of other studies suggesting greater human exposure risk in peridomestic versus exposure in sylvan settings (Armstrong et al. 1995; Gedeon et al. 2010).
Contrary to our initial hypothesis, we found that walking aerosolized significantly more small particulate matter (≥2.5 µm) at the breathing zone than sweeping. This finding has important implications for exposure risk to SNV. If human exposure is a function of the amount of SNV shed into the environment, the amount of particulate exposure (Carver et al. 2010) and persistence of virus in the environment (Kallio et al. 2006), then simply moving around in buildings (which is more common than sweeping) may pose a significantly greater infection risk than sweeping. We acknowledge that this result appears counterintuitive, because sweeping produces a visually greater amount of particulates, and other studies suggest sweeping may be an important determinant of human exposure to SNV (Armstrong et al. 1995). Our results suggest that walking lifts more small particulate matter higher (up to the 1.5 m height of the sampler), whereas sweeping appears to aerosolize particulates at a lower elevation. It is important to note that both activities produce large amounts of small particulate matter at the breathing zone, and the difference between walking and sweeping does not necessarily exclude potential exposure to SNV by one method or the other. In addition, our experimental design assumed that particulate exposure took place at 1.5 m above the ground. This altitude will likely vary among individuals performing the activities, thus possibly submitting individuals varied particle exposure. We hypothesize that sweeping produces significantly more particulate matter at lower vertical profiles, and that there may be an interaction between activity type and height of SNV exposure risk.
This study also demonstrates that small particle aerosolization is greatest in summer and spring. This is an important finding because spring and summer is when most SNV transmission takes place among deer mice, and when most human cases of HPS occur (Zeitz et al. 1995; Douglass et al. 2001; Kuenzi et al. 2001). Furthermore, it has been shown that humans are likely to enhance the frequency of activities within peridomestic settings that aerosolize particulate matter during these seasons, such as walking in and out of buildings, sweeping and cleaning (Cline et al. 2010). Taken together, these findings suggest that human exposure to virally contaminated particulates may result from the additive effect of increased seasonal transmission in the reservoir host, increased seasonal use of buildings and activities within buildings, and increased propensity of particulates to be aerosolized during the spring and summer months. Additional research that would be valuable to further understand human exposure risk would be to determine which size particulates SNV typically binds. It is also unclear how far the SNV contaminated particles must penetrate into respiratory system for humans to become infected.
We experienced two challenges in this study that had some influence on data collection. We were unable to use the particle sampler when there was precipitation—as a result we avoided sampling when environmental conditions produced rain or snow. However, we were not always able to avoid such conditions and on occasion were unable to collect data from some sites, particularly outside during winter. This likely inflated the standard deviations in our data, but we believe the data are still broadly representative. The second challenge we encountered was occasional equipment malfunctions in the recording of data. This problem was rarely encountered and every effort was made to re-sample the site within the season when these instances occurred. Nevertheless, there were three occasions where we were unable to re-sample, due to the remoteness of some of our sites. Despite these challenges the majority of sampling was successful, and the impact of weather and equipment failures on the outcomes of this study appear minimal.
In conclusion, this study contributes to a broader understanding of human exposure to particulate matter potentially contaminated with SNV. In particular, particulate exposure at the breathing zone (1.5 m) appears greatest while walking in peridomestic settings during summer and spring. Greater particle exposure while walking compared to sweeping in buildings, suggests an elevated potential for SNV exposure than previously recognized. Differences in particle exposure among seasons and between peridomestic and sylvan environments coincide with known timing and locations of human exposure to SNV. Our results suggest there may be an additive effect of SNV dynamics in the deer mouse reservoir, human activities and propensity of particulate matter to be aerosolized that contribute to HPS incidence. In light of findings in this study, it would be valuable for future research on human exposure risk to SNV to (1) examine how vertical profile influences particulate exposure, (2) to screen particulate matter for SNV contamination, and (3) determine particle sizes to which SNV is most likely to bind.
Acknowledgments
We thank the private ranch owners at Cascade, Twin Bridges, Glen, and Gregson for allowing us access to their property. This project was supported by grants from the National Center for Research Resources (5P20RR016455-11) and the National Institute of General Medical Sciences (8 P20 GM103474-11) from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health. This work followed all relevant environmental and institutional regulations in the collection of data presented here.
References
- Armstrong LR, Zaki SR, Goldoft MJ, Todd RL, Khan AS, Khabbaz RF, et al. Hantavirus pulmonary syndrome associated with entering or cleaning rarely used, rodent-infested structures. Journal of Infectious Diseases. 1995;172:1166. doi: 10.1093/infdis/172.4.1166. [DOI] [PubMed] [Google Scholar]
- Carver S, Kilpatrick AM, Kuenzi A, Douglass R, Ostfeld RS, Weinstein P. Integration of environmental monitoring to enhance comprehension and control of infectious diseases. Journal of Environmental Monitoring. 2010;12:2048–2055. doi: 10.1039/c0em00046a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs JE, Krebs JW, Ksiazek TG, Maupin GO, Gage KL, Rollin PE, et al. A household-based, case–control study of environmental-factors associated with hantavirus pulmonary syndrome in the southwestern United States. American Journal of Tropical Medicine and Hygiene. 1995;52:393–397. doi: 10.4269/ajtmh.1995.52.393. [DOI] [PubMed] [Google Scholar]
- Childs JE, Mills JN, Glass GE. Rodent-borne hemorrhagic-fever viruses—a special risk for mammalogists. Journal of Mammalogy. 1995;76:664–680. [Google Scholar]
- Cline B, Carver S, Douglass R. Relationship of human behavior within outbuildings to potential exposure to Sin Nombre virus in western Montana. EcoHealth. 2010;7:389–393. doi: 10.1007/s10393-010-0318-x. [DOI] [PubMed] [Google Scholar]
- Douglass RJ, Wilson T, Semmens WJ, Zanto SN, Bond CW, Van Horn RC, et al. Longitudinal studies of Sin Nombre virus in deer mouse-dominated ecosystems of Montana. American Journal of Tropical Medicine and Hygiene. 2001;65:33–41. doi: 10.4269/ajtmh.2001.65.33. [DOI] [PubMed] [Google Scholar]
- Gedeon T, Bodelon C, Kuenzi A. Hantavirus transmission in sylvan and peridomestic environments. Bulletin of Mathematical Biology. 2010;72:541–564. doi: 10.1007/s11538-009-9460-4. [DOI] [PubMed] [Google Scholar]
- Jay M, Ascher MS, Chomel BB, Madon M, Sesline D, Enge BA, et al. Seroepidemiologica studies of hantavirus infection among wild rodents in California. Emerging Infectious Diseases. 1997;3:183–190. doi: 10.3201/eid0302.970213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio ER, Poikonen A, Vaheri A, Vapalahti O, Henttonen H, Koskela E, et al. Maternal antibodies postpone hantavirus infection and enhance individual breeding success. Proceedings of the Royal Society of London B: Biological Sciences. 2006;273:2771–2776. doi: 10.1098/rspb.2006.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzi AJ, Douglass RJ, White D, Bond CW, Mills JN. Antibody to Sin Nombre virus in rodents associated with peridomestic habitats in west central Montana. American Journal of Tropical Medicine and Hygiene. 2001;64:137–146. doi: 10.4269/ajtmh.2001.64.137. [DOI] [PubMed] [Google Scholar]
- Mills JN. Regulation of rodent-borne viruses in the natural host: implications for human disease. Archives of Virology. 2005;(Suppl 19):45–57. doi: 10.1007/3-211-29981-5_5. [DOI] [PubMed] [Google Scholar]
- Mills JN, Johnson JM, Ksiazek TG, Ellis BA, Rollin PE, Yates TL, et al. A survey of hantavirus antibody in small-mammal populations in selected United States National Parks. American Journal of Tropical Medicine and Hygiene. 1998;58:525–532. doi: 10.4269/ajtmh.1998.58.525. [DOI] [PubMed] [Google Scholar]
- Mills JN, Ksiazek TG, Peters CJ, Childs JE. Long-term studies of hantavirus reservoir populations in the southwestern United States: a synthesis. Emerging Infectious Diseases. 1999;5:135–142. doi: 10.3201/eid0501.990116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol ST, Spiropoulou CF, Morzunov S, Rollin PE, Ksiazek TG, Feldmann H, et al. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science. 1993;262:914–917. doi: 10.1126/science.8235615. [DOI] [PubMed] [Google Scholar]
- Otteson EW, Riolo J, Rowe JE, Nichol ST, Ksiazek TG, Rollin PE, et al. Occurrence of hantavirus within the rodent population of northeastern California and Nevada. American Journal of Tropical Medicine and Hygiene. 1996;54:127–133. doi: 10.4269/ajtmh.1996.54.127. [DOI] [PubMed] [Google Scholar]
- Schmaljohn C, Juggins J, Calisher CH. Laboratory and field safety. In: Schmaljohn C, Calisher CH, Lee HW, editors. Manual of Hemorrhagic Fever with Renal Syndrome and Hantavirus Pulmonary Syndrome. 2nd ed. Seoul: WHO Collaborating Center for Virus Reference and Research (Hantaviruses) Asian Institute for Life Sciences; 1998. pp. 192–198. [Google Scholar]
- Zeitz PS, Butler JC, Cheek JE, Samuel MC, Childs JE, Shands LA, et al. A case control study of hantavirus pulmonary syndrome during an outbreak in the southwestern United States. Journal of Infectious Diseases. 1995;171:864–870. doi: 10.1093/infdis/171.4.864. [DOI] [PubMed] [Google Scholar]