Abstract
In the natural environment bacteria predominantly live adhered to a surface as part of a biofilm. While many of the components needed for biofilm assembly are known, the mechanism by which microbes sense and respond to contact with a surface is poorly understood. Bacillus subtilis is a Gram-positive model for biofilm formation. The DegS–DegU two-component system controls several multicellular behaviours in B. subtilis, including biofilm formation. Here we identify the B. subtilis flagellum as a mechanosensor that activates the DegS–DegU regulatory pathway. Inhibition of flagellar rotation by deletion or mutation of the flagellar stator gene, motB, results in an increase in both degU transcription and DegU∼P driven processes, namely exoprotease production and poly-γ-dl-glutamic acid biosynthesis. Similarly, inhibition of flagellar rotation by engaging the flagellar clutch or by tethering the flagella with antibodies also promotes an increase in degU transcription that is reflective of increased DegU∼P levels in the cell. Collectively, these findings strongly indicate that inhibition of flagellar rotation acts as a mechanical trigger to activate the DegS–DegU two-component signal transduction system. We postulate that inhibition of flagellar rotation could function as a mechanical trigger to activate bacterial signal transduction cascades in many motile bacteria upon contact with a surface.
Introduction
Persistent adhesion of bacterial cells to a surface is the first step in the formation of a biofilm – a complex community of bacteria encased in a self-produced exopolymeric matrix (Flemming and Wingender, 2010). The settlement of microbes on a surface within the confines of a biofilm can confer many advantages to the population, including increased access to nutrients and protection from environmental stress (Costerton et al., 1995). Despite significant recent advances in our understanding of the regulatory pathways and key building blocks required for the nucleation and growth of biofilms for many species of bacteria (Flemming and Wingender, 2010; Lopez et al., 2010; Vlamakis et al., 2013), it is not fully understood how a motile cell senses and responds to a surface.
The bacterial flagellum is a complex molecular machine comprised of over 30 different proteins and is organized into three main structural domains: the basal body, hook and filament (Chevance and Hughes, 2008). The filament acts as a propeller to drive movement and is powered by a rotary motor that comprises stator and rotor protein complexes and can be driven by proton-motive or sodium-motive force (Manson et al., 1977; Chernyak et al., 1983). Torque is generated by specific interactions between the rotor and stator components (Zhou et al., 1998a). The stator complex, which in Escherichia coli comprises a complex of four MotA proteins and two MotB proteins, forms two proton channels (Braun et al., 1999; Kojima and Blair, 2004). It is thought that proton flux through the channels triggers a conformational change that alters the electrostatic interactions between MotA and the rotor protein FliG, resulting in torque generation (Zhou et al., 1998a). As well as being required as a mechanical device for propulsion, the flagellum is also crucial for biofilm development in many bacterial species due to its role in the initial stages of surface adhesion (O'Toole et al., 2000).
Bacillus subtilis is a Gram-positive, non-pathogenic, soil-dwelling bacterium that has emerged as a model organism for the study of biofilm formation (Vlamakis et al., 2013). The development of the B. subtilis biofilm is tightly controlled and requires the activation of three transcriptional regulators: ComA (Lopez et al., 2009c), Spo0A (Branda et al., 2001; Hamon and Lazazzera, 2001) and DegU (Stanley and Lazazzera, 2005). Previous studies have delineated many of the signals required to activate both ComA and Spo0A. At high cell density the quorum sensing transcription factor, ComA is phosphorylated, resulting in production of the lipopeptide, surfactin (Nakano et al., 1991). The production of surfactin, along with other signals that induce potassium leakage (Lopez et al., 2009a; Shank and Kolter, 2011) and osmotic stress (Rubinstein et al., 2012), triggers activation of a multicomponent phosphorelay which begins with the phosphorylation of one or more sensor histidine kinases (namely KinA to KinE, each of which has a unique role in signal perception) (see Vlamakis et al., 2013) and culminates in phosphorylation of Spo0A (Burbulys et al., 1991). The level of phosphorylated Spo0A (hereafter Spo0A∼P) within the cell dictates which bacterial behaviour will be stimulated or repressed (Fujita et al., 2005; Lopez et al., 2009b). Biofilm formation by B. subtilis requires a low level of Spo0A∼P to indirectly promote the transcription of the tapA-sipW-tasA and epsA-O operons (Fujita et al., 2005; Chai et al., 2011), which encode the extracellular biofilm matrix amyloid protein, TasA and proteins required for the synthesis of the biofilm matrix exopolysaccharide (EPS) respectively (Vlamakis et al., 2013). The third component required for B. subtilis biofilm formation is the hydrophobic coat protein, BslA (formerly YuaB) (Kobayashi and Iwano, 2012; Hobley et al., 2013). Transcription of the bslA gene is indirectly activated by phosphorylated DegU (hereafter DegU∼P) (Kobayashi, 2007; Ostrowski et al., 2011).
DegU is a response regulator that is phosphorylated by its cytoplasmic cognate sensor histidine kinase, DegS (Dahl et al., 1991). DegU∼P is a pleiotropic regulator that controls a myriad of processes, including flagella-based motility (Amati et al., 2004; Verhamme et al., 2007; Hsueh et al., 2011; Patrick and Kearns, 2012), biofilm formation (Kobayashi, 2007; Verhamme et al., 2007), exoprotease production (Dahl et al., 1992) and biosynthesis of the exopolymer poly-γ-dl-glutamic acid (hereafter γ-PGA) (Stanley and Lazazzera, 2005). The ability of DegU∼P to promote both motility and γ-PGA production is dependent on the small protein SwrA (Kearns et al., 2004; Stanley and Lazazzera, 2005; Calvio et al., 2008; Osera et al., 2009). The main role of SwrA is to regulate the number of flagellar hook-basal bodies in the cell (Kearns and Losick, 2005; Guttenplan et al., 2013). It is thought that the ability of DegU∼P to regulate several different processes is underpinned by variation in promoter affinities (Kobayashi, 2007; Murray et al., 2009). A small protein, DegQ, aids the transfer of the phosphoryl moiety from DegS to DegU (Kobayashi, 2007) with recent work suggesting that this is due to the ability of DegQ to stabilize the phosphorylated form of DegS in the presence of DegU (Do et al., 2011). Transcription of degQ is positively regulated by ComA and thus increases in response to cell density, thereby ensuring that DegU∼P levels also rise as growth approaches stationary phase (Msadek et al., 1991).
While many aspects of DegU activation and regulation are understood (for a review see Murray et al., 2009), the signal sensed by DegS to trigger phosphorylation of DegU has remained somewhat elusive. Previous studies have identified a link between the activity of the DegS–DegU system and osmolarity (Ruzal and Sanchez-Rivas, 1998), the structural maintenance of the chromosome (SMC)–ScpA–ScpB complex (Dervyn et al., 2004), ClpCP mediated proteolytic degradation (Ogura and Tsukahara, 2010), the RapG-PhrG quorum sensing system (Ogura et al., 2003) and, more recently, the completion status of the flagellar basal body (Hsueh et al., 2011). The aim of this work was to investigate the link between flagellar assembly and phosphorylation of DegU. We hypothesized that inhibition of flagellar rotation might trigger activation of the DegS–DegU two-component system. This would provide a mechanism to allow motile cells to detect and respond to the presence of a surface during the initial stages of biofilm formation. The data presented here identifies the B. subtilis flagellum as a mechanosensor. Deletion of the flagellar stator gene, motB, triggered an increase in DegU∼P levels, exemplified by an upregulation of degU transcription and two distinct DegU∼P driven processes, namely exoprotease production and γ-PGA biosynthesis. Further experiments designed to perturb flagellar rotation by genetic and non-genetic methods also resulted in elevated DegU∼P levels within the cell. We conclude that the DegS–DegU two-component regulatory system is activated by the lack of flagellar rotation. As the flagellar structure is highly conserved between microbial species, the arrest of flagellar rotation may present a mechanism by which many flagellated organisms detect and respond to a surface.
Results
Deletion of motB is associated with increased γ-PGA biosynthesis
To test if flagellar rotation was linked to the activity of the DegS–DegU two-component system, an in-frame non-polar deletion in the flagellar stator gene, motB was constructed (NRS3494). Disruption of the flagellar stator genes perturbs motility but has no effect on biosynthesis of the flagellum itself (Chevance and Hughes, 2008). Consistent with this, the ΔmotB strain synthesized flagella but displayed a non-motile phenotype (Fig. S1A and C). The observed motility defect was complemented upon re-introduction of the motB coding sequence on the chromosome under the control of an IPTG-inducible promoter (Phy-spank) at the non-essential amyE locus (NRS3775) verifying the specificity in the deletion (Fig. S1B). Strikingly, as shown in Fig. 1A, the ΔmotB strain displayed a mucoid colony phenotype on LB plates after growth overnight. The mucoid colony morphology was specific to deletion of motB as the colony morphology reverted to the flat dry phenotype exhibited by the wild-type strain upon heterologous expression of motB (Fig. 1A). Production of the exopolymer γ-PGA has been linked with mucoid colony morphology in B. subtilis (Stanley and Lazazzera, 2005). The relationship between the mucoid colony morphology of the motB deletion strain and γ-PGA production was confirmed as γ-PGA could be biochemically extracted from the culture supernatant collected at the onset of stationary phase upon deletion of motB (Fig. 1C and Fig. S1D).
Figure 1.
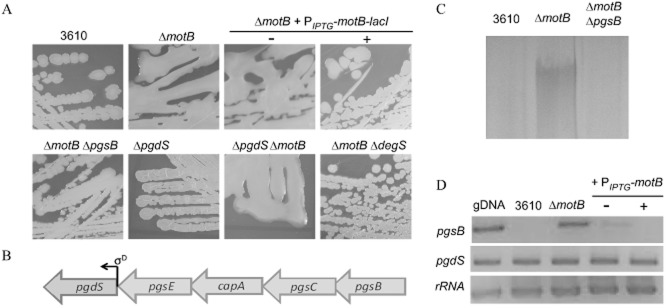
Deletion of motB from the chromosome is associated with γ-PGA production.A. Colony morphology of 3610 (wild-type), ΔmotB (NRS3494), ΔmotB + amyE::PIPTG-motB-lacI (NRS3775) grown on LB agar plate in the absence or presence of 50 μM IPTG, ΔmotB + pgsB::spc (NRS3434), ΔpgdS (NRS3347), ΔpgdSΔmotB (NRS3348) and ΔmotB + degS::cml (NRS3398).B. Schematic diagram of the γ-PGA synthesis operon and γ-PGA hydrolase gene. Arrows represent open reading frames (ORF), with the direction of the arrow indicating the direction of the ORF. The bent arrow represents the promoter located before the pgdS gene, which is driven by the alternative sigma factor, σD, as indicated.C. SDS-PAGE of γ-PGA collected from cultures of NCIB3610, ΔmotB (NRS3494) and ΔmotB + pgsB::spc (NRS3434) grown to the onset of stationary phase.D. Reverse-transcription-PCR analysis of pgsB and pgdS. Regions of DNA internal to pgsB and pgdS were amplified from cDNA generated from the wild-type (NCIB3610), ΔmotB (NRS3494) and ΔmotB + amyE::PIPTG-motB-lacI (NRS3775) grown in the absence and presence of 50 μM IPTG. Genomic DNA (gDNA) was used as a positive control for the PCR reaction and the ribosomal 16S rRNA was amplified as an internal control.
Increased γ-PGA biosynthesis in the absence of motB was predicted to be the consequence of: (i) decreased hydrolysis of γ-PGA and/or (ii) increased biosynthesis of γ-PGA. γ-PGA biosynthesis is driven by the protein products of the pgsB operon, while turnover is catalysed by the endo-γ-glutamyl peptidase, PgdS (Fig. 1B) (Candela and Fouet, 2006). Consistent with deletion of motB triggering increased γ-PGA biosynthesis, reverse-transcription (RT)-PCR analysis showed that transcription of the pgsB coding region could not be detected in NCIB3610, but was detectable upon deletion of motB (Fig. 1D). Furthermore, the mucoid phenotype of the ΔmotB strain was abolished upon disruption of pgsB (Fig. 1A). In contrast, the pgdS coding region was transcribed in NCIB3610, ΔmotB and the complemented strain (Fig. 1D). Consistent with these data, deletion of pgdS did not result in a mucoid colony morphology on LB agar plates indicating that the pgdS deletion strain did not phenocopy the motB strain (Fig. 1A). Collectively, these data demonstrate that deletion of motB triggers increased biosynthesis of the exopolymer γ-PGA.
Biosynthesis of γ-PGA is triggered by high levels of DegU∼P in B. subtilis NCIB3610
Consistent with our hypothesis that flagellar rotation might control the activity of the DegS–DegU two-component system, production of γ-PGA is linked with high levels of DegU∼P (Stanley and Lazazzera, 2005; Osera et al., 2009). Transcription of the γ-PGA synthetase gene, pgsB is directly regulated by DegU∼P (Ohsawa et al., 2009), supporting the hypothesis that γ-PGA biosynthesis is increased in the ΔmotB strain due to an increase in the level of DegU∼P. It is worth noting that in certain B. subtilis isolates, such as R0-FF-1, γ-PGA is a component of the biofilm matrix (Stanley and Lazazzera, 2005; Morikawa et al., 2006). However, in NCIB3610 this is not the case as γ-PGA is not synthesized, despite the presence of an intact γ-PGA biosynthetic operon on the chromosome (Branda et al., 2006; Srivatsan et al., 2008; Earl et al., 2012). We therefore hypothesized that in NCIB3610 the DegU∼P levels are suppressed and that this suppression was alleviated upon deletion of motB. To test if directly increasing the level of DegU∼P was sufficient to allow biosynthesis of γ-PGA by NCIB3610, we used a synthetic strain of NCIB3610 that contained a disruption in the native degU gene and carried an allele of degU containing an H12L amino acid mutation (degU32hy) under the control of an IPTG-inducible promoter at the non-essential amyE locus (NRS1325) (Verhamme et al., 2007) (Fig. 2A). The degU32 hy gene encodes a DegU variant that is described as exhibiting a slower rate of dephosphorylation by comparison with the wild-type protein, thus increasing the level of DegU∼P in the cell (Dahl et al., 1992). As shown in Fig. 2B, induction of degU32hy expression with 25 μM IPTG resulted in a highly mucoid colony phenotype. The relationship between the mucoid colony morphology and γ-PGA production was confirmed as γ-PGA could be biochemically extracted from culture supernatant collected at the onset of stationary phase upon induction of degU32 hy expression (Fig. 2C). Moreover, disruption of the γ-PGA synthetase gene, pgsB, in this background abolished γ-PGA production as assessed by the presence of flat dry colonies (data not shown) and a lack of exopolymer extraction from the culture supernatant (Fig. 2C). Intriguingly, a strain carrying the degU32 hy allele retained γ-PGA biosynthesis in the absence of degS as defined by a mucoid colony morphology (data not shown). These findings indicate that DegU H12L can be phosphorylated by another kinase or by acetyl phosphate (Wolfe et al., 2003; 2008) as has been suggested previously for the accumulation of low levels of native DegU∼P in the absence of degS (Kobayashi, 2007; Verhamme et al., 2007). These data highlight that increasing the level of DegU∼P in NCIB3610 results in γ-PGA production. This is replicated upon deletion of motB, suggesting that upon inhibition of flagellar rotation DegU∼P levels are increased.
Figure 2.
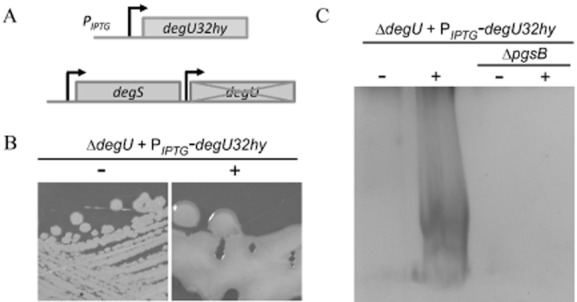
Increasing DegU∼P levels allows γ-PGA production in NCIB3610.A. Schematic diagram of the construction of the ΔdegU + amyE::PIPTG-degU32hy-lacI strain (NRS1325). Bent arrows represent the promoters located before each gene.B. Colony morphology of the ΔdegU + amyE::PIPTG-degU32hy-lacI (NRS1325) strain without and with induction with 25 μM IPTG.C. SDS-PAGE of γ-PGA collected from cultures of ΔdegU + amyE::PIPTG-degU32hy-lacI (NRS1325) and ΔdegU + amyE::PIPTG-degU32hy-lacI + pgsB::spc (NRS3770) grown to the onset of stationary phase in the absence or presence of 25 μM IPTG.
The ΔmotB strain shows increased degU transcription and protease production
To determine if DegU∼P levels were increased in the ΔmotB strain two approaches were taken. First, transcription of degU was quantified upon deletion of motB and second, protease production was quantified as an indirect measure of DegU∼P levels. DegU∼P positively autoregulates transcription of degU, therefore measuring the level of activity from the degU promoter provides an indirect measurement of DegU∼P in the cell (Kobayashi, 2007; Veening et al., 2008). To this end, a PdegU–lacZ transcriptional reporter fusion was constructed and integrated into the wild-type, ΔmotB and ΔmotB complemented strains. Cells were harvested from cultures grown to the onset of stationary phase, and β-galactosidase assays performed. As shown in Fig. 3A, in ΔmotB transcription of degU is increased approximately fourfold when compared with the wild-type (P < 0.01). This effect is specific to deletion of motB as transcription of degU can be restored to wild-type levels by expression of motB from an IPTG-inducible promoter integrated at a non-essential locus (Fig. 3A).
Figure 3.
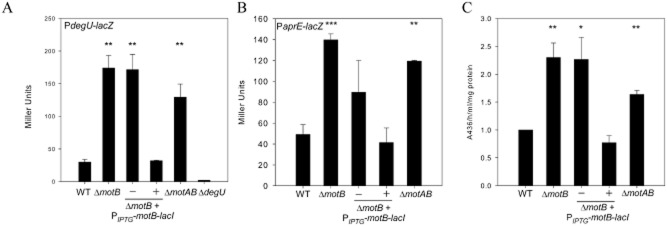
Transcription of degU and aprE is increased alongside total protease activity in ΔmotB.A. β-Galactosidase assays of strains carrying the PdegU–lacZ transcriptional reporter fusion. Strains shown are WT (wild-type NRS4351), ΔmotB (NRS4345), motB + amyE::PIPTG-motB-lacI (NRS4396) grown in the absence or presence of 50 μM IPTG, ΔmotAB (NRS4354) and ΔdegU (NRS4373). All cells were collected at the onset of stationary phase.B. β-Galactosidase assays of strains carrying the PaprE–lacZ transcriptional reporter fusion. Strains shown are WT (wild-type NRS1561), ΔmotB (NRS3440), ΔmotB + amyE::PIPTG-motB-lacI (NRS3858) grown in the absence or presence of 50 μM IPTG and ΔmotAB (NRS4093). All cells were collected at the onset of stationary phase.C. Total protease activity assays performed with the supernatant collected from cells grown in (B).Data in parts (A), (B) and (C) are plotted as the average of at least three independent replicates; in (C) data are represented as a fold change relative to the wild-type strain which was assigned value of 1. Error bars represent standard error of the mean. An asterisk denotes significance as calculated by the Student's t-test where * represents P < 0.05, ** P < 0.01 and *** P < 0.001.
To test the effect of deletion of both stator components a ΔmotAB (NRS3744) strain was constructed. Consistent with inhibition of flagellar rotation promoting an increase in DegU∼P levels, the phenotypes reported for ΔmotB were replicated in the double mutant strain. We first established that the non-polar motAB deletion strain synthesized flagella and exhibited a motility defect that could be specifically complemented by heterologous induction of motAB transcription using an IPTG-dependent promoter (Fig. S1A and C). Next, we examined the phenotype when grown on LB agar plates. The ΔmotAB deletion strain exhibited a mucoid colony phenotype that could be complemented upon heterologous expression of motAB (Fig. S1B). Consistent with these findings, transcriptional analysis demonstrated that the motAB strain showed an increase in the level of degU expression (Fig. 3A). These data suggest that DegU∼P levels are elevated in the both ΔmotB and ΔmotAB strains.
High levels of DegU∼P are closely associated with exoprotease biosynthesis (Dahl et al., 1992; Kobayashi, 2007; Verhamme et al., 2007). DegU∼P positively regulates transcription of the aprE gene, which encodes the alkaline protease subtilisin (Mukai et al., 1990). We therefore hypothesized that if DegU∼P levels were high in the absence of motB this would correlate with an increase in aprE transcription and, moreover, an increase in the total protease activity in the extracellular environment. To establish if aprE transcription was altered in the ΔmotB and ΔmotAB strains, a PaprE–lacZ transcriptional reporter fusion was integrated at the non-essential thrC locus. Cells were harvested for β-galactosidase activity assays and the culture supernatant collected for measurement of extracellular protease activity (see Experimental procedures). Transcription of aprE was increased approximately threefold in the ΔmotB (P < 0.001) and twofold in the ΔmotAB (P < 0.01) strains when compared with the wild-type (Fig. 3B). The increase in transcription was specific as induction of motB transcription in the motB deletion strain reduced aprE transcription levels back to wild-type levels (Fig. 3B). In accordance with these data, total protease activity was increased by twofold upon deletion of motB and 1.6-fold in the ΔmotAB strain (Fig. 3C) (P < 0.01).
Previous work has indicated that DegU can be phosphorylated in the absence of DegS (Kobayashi, 2007; Verhamme et al., 2007). To demonstrate that the upregulation in DegU∼P processes seen in the ΔmotB strain was transmitted through DegS, the degS gene was disrupted in the ΔmotB background and γ-PGA production assessed by colony morphology. As seen in Fig. 1A, the colony morphology of the ΔmotB ΔdegS strain reverted to that of the wild-type. Collectively these findings demonstrate that deletion of the flagellar stator gene, motB, causes an increase in the DegU∼P level within the population, leading to an upregulation of at least two distinct DegU∼P regulated processes. This is reliant on the presence of DegS.
Mutation of motB to disturb proton flux phenocopies the ΔmotB strain
Given the importance of MotB in the generation of torque, we hypothesized that the increase in DegU∼P regulated processes in the ΔmotB background might be linked with the inhibition of flagellar rotation. Although the precise mechanisms by which torque is generated are not yet fully understood, it has been shown that protonation of a conserved aspartate in the MotB transmembrane domain is essential (Sharp et al., 1995; Zhou et al., 1998b). To test if disruption of proton flux through the MotAB complex, and therefore inhibition of flagellar rotation, were key to triggering an increase in DegU∼P levels, the conserved aspartate residue of motB (aspartate 24 in B. subtilis; Fig. S2A) was mutated to alanine (motB D24A) by site-directed mutagenesis. This construct was first integrated into the wild-type strain at a non-essential site on the chromosome under the control of an IPTG-inducible promoter. Upon induction of expression with 1 mM IPTG, the mutated motB allele conferred a dominant-negative phenotype with respect to both swarming motility (Fig. S2B) and γ-PGA production (Fig. S2D). These findings demonstrate that the MotB-D24A protein is synthesized and is functional as it has a dominant phenotype over the native MotB. Next, the construct was integrated into the ΔmotB strain background. As expected, in contrast to complementation of the motB strain with the wild-type allele of motB, induction of motB D24A transcription did not restore motility to the motB deletion strain (Fig. S2C). Next the effect of the motB D24A mutation with regard to degU and aprE transcription, protease activity and γ-PGA biosynthesis was assessed.
It was determined that induction of motB D24A expression was unable to complement ΔmotB with respect to both degU and aprE transcription, which remained fivefold and threefold higher than in the wild-type respectively (Fig. 4A and B). Similarly protease activity was maintained at a higher level in the presence of motB D24A (Fig. 4C). γ-PGA production was confirmed based on the mucoid colony morphology and analysis of exopolymers extracted from culture supernatant (Fig. 4D). These data support the hypothesis that perturbation of proton flux, and therefore flagellar rotation, is necessary to trigger an increase in DegU∼P activity.
Figure 4.
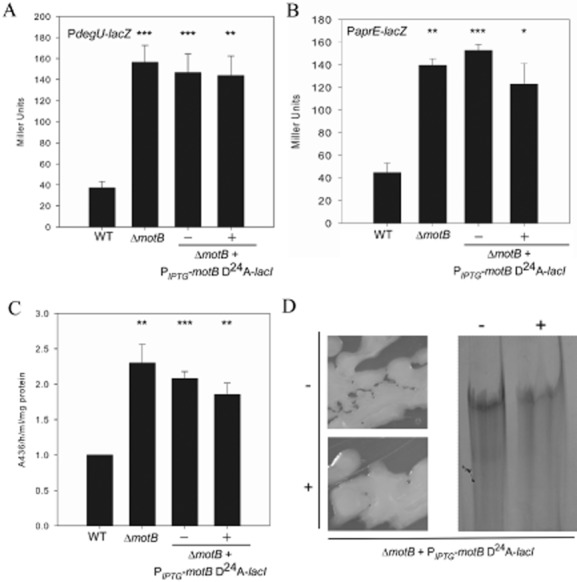
The motB D24A allele cannot complement ΔmotB.A and B. β-Galactosidase assays of strains carrying the (A) PdegU–lacZ or (B) PaprE–lacZ transcriptional reporter fusion. Strains shown are WT (wild-type NRS4351), ΔmotB (NRS4345), motB + amyE::PIPTG-motB-D24A-lacI (NRS4397) grown in the absence or presence of 50 μM IPTG and ΔdegU (NRS4373).B. β-Galactosidase assays of strains carrying the PaprE–lacZ transcriptional reporter fusion. Strains shown are WT (wild-type NRS1561), ΔmotB (NRS3440), ΔmotB + amyE::PIPTG-motB-D24A-lacI (NRS3870) grown in the absence or presence of 50 μM IPTG. All cells in (A) and (B) were collected at the onset of stationary phase.C. Total protease activity assays performed with supernatants collected from cells grown in (B).Data in (A), (B) and (C) are plotted as the average of at least three independent replicates. In (C) data are represented as a fold change relative to the wild-type strain which was assigned value of 1. Error bars represent standard error of the mean. An asterisk denotes significance as calculated by the Student's t-test where * represents P < 0.05; **P < 0.01; and *** P < 0.001.D. Colony morphology of ΔmotB + amyE::PIPTG-motB-D24A-lacI (NRS3870) grown on LB agar in the absence and presence of 50 μM IPTG. SDS-PAGE analysis of γ-PGA collected from cultures of ΔmotB + amyE::PIPTG-motB-D24A-lacI (NRS3870) grown in the absence or presence of 50 μM IPTG.
Engaging the flagellar clutch causes an increase in degU transcription
To assess if the increase in DegU∼P regulated processes was specific to deletion or mutation of the flagellar stator genes the effect of perturbing flagellar rotation by an alternative genetic means was tested. The epsE gene encodes a bi-functional protein, EpsE, that can act as (i) a flagellar clutch to disable flagellar rotation and (ii) a glycosyltransferase enzyme required for the formation of robust biofilms (Blair et al., 2008; Guttenplan et al., 2010). Overexpression of epsE under the control of an IPTG-inducible promoter inhibits motility by interaction with FliG, thereby disengaging the rotor from the stator (Blair et al., 2008; Guttenplan et al., 2010). To test if inhibition of flagellar rotation by overexpression of epsE would also trigger an increase in DegU∼P levels, the coding region of epsE was integrated at a non-essential locus on the chromosome under the control of an IPTG-inducible promoter. To ensure that any effect on DegU∼P was specific to the clutch activity of epsE and not due to the glycosyltransferase function, site-directed mutagenesis was used to mutate aspartate 94 to alanine (epsE D94A) to yield a protein variant that retained clutch activity but lost glycosyltransferase functionality. Simultaneously, lysine 106 was mutated to glutamic acid (epsE K106E) to yield a protein variant that lost clutch activity but possessed glycosyltransferase activity. Both single amino acid mutations have been previously characterized by Guttenplan et al. (2010).
First, the motility phenotypes of these strains were assessed to ensure that the point mutations introduced functioned as expected. As shown in Fig. S3A, induction of either the epsE WT or epsE D94A coding regions inhibited swarming motility, while induction of the epsE K106E coding region in the otherwise wild-type strain had no impact on swarming motility as compared with NCIB3610. As these phenotypes are entirely in line with the previous report (Guttenplan et al., 2010), we proceeded to assess the effect of these mutations on degU transcription. The strains were grown to mid-exponential phase, at which point expression of epsE (or the mutant alleles) was induced by the addition of IPTG. Samples were collected over time to assess β-galactosidase activity. Induction of epsE WT or epsE D94A expression resulted in a fourfold increase in degU transcription by comparison with the wild-type, while induction of epsE K106E phenocopied the wild-type (Fig. 5A and Fig. S3B).
Figure 5.
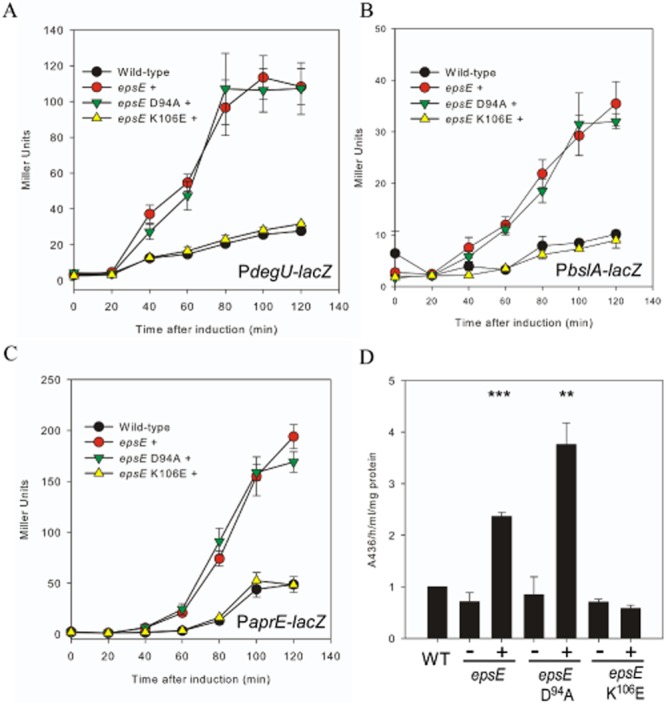
Engaging the flagellar clutch causes an increase in DegU∼P levels.A–C. β-Galactosidase assays of strains carrying the (A) PdegU–lacZ, (B) PbslA–lacZ or (C) PaprE–lacZ transcriptional reporter fusion. Cells were grown to 0.5 OD600 and induced with 1 mM IPTG (final concentration). Strains shown are: (A) wild-type (NRS4351), epsE + (PIPTG-epsE-lacI (NRS4374)), epsE D94A + (PIPTG-epsE-D94A-lacI (NRS4392)) and epsE K106E (PIPTG-epsE-K106E-lacI (NRS4394)); (B) Wild-type (NRS2052), PIPTG-epsE-lacI (NRS4405), PIPTG-epsE-D94A-lacI (NRS4406) and PIPTG-epsE-K106E-lacI (NRS4407); (C) Wild-type (NRS1561), PIPTG-epsE-lacI (NRS4345), PIPTG-epsE-D94A-lacI (NRS4393) and PIPTG-epsE-K106E-lacI (NRS4395). Data shown in (A), (B) and (C) are plotted as the average of at least three independent replicates. Error bars represent standard error of the mean.D. Total protease activity assays performed with supernatants collected from cells grown in (C) after 120 min of induction in the absence or presence of 1 mM IPTG. Data are plotted as the average of at least three independent replicates and are represented as a fold change relative to the wild-type strain (WT) which was assigned value of 1. Error bars represent standard error of the mean. An asterisk denotes significance as calculated by the Student's t-test, where ** represents P < 0.01; and *** P < 0.001.
Engaging the flagellar clutch causes an increase in DegU∼P regulated processes
To test if the upregulation of degU transcription observed upon induction of epsE translated to an increase in DegU∼P-regulated processes, transcription of the bslA gene was measured. The bslA promoter is the main target of DegU∼P during biofilm formation by B. subtilis (Kobayashi, 2007; Verhamme et al., 2009; Ostrowski et al., 2011). Therefore, it was hypothesized that an increase in DegU∼P levels by inhibition of flagellar rotation would result in increased bslA transcription. A PbslA–lacZ transcriptional reporter fusion was integrated into the epsE WT, D94A and K106E strains and activity measured over time by β-galactosidase assays. A similar trend to that reported for degU transcription was observed. Induction of expression of epsE WT or epsE D94A resulted in a threefold increase in transcription by comparison with the wild-type (Fig. 5B and Fig. S3C). As predicted from the degU transcription analysis results, induction of epsE K106E phenocopied the wild-type strain.
The effect of overexpression of epsE on aprE transcription and total protease activity was then tested. As seen for degU and bslA transcription, aprE transcription was increased threefold upon inhibition of flagellar rotation (Fig. 5C and Fig. S3D). In accordance with the aprE transcription analysis data, 120 min after induction of epsE or epsE D94A, total protease activity was twofold higher than the wild-type levels, while the protease activity of the epsE K106E strain was not significantly different from the wild-type (Fig. 5C). Consistent with DegU∼P directing both bslA transcription (Kobayashi, 2007; Verhamme et al., 2009) and exoprotease production (Mukai et al., 1990), we noted that the level of degU transcription started to increase 20 min after IPTG induction of epsE transcription, whereas both bslA and aprE transcription began to rise 40 min post induction (compare Fig. 5A with Fig. 5B and C). These data demonstrate that bslA and aprE are only transcribed after the level of DegU∼P increases. It is important to note that at this point the transcriptional repressor AbrB will have been removed from the promoter elements (Olmos et al., 1996; Verhamme et al., 2009).
γ-PGA production was then assessed. Induction of either epsE WT or epsE D94A expression overnight on LB-agar plates containing 1 mM IPTG yielded a mucoid colony phenotype (Fig. 6A). The mucoid phenotype was not seen when IPTG was lacking or when either the wild-type or epsE K106E expression strain was examined (Fig. 6A). Consistent with the colony phenotypes, γ-PGA was extracted from the culture supernatant and analysed by SDS-PAGE for both the epsE WT and epsE D94A expression strains (Fig. 6C). To determine if the γ-PGA production, and hence DegU∼P levels, was reliant on input from DegS, the degS gene was disrupted in the epsE WT, epsE D94A and epsE K106E expression strains and γ-PGA production monitored using colony phenotype in the absence and presence of IPTG. A dry, flat colony morphology was observed for all strains carrying the degS disruption upon heterologous expression of epsE and the mutant epsE alleles with IPTG (Fig. 6B). Taken together, these data indicate that inhibition of flagellar rotation by EpsE results in an increase in DegU∼P levels, which is reflected by an upregulation of bslA transcription, exoprotease activity and γ-PGA production. As proven for the motB strain (Fig. 1), DegS is responsible for increasing the levels of DegU∼P upon induction of EpsE. Thus the signal generated by inhibition of flagellar rotation when EpsE is present is likely to be the same as when flagellar rotation is inhibited due to mutation or disruption of MotB.
Figure 6.
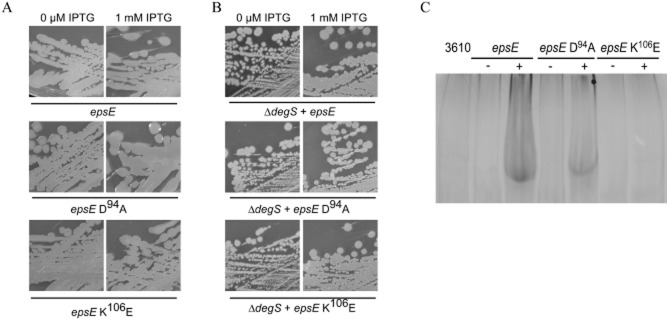
Engaging the flagellar clutch triggers an increase in γ-PGA production that requires DegS.A. Colony morphology of PIPTG-epsE-lacI (NRS4085), PIPTG-epsE-D94A-lacI (NRS4388) and PIPTG-epsE-K106E-lacI (NRS4389) in the absence and presence of 1 mM IPTG after growth overnight at 37°C.B. Colony morphology of PIPTG-epsE-lacI + degS::cml (NRS4399), PIPTG-epsE-D94A-lacI + degS::cml (NRS4400) and PIPTG-epsE-K106E-lacI + degS::cml (NRS4401) in the absence and presence of 1 mM IPTG after growth overnight at 37°C.C. SDS-PAGE of γ-PGA collected from cultures of 3610 (wild-type NRS1561), PIPTG-epsE-lacI (NRS4345) and PIPTG-epsE-D94A-lacI (NRS4393) and PIPTG-epsE-K106E-lacI (NRS4395), grown in the absence or presence of 1 mM IPTG, at the onset of stationary phase.
Tangling of flagella increases DegU∼P activity
Data presented thus far indicate that inhibition of flagellar rotation by genetic manipulation activates the DegS–DegU two-component signal transduction system. We next used a non-genetic method to block flagellar rotation (Meister et al., 1987). An antibody raised against the flagellar filament protein, Hag, was used to tangle flagella (see Fig. 7A and Movies S1–S4) and the impact on degU transcription measured. Prior to this we used Western blot analysis to check the specificity of the Hag antibody (Fig. S4). To ensure that any effects seen were specific to the Hag antibody, and not due to off-target effects of the serum, three independent pre-immune sera were used as controls. The wild-type strain carrying the PdegU–lacZ transcriptional reporter fusion was grown in liquid culture to mid-exponential phase at which point either pre-immune sera or the Hag antibody in sera was added (1:20 dilution). Swimming motility was immediately checked by real-time live single cell microscopy (see Fig. 7A and Movies S1–S4) and samples were collected over time for β-galactosidase assays. Analysis demonstrated that transcription from the degU promoter was upregulated only 15 min after addition of the antibody, and increased fourfold by comparison with the pre-immune sera controls after 30 min (Fig. 7B) (P < 0.001). This trend of increased transcription was maintained over the course of the experiment (Fig. 7B). To further validate these data, exoprotease activity assays were undertaken with samples collected at the end of each time-course. The mean level of exoprotease activity for the three pre-immune sera controls was calculated and compared with that of the experimental sample incubated with Hag anti-sera. Analysis demonstrated a statistically significant (P = 0.01) 13-fold increase in exoprotease activity upon inhibition of flagellar rotation by tangling of the flagella. The mean level of protease activity in the presence of sera alone was 0.004 ± 0.001 ΔA436 h−1 ml−1 per mg of total protein compared with 0.05 ± 0.001 ΔA436 h−1 ml−1 per mg of total protein in the presence of the sera containing the anti-hag antibody. Collectively, these data unequivocally demonstrate that inhibition of rotation of the B. subtilis flagellum either genetically or mechanically results in an increase in degU transcription and DegU∼P regulated processes, findings that are consistent with the activation of the sensor kinase, DegS.
Figure 7.
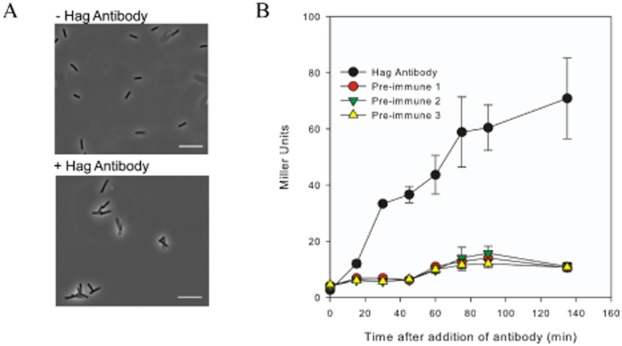
Tethering the flagella triggers an increase in degU transcription.A. Micrographs of cells containing the PdegU–lacZ transcriptional reporter fusion (NRS4351) grown presence or absence of Hag antibody in sera. Micrographs are static images taken from movies filmed 5 min after the addition of antibody to the culture (see Movies S1 and S2). Scale bars represent 100 pixels.B. β-Galactosidase assays of the wild-type strain carrying the PdegU–lacZ transcriptional reporter fusion (NRS4351) grown in the presence of a 1:20 dilution of either pre-immune sera or the Hag-specific antibody. Three independent pre-immune sera were used as controls. Data are plotted as the average of at least three independent replicates. Error bars represent standard error of the mean.
Discussion
In the natural environment bacteria predominantly live adhered to a surface as part of a biofilm (Costerton et al., 1995; Vlamakis et al., 2013). However, how a motile cell is able to sense and respond to the presence of a surface is poorly understood. Here, we show that inhibition of flagellar rotation acts as a signal to trigger an increase in the level of DegU∼P via the sensor kinase DegS. This is a regulatory pathway that is needed for biofilm formation by the Gram-positive bacterium B. subtilis (Stanley and Lazazzera, 2005; Kobayashi, 2007; Verhamme et al., 2007). These findings clearly demonstrate that B. subtilis can effectively use the flagellum for both signal transduction purposes as well as a mechanical device for propulsion. These findings add to the small but growing body of evidence indicating that the flagellum is utilized by the cell to explore and respond to external stimuli in diverse bacterial species (Belas and Suvanasuthi, 2005; Wang et al., 2005; Gode-Potratz et al., 2011; Friedlander et al., 2013). We propose that inhibition of flagellar rotation would occur due to physical contacts with a surface, and that the consequential activation of a signal transduction pathway may present a mechanism by which flagellated organisms detect and respond to a surface.
Activation of the DegS–DegU two-component regulatory system
Previous work has highlighted a role of DegU∼P in controlling flagellar assembly (Amati et al., 2004; Hsueh et al., 2011). Indeed it has been shown that DegU is preferentially activated in genetic backgrounds that promote cell chaining, with further experiments tentatively suggesting that DegU might play a role in sensing the status of flagellar assembly (Hsueh et al., 2011). The data presented herein demonstrate that the DegS–DegU two-component regulatory system is activated when the flagellum stops rotating. Therefore DegU∼P has a role both in flagellar biosynthesis and in responding to signals inputted by the flagellum. Data demonstrating that inhibition of flagellar rotation acts as a trigger to activate the DegS–DegU two-component system allows the B. subtilis flagellum to be classified as a mechanosensor that controls bacterial cell behaviour. To the best of our knowledge this is the first example of a classical two-component system being activated in response to a mechanical signal: namely inhibition of flagellar rotation. We suggest that repression of flagellar rotation occurs when the flagellum encounters a surface; a process that is mimicked in our study by flagella tangling experiments (Fig. 7).
Surface sensing by the flagellum
Our findings add B. subtilis to the small list of microorganisms for which surface sensing has been linked with downstream alterations in gene transcription and protein synthesis (McCarter et al., 1988; Kawagishi et al., 1996). For example, transcriptomic and proteomic screens have shown that genes and proteins are differentially regulated between liquid and surface grown bacteria (Kim and Surette, 2004; Wang et al., 2004). Moreover, in Vibrio parahaemolyticus, which possesses a dual flagellar system, it has been shown that slowing the rotational speed of the polar flagellum triggers gene expression changes that result in the transcription of genes essential for the synthesis of lateral flagella (McCarter et al., 1988; Kawagishi et al., 1996). Furthermore, inhibition of rotation of the polar flagellum also impacts genes associated with cyclic-di-GMP signalling and virulence, thereby suggestive of a global role for flagellar mediated surface sensing in controlling bacterial cell physiology (Gode-Potratz et al., 2011).
The transition from motility to attachment
The attachment of bacteria to a surface is the first step in the formation of a biofilm, where the initial stage of adhesion is often mediated by flagella-or pili-based motility (O'Toole et al., 2000). Indeed, several studies have identified flagella, or flagellar motility, as a key aspect of biofilm development and biofilm ‘microanatomy’ (O'Toole and Kolter, 1998; Pratt and Kolter, 1998; Watnick and Kolter, 1999; Lemon et al., 2007; Friedlander et al., 2013; Serra et al., 2013). The subsequent transition to persistent adhesion is often mediated by polysaccharides (Watnick and Kolter, 2000). In Caulobacter crescentus a direct link between surface-sensing by the flagellum and irreversible adhesion has been shown, where upon reaching the surface, pili-dependent inhibition of flagellum rotation stimulates concomitant synthesis of the holdfast, promoting permanent attachment (Li et al., 2012). Moreover, exopolysaccharides have been shown to ‘wheel-lock’ flagellar rotation resulting in co-ordination of motility inhibition and stimulation of biofilm formation (Zorraquino et al., 2013). Alternatively, or indeed additionally, flagellar brake or clutch activity can play a role in the transition between motility and biofilm formation to ensure separation of these mutually exclusive cell behaviours (Blair et al., 2008; Pilizota et al., 2009; Boehm et al., 2010; Paul et al., 2010). This can be exemplified by the B. subtilis flagellar clutch, EpsE that was utilized in this study. It is of interest to note that EpsE is encoded within the epsA-O operon that is required for the synthesis of one of the major structural components of the biofilm, the exopolysaccharide. EpsE therefore links inhibition of motility to exopolysaccharide production and biofilm formation (Blair et al., 2008; Guttenplan et al., 2010; Guttenplan and Kearns, 2013). Here we provide evidence that induction of EpsE will reinforce biofilm formation through increased biosynthesis of BslA (Fig. 5B), a bacterial hydrophobin that coats the B. subtilis biofilm (Kobayashi and Iwano, 2012; Hobley et al., 2013) and functions synergistically with the exopolysaccharide and TasA amyloid fibres to facilitate assembly of the biofilm matrix (Ostrowski et al., 2011). Proteins functionally similar to EpsE have been classed as flagellar ‘brakes’ in E. coli (Boehm et al., 2010; Paul et al., 2010) and Rhodobacter sphaeroides (Pilizota et al., 2009). Therefore, the ability of a single protein to inhibit flagellar rotation appears to be a general mechanism used by bacteria to facilitate the transition to a sessile state.
Concluding remarks
There are, of course, outstanding questions that follow our discovery; the first being how a slowing or lack of flagellar rotation is sensed. There are several mechanisms by which this might occur. One possibility is that DegS, as a cytoplasmic sensor kinase, interacts with components of the flagellar motor when rotation is stopped. This postulated mechanism is similar to that proposed for the cyclic-di-GMP (c-di-GMP)-binding protein, YcgR, which interacts with the flagellar motor proteins FliG, FliM (Paul et al., 2010) and MotA (Boehm et al., 2010), when c-di-GMP levels are high resulting in the inhibition of motility. Consistent with this, in Pseudomonas aeruginosa the chemotaxis-like Wsp signal transduction system, which is essential for biofilm formation, has been shown to produce c-di-GMP in response to surface growth (Guvener and Harwood, 2007; O'Connor et al., 2012). A second possible mechanism may involve the stator-associated transmembrane protein, FliL. Previous studies have identified FliL as a possible intermediary between the inhibition of rotation and signal transduction (Belas and Suvanasuthi, 2005; Cusick et al., 2012). Moreover, recent studies in different bacterial species have identified several roles for FliL in bacterial motility and surface-sensing (Lee et al., 2013). For example, FliL has been shown to be associated with (Motaleb et al., 2011) and enhance the function of the flagellar stator (Suaste-Olmos et al., 2010), with evidence now suggesting that overexpression of FliL alongside MotAB is sufficient to overcome surface friction associated with swarming on hard agar (Partridge and Harshey, 2013). Therefore, given that the function of FliL does not appear to be strictly conserved between bacterial species it will be of interest to determine if FliL is also a key player in surface-sensing and flagellar rotation in B. subtilis. Alternatively, we cannot exclude the possibility that DegS might directly sense changes in the intracellular environment that are triggered by a lack of flagellar rotation, such as ion flux (Kawagishi et al., 1996) or potentially energy status (Watson and Fedor, 2012). Intriguingly, recent work in E. coli has identified the flagellar stator, not the flagellar filament, as a mechanosensor that is able to remodel itself in a load-dependent manner (Lele et al., 2013). It is therefore possible that the increase in the number of stators required to drive flagellar torque under high loads might itself act as a signal to impact downstream signalling pathways. It will be of interest in the future to determine the underlying molecular detail of DegS activation, and moreover to clarify if our hypothesis that the flagellum acts as a mechanosensor to allow a sessile lifestyle to be adopted by other flagellated bacterial species holds true.
Experimental procedures
Growth conditions and strain construction
Escherichia coli and B. subtilis strains were routinely grown in Luria–Bertani (LB) broth (10 g NaCl, 5 g yeast extract, 10 g tryptone per litre) or on LB plates supplemented with 1.5% select agar (Invitrogen) at 37°C unless otherwise stated. When appropriate, isopropyl β-d-1-thiogalactopyranoside (IPTG) was added at the indicated concentrations. E. coli strain MC1061 [F'lacIQ lacZM15 Tn10 (tet)] was used for the routine construction and maintenance of plasmids. When required, antibiotics were used at the following concentrations: 100 μg ml−1 ampicillin, 100 μg ml−1 spectinomycin, 1 μg ml−1 erythromycin and 25 μg ml−1 lincomycin. Strains were constructed using standard protocols. Phage transductions were carried out as previously described (Verhamme et al., 2007). A full list of strains used in this study is provided in Table S1.
Construction of deletion strains
To construct the in-frame deletion of motB an approach similar to that previously described was used (Kiley and Stanley-Wall, 2010). The upstream region of motB was amplified from genomic DNA using primers NSW874 and NSW875, purified and digested with BamHI and XbaI, using the restriction sites engineered into the primers and ligated into pUC19 (Vieira and Messing, 1982) cut the same to yield pNW651. The downstream region of motB was amplified using primers NSW876 and NSW877, purified, and digested with XbaI and BamHI, using the restriction sites engineered into the primers and ligated into pUC19 cut the same to yield pNW652. The upstream and downstream regions of motB were released from pNW651 and pNW652 with BamHI and XbaI and XbaI and EcoRI, respectively, and ligated into pUC19 cut with BamHI and EcoRI to produce plasmid pNW653. The ΔmotB region was then cut from pNW653 with BamHI and EcoRI and ligated into pMAD cut the same (Arnaud et al., 2004). Strain NRS3494 (NCIB3610 ΔmotB) was generated by integration and curing of the region contained in pNW654 in strain NCIB3610. An in-frame deletion of motAB was constructed in a similar manner. The upstream region of motA was amplified from genomic DNA using primers NSW965 and NSW966, purified and digested with BamHI and XbaI, using restriction sites engineered into the primers. The downstream region of motB was excised from pNW652 with XbaI and EcoRI. The upstream region of motA and downstream region of motB were ligated into pUC19 cut with BamHI and EcoRI to yield pNW1019. The ΔmotAB region was then cloned into pMAD cut with BamHI and EcoRI to yield pNW1021. Strain NRS3744 (NCIB3610 ΔmotAB) was generated by integration and curing of the region contained in pNW1021 in strain NCIB3610. All primers and plasmids used in this study are listed in Tables S2 and S3.
Reverse transcription (RT)-PCR
RNA was isolated from the following strains grown to mid-exponential phase: NCIB3610 (wild-type), NRS3494 (ΔmotB) and NRS3775 (ΔmotB + Pspankhy-motB-lacI) with or without 50 μM IPTG. RNA isolation was carried out as described previously (Kiley and Stanley-Wall, 2010) using the RiboPure Bacteria RNA Isolation Kit (Ambion), according to manufacturer's instructions. cDNA was synthesized using random hexamers and subsequently treated with Rnase H for 20 min at 37°C. To amplify internal gene products the following primer pairs were used: NSW1474 and NSW1475 (pgsB), NSW1604 and NSW1605 (pgdS) and DEN5 and DEN7 (16S rRNA).
Motility assays
Swimming and swarming analyses were performed as described before (Verhamme et al., 2007) using low-salt LB (5 g NaCl, 5 g yeast extract, 10 g tryptone per litre) supplemented with 0.4% or 0.7% Bacto agar (Invitrogen) respectively. Plates were incubated at 37°C and the extent of swimming or swarming noted at defined intervals.
Whole-cell analysis of Hag
Proteins were collected from planktonic cultures grown to mid-exponential phase. Briefly, cells were harvested by centrifugation at 4700 g. Cells were suspended in 1× Bugbuster Master Mix (Novagen) and lysed according to manufacturer's instructions. Seven micrograms of proteins were resolved by SDS-PAGE and stained with Coomassie Brilliant Blue. Hag protein was identified by 1D SDS-PAGE analysis of the total cellular proteins by comparison with the Δhag strain (DS1677). The protein identity was confirmed by mass spectrometry (FingerPrints Proteomics and Mass Spectrometry Facility, University of Dundee) (Diethmaier et al., 2011).
Inhibition of flagellar rotation with an anti-Hag antibody
A wild-type strain carrying the PdegU–lacZ transcriptional reporter fusion (NRS4351) was grown to OD600 of 0.4 in LB prior to addition of a 1 in 20 dilution of Hag antibody (Prof. Kursad Turgay) or pre-immune sera. To check the motility of the cells, a small sample of each culture was imaged by microscopy at each time point. A thin channel was generated between a glass slide and the coverslip using double sided sticky tape. Cells were injected into the viewing chamber and visualized using a Zeiss Axio10 Imager.M10 under a Zeiss 40× EC Plan-NEO FLUAR objective and recorded using the high speed digital recorder function in the Zen lite software (Zeiss). Samples (0.5 ml) were collected by centrifugation over the course of the experiment, frozen at −20°C and later analysed by β-galactosidase assay.
β-Galactosidase assays
The β-galactosidase activity of strains harbouring lacZ promoter reporter fusions was measured as previously described (Verhamme et al., 2007; 2009). The values presented are the average β-galactosidase activities in Miller Units (Miller, 1972) determined from at least three independent samples. Error bars represent the standard error of the mean.
Protease plate assays
Analysis of protease production was carried out as previously described (Verhamme et al., 2007). Briefly, secreted protease production was analysed using LB agar plates supplemented with 1.5% (w/v) milk. B. subtilis cultures were grown to mid-late exponential phase in LB and 10 μl of culture spotted on to each plate (containing IPTG as required) and incubated at 37°C for 18 h prior to being photographed.
Secreted protease activity assay
Culture samples were collected by centrifugation (17 000 g for 5 min) after which the supernatant was removed and stored at −20°C until use. To determine extracellular protease activity the azocasein assay (Braun and Schmitz, 1980) was performed. A 150 μl aliquot of thawed supernatant was mixed with 500 μl of 2% w/v azocasein (Sigma), along with 100 μl of Tris-HCl (pH 8.0) and 650 μl of ddH2O. A blank sample was prepared containing ddH2O in the place of the supernatant and a media only control sample containing LB in the place of the supernatant was also prepared. The samples were incubated for 1 h at 30°C, after which 375 μl of 14% v/v perchloric acid was added to stop each reaction. The samples were centrifuged (17 000 g for 5 min) and 750 μl of the supernatant was mixed directly in a cuvette with 75 μl of 10 M NaOH and the absorbance at 436 nm was measured using a spectrophotometer. The background activity of the medium-only control was subtracted and activity was calculated as ΔA436 h−1 ml−1 per mg of total protein.
γ-PGA isolation
The method used for γ-PGA isolation was adapted from Stanley and Lazazzera, (2005). Briefly, cells were grown overnight on LB lawn plates, collected in 5 ml LB and diluted to an OD600 of 0.01. Cells were grown to stationary phase in 25 ml LB and harvested by centrifugation. A total of 10 ml of the culture supernatant was retained and the cell pellet suspended in 2.5 ml of 0.14 mM NaCl. The cells were again harvested by centrifugation and the supernatant from the wash added to the previously collected supernatant. The combined supernatants were brought to pH 2.0 with concentrated sulphuric acid and incubated at 4°C overnight. To precipitate γ-PGA, 40 ml 100% ethanol was added to the supernatant and the sample incubated at −20°C for a minimum of 10 min. The γ-PGA was harvested by centrifugation and the resulting pellet suspended in 1 ml 10 mM Tris-HCl pH 8.0 and concentrated in a vacuum concentrator. The resulting γ-PGA pellet was suspended in 200 μl 10 mM Tris-HCl pH 8.0 and analysed by SDS-PAGE. Gels were stained with 0.5% methylene blue in 3% acetic acid for 30 min and de-stained in H2O.
Acknowledgments
L.S.C. is the recipient of a Wellcome Trust PhD studentship (093714/Z/10/Z). V.L.M. was funded by Biotechnology and Biological Sciences Research Council grant number (BB/I006915/1). E.B. was funded by a College of Life Sciences James Black Scholarship, through the Wellcome Trust ISSF grant (097818/Z/11/A). A.O. was the recipient of a Biotechnology and Biological Sciences Research Council Doctoral Training Account grant (BB/D526161/1). The proteomic facilities in the College of Life Sciences are supported by a Wellcome Trust Strategic Award (097945/B/11/Z). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We thank Zoe Landsborough for construction of pNW1060, Prof. Kürsad Turgay for the kind gift of the anti-Hag antibodies, and Dr Robert Ryan and members of the NSW laboratory for critical review of the manuscript. Finally, we thank Prof. Judy Armitage for many helpful and informative discussions.
Supporting information
Additional supporting information may be found in the online version of this article at the publisher's web-site.
References
- Amati G, Bisicchia P, Galizzi A. DegU-P represses expression of the motility fla-che operon in Bacillus subtilis. J Bacteriol. 2004;186:6003–6014. doi: 10.1128/JB.186.18.6003-6014.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud M, Chastanet A, Debarbouille M. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, gram-positive bacteria. Appl Environ Microbiol. 2004;70:6887–6891. doi: 10.1128/AEM.70.11.6887-6891.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belas R, Suvanasuthi R. The ability of Proteus mirabilis to sense surfaces and regulate virulence gene expression involves FliL, a flagellar basal body protein. J Bacteriol. 2005;187:6789–6803. doi: 10.1128/JB.187.19.6789-6803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KM, Turner L, Winkelman JT, Berg HC, Kearns DB. A molecular clutch disables flagella in the Bacillus subtilis biofilm. Science. 2008;320:1636–1638. doi: 10.1126/science.1157877. [DOI] [PubMed] [Google Scholar]
- Boehm A, Kaiser M, Li H, Spangler C, Kasper CA, Ackermann M, et al. Second messenger-mediated adjustment of bacterial swimming velocity. Cell. 2010;141:107–116. doi: 10.1016/j.cell.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Branda SS, Gonzalez-Pastor JE, Ben-Yehuda S, Losick R, Kolter R. Fruiting body formation by Bacillus subtilis. Proc Natl Acad Sci USA. 2001;98:11621–11626. doi: 10.1073/pnas.191384198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda SS, Chu F, Kearns DB, Losick R, Kolter R. A major protein component of the Bacillus subtilis biofilm matrix. Mol Microbiol. 2006;59:1229–1238. doi: 10.1111/j.1365-2958.2005.05020.x. [DOI] [PubMed] [Google Scholar]
- Braun TF, Poulson S, Gully JB, Empey JC, Van Way S, Putnam A, Blair DF. Function of proline residues of MotA in torque generation by the flagellar motor of Escherichia coli. J Bacteriol. 1999;181:3542–3551. doi: 10.1128/jb.181.11.3542-3551.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V, Schmitz G. Excretion of a protease by Serratia marcescens. Arch Microbiol. 1980;124:55–61. doi: 10.1007/BF00407028. [DOI] [PubMed] [Google Scholar]
- Burbulys D, Trach KA, Hoch JA. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell. 1991;64:545–552. doi: 10.1016/0092-8674(91)90238-t. [DOI] [PubMed] [Google Scholar]
- Calvio C, Osera C, Amati G, Galizzi A. Autoregulation of swrAA and motility in Bacillus subtilis. J Bacteriol. 2008;190:5720–5728. doi: 10.1128/JB.00455-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candela T, Fouet A. Poly-gamma-glutamate in bacteria. Mol Microbiol. 2006;60:1091–1098. doi: 10.1111/j.1365-2958.2006.05179.x. [DOI] [PubMed] [Google Scholar]
- Chai Y, Norman T, Kolter R, Losick R. Evidence that metabolism and chromosome copy number control mutually exclusive cell fates in Bacillus subtilis. EMBO J. 2011;30:1402–1413. doi: 10.1038/emboj.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyak BV, Dibrov PA, Glagolev AN, Sherman MY, Skulachev VP. A novel type of energetics in a marine alkali-tolerant bacterium delta-muna-driven motility and sodium cycle. FEBS Lett. 1983;164:38–42. [Google Scholar]
- Chevance FF, Hughes KT. Coordinating assembly of a bacterial macromolecular machine. Nat Rev Microbiol. 2008;6:455–465. doi: 10.1038/nrmicro1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- Cusick K, Lee YY, Youchak B, Belas R. Perturbation of FliL interferes with Proteus mirabilis swarmer cell gene expression and differentiation. J Bacteriol. 2012;194:437–447. doi: 10.1128/JB.05998-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl MK, Msadek T, Kunst F, Rapoport G. Mutational analysis of the Bacillus subtilis DegU regulator and its phosphorylation by the DegS protein kinase. J Bacteriol. 1991;173:2539–2547. doi: 10.1128/jb.173.8.2539-2547.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl MK, Msadek T, Kunst F, Rapoport G. The phosphorylation state of the DegU response regulator acts as a molecular switch allowing either degradative enzyme synthesis or expression of genetic competence in Bacillus subtilis. J Biol Chem. 1992;267:14509–14514. [PubMed] [Google Scholar]
- Dervyn E, Noirot-Gros MF, Mervelet P, McGovern S, Ehrlich SD, Polard P, Noirot P. The bacterial condensin/cohesin-like protein complex acts in DNA repair and regulation of gene expression. Mol Microbiol. 2004;51:1629–1640. doi: 10.1111/j.1365-2958.2003.03951.x. [DOI] [PubMed] [Google Scholar]
- Diethmaier C, Pietack N, Gunka K, Wrede C, Lehnik-Habrink M, Herzberg C, et al. A novel factor controlling bistability in Bacillus subtilis: the YmdB protein affects flagellin expression and biofilm formation. J Bacteriol. 2011;193:5997–6007. doi: 10.1128/JB.05360-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do TH, Suzuki Y, Abe N, Kaneko J, Itoh Y, Kimura K. Mutations suppressing the loss of DegQ function in Bacillus subtilis (natto) poly-gamma-glutamate synthesis. Appl Environ Microbiol. 2011;77:8249–8258. doi: 10.1128/AEM.05827-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl AM, Eppinger M, Fricke WF, Rosovitz MJ, Rasko DA, Daugherty S, et al. Whole-genome sequences of Bacillus subtilis and close relatives. J Bacteriol. 2012;194:2378–2379. doi: 10.1128/JB.05675-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- Friedlander RS, Vlamakis H, Kim P, Khan M, Kolter R, Aizenberg J. Bacterial flagella explore microscale hummocks and hollows to increase adhesion. Proc Natl Acad Sci USA. 2013;110:5624–5629. doi: 10.1073/pnas.1219662110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Gonzalez-Pastor JE, Losick R. High-and low-threshold genes in the Spo0A regulon of Bacillus subtilis. J Bacteriol. 2005;187:1357–1368. doi: 10.1128/JB.187.4.1357-1368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gode-Potratz CJ, Kustusch RJ, Breheny PJ, Weiss DS, McCarter LL. Surface sensing in Vibrio parahaemolyticus triggers a programme of gene expression that promotes colonization and virulence. Mol Microbiol. 2011;79:240–263. doi: 10.1111/j.1365-2958.2010.07445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttenplan SB, Kearns DB. Regulation of flagellar motility during biofilm formation. FEMS Microbiol Rev. 2013 doi: 10.1111/1574-6976.12018. doi: 10.1111/1574-6976.12018 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttenplan SB, Blair KM, Kearns DB. The EpsE flagellar clutch is bifunctional and synergizes with EPS biosynthesis to promote Bacillus subtilis biofilm formation. PLoS Genet. 2010;6:e1001243. doi: 10.1371/journal.pgen.1001243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttenplan SB, Shaw S, Kearns DB. The cell biology of peritrichous flagella in Bacillus subtilis. Mol Microbiol. 2013;87:211–229. doi: 10.1111/mmi.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guvener ZT, Harwood CS. Subcellular location characteristics of the Pseudomonas aeruginosa GGDEF protein, WspR, indicate that it produces cyclic-di-GMP in response to growth on surfaces. Mol Microbiol. 2007;66:1459–1473. doi: 10.1111/j.1365-2958.2007.06008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon MA, Lazazzera BA. The sporulation transcription factor Spo0A is required for biofilm development in Bacillus subtilis. Mol Microbiol. 2001;42:1199–1209. doi: 10.1046/j.1365-2958.2001.02709.x. [DOI] [PubMed] [Google Scholar]
- Hobley L, Ostrowski A, Rao F, Bromley K, Porter M, Prescott A, et al. BslA is a self-assembling bacterial hydrophobin that coats the Bacillus subtilis biofilm. Proc Natl Acad Sci USA. 2013 doi: 10.1073/pnas.1306390110. doi: 10.1073/pnas.130639110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh YH, Cozy LM, Sham LT, Calvo RA, Gutu AD, Winkler ME, Kearns DB. DegU-phosphate activates expression of the anti-sigma factor FlgM in Bacillus subtilis. Mol Microbiol. 2011;81:1092–1108. doi: 10.1111/j.1365-2958.2011.07755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagishi I, Imagawa M, Imae Y, McCarter L, Homma M. The sodium-driven polar flagellar motor of marine Vibrio as the mechanosensor that regulates lateral flagellar expression. Mol Microbiol. 1996;20:693–699. doi: 10.1111/j.1365-2958.1996.tb02509.x. [DOI] [PubMed] [Google Scholar]
- Kearns DB, Losick R. Cell population heterogeneity during growth of Bacillus subtilis. Genes Dev. 2005;19:3083–3094. doi: 10.1101/gad.1373905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns DB, Chu F, Rudner R, Losick R. Genes governing swarming in Bacillus subtilis and evidence for a phase variation mechanism controlling surface motility. Mol Microbiol. 2004;52:357–369. doi: 10.1111/j.1365-2958.2004.03996.x. [DOI] [PubMed] [Google Scholar]
- Kiley TB, Stanley-Wall NR. Post-translational control of Bacillus subtilis biofilm formation mediated by tyrosine phosphorylation. Mol Microbiol. 2010;78:947–963. doi: 10.1111/j.1365-2958.2010.07382.x. [DOI] [PubMed] [Google Scholar]
- Kim W, Surette MG. Metabolic differentiation in actively swarming Salmonella. Mol Microbiol. 2004;54:702–714. doi: 10.1111/j.1365-2958.2004.04295.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K. Gradual activation of the response regulator DegU controls serial expression of genes for flagellum formation and biofilm formation in Bacillus subtilis. Mol Microbiol. 2007;66:395–409. doi: 10.1111/j.1365-2958.2007.05923.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Iwano M. BslA (YuaB) forms a hydrophobic layer on the surface of Bacillus subtilis biofilms. Mol Microbiol. 2012;85:51–66. doi: 10.1111/j.1365-2958.2012.08094.x. [DOI] [PubMed] [Google Scholar]
- Kojima S, Blair DF. Solubilization and purification of the MotA/MotB complex of Escherichia coli. Biochemistry. 2004;43:26–34. doi: 10.1021/bi035405l. [DOI] [PubMed] [Google Scholar]
- Lee YY, Patellis J, Belas R. Activity of Proteus mirabilis FliL is viscosity dependent and requires extragenic DNA. J Bacteriol. 2013;195:823–832. doi: 10.1128/JB.02024-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lele PP, Hosu BG, Berg HC. Dynamics of mechanosensing in the bacterial flagellar motor. Proc Natl Acad Sci USA. 2013;110:11839–11844. doi: 10.1073/pnas.1305885110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon KP, Higgins DE, Kolter R. Flagellar motility is critical for Listeria monocytogenes biofilm formation. J Bacteriol. 2007;189:4418–4424. doi: 10.1128/JB.01967-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Brown PJ, Tang JX, Xu J, Quardokus EM, Fuqua C, Brun YV. Surface contact stimulates the just-in-time deployment of bacterial adhesins. Mol Microbiol. 2012;83:41–51. doi: 10.1111/j.1365-2958.2011.07909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez D, Fischbach MA, Chu F, Losick R, Kolter R. Structurally diverse natural products that cause potassium leakage trigger multicellularity in Bacillus subtilis. Proc Natl Acad Sci USA. 2009a;106:280–285. doi: 10.1073/pnas.0810940106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez D, Vlamakis H, Kolter R. Generation of multiple cell types in Bacillus subtilis. FEMS Microbiol Rev. 2009b;33:152–163. doi: 10.1111/j.1574-6976.2008.00148.x. [DOI] [PubMed] [Google Scholar]
- Lopez D, Vlamakis H, Losick R, Kolter R. Paracrine signaling in a bacterium. Genes Dev. 2009c;23:1631–1638. doi: 10.1101/gad.1813709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez D, Vlamakis H, Kolter R. Biofilms. Cold Spring Harb Perspect Biol. 2010;2:a000398. doi: 10.1101/cshperspect.a000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter L, Hilmen M, Silverman M. Flagellar dynamometer controls swarmer cell differentiation of V. parahaemolyticus. Cell. 1988;54:345–351. doi: 10.1016/0092-8674(88)90197-3. [DOI] [PubMed] [Google Scholar]
- Manson MD, Tedesco P, Berg HC, Harold FM, Van der Drift C. A protonmotive force drives bacterial flagella. Proc Natl Acad Sci USA. 1977;74:3060–3064. doi: 10.1073/pnas.74.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister M, Lowe G, Berg HC. The proton flux through the bacterial flagellar motor. Cell. 1987;49:643–650. doi: 10.1016/0092-8674(87)90540-x. [DOI] [PubMed] [Google Scholar]
- Miller J. Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- Morikawa M, Kagihiro S, Haruki M, Takano K, Branda S, Kolter R, Kanaya S. Biofilm formation by a Bacillus subtilis strain that produces gamma-polyglutamate. Microbiology. 2006;152:2801–2807. doi: 10.1099/mic.0.29060-0. [DOI] [PubMed] [Google Scholar]
- Motaleb MA, Pitzer JE, Sultan SZ, Liu J. A novel gene inactivation system reveals altered periplasmic flagellar orientation in a Borrelia burgdorferi fliL mutant. J Bacteriol. 2011;193:3324–3331. doi: 10.1128/JB.00202-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Msadek T, Kunst F, Klier A, Rapoport G. DegS–DegU and ComP–ComA modulator-effector pairs control expression of the Bacillus subtilis pleiotropic regulatory gene degQ. J Bacteriol. 1991;173:2366–2377. doi: 10.1128/jb.173.7.2366-2377.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai K, Kawata M, Tanaka T. Isolation and phosphorylation of the Bacillus subtilis degS and degU gene products. J Biol Chem. 1990;265:20000–20006. [PubMed] [Google Scholar]
- Murray EJ, Kiley TB, Stanley-Wall NR. A pivotal role for the response regulator DegU in controlling multicellular behaviour. Microbiology. 2009;155:1–8. doi: 10.1099/mic.0.023903-0. [DOI] [PubMed] [Google Scholar]
- Nakano MM, Xia LA, Zuber P. Transcription initiation region of the srfA operon, which is controlled by the comP–comA signal transduction system in Bacillus subtilis. J Bacteriol. 1991;173:5487–5493. doi: 10.1128/jb.173.17.5487-5493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JR, Kuwada NJ, Huangyutitham V, Wiggins PA, Harwood CS. Surface sensing and lateral subcellular localization of WspA, the receptor in a chemosensory-like system leading to c-di-GMP production. Mol Microbiol. 2012;86:720–729. doi: 10.1111/mmi.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole G, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- O'Toole GA, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annu Rev Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- Ogura M, Tsukahara K. Autoregulation of the Bacillus subtilis response regulator gene degU is coupled with the proteolysis of DegU-P by ClpCP. Mol Microbiol. 2010;75:1244–1259. doi: 10.1111/j.1365-2958.2010.07047.x. [DOI] [PubMed] [Google Scholar]
- Ogura M, Shimane K, Asai K, Ogasawara N, Tanaka T. Binding of response regulator DegU to the aprE promoter is inhibited by RapG, which is counteracted by extracellular PhrG in Bacillus subtilis. Mol Microbiol. 2003;49:1685–1697. doi: 10.1046/j.1365-2958.2003.03665.x. [DOI] [PubMed] [Google Scholar]
- Ohsawa T, Tsukahara K, Ogura M. Bacillus subtilis response regulator DegU is a direct activator of pgsB transcription involved in gamma-poly-glutamic acid synthesis. Biosci Biotechnol Biochem. 2009;73:2096–2102. doi: 10.1271/bbb.90341. [DOI] [PubMed] [Google Scholar]
- Olmos J, Bolanos V, Causey S, Ferrari E, Bollvar F, Valle F. A functional Spo0A is required for maximal aprE expression in Bacillus subtilis. FEBS Lett. 1996;381:29–31. doi: 10.1016/0014-5793(96)00070-1. [DOI] [PubMed] [Google Scholar]
- Osera C, Amati G, Calvio C, Galizzi A. SwrAA activates poly-gamma-glutamate synthesis in addition to swarming in Bacillus subtilis. Microbiology. 2009;155:2282–2287. doi: 10.1099/mic.0.026435-0. [DOI] [PubMed] [Google Scholar]
- Ostrowski A, Mehert A, Prescott A, Kiley TB, Stanley-Wall NR. YuaB functions synergistically with the exopolysaccharide and TasA amyloid fibers to allow biofilm formation by Bacillus subtilis. J Bacteriol. 2011;193:4821–4831. doi: 10.1128/JB.00223-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge JD, Harshey RM. More than motility: Salmonella flagella contribute to overriding friction and facilitating colony hydration during swarming. J Bacteriol. 2013;195:919–929. doi: 10.1128/JB.02064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick JE, Kearns DB. Swarming motility and the control of master regulators of flagellar biosynthesis. Mol Microbiol. 2012;83:14–23. doi: 10.1111/j.1365-2958.2011.07917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul K, Nieto V, Carlquist WC, Blair DF, Harshey RM. The c-di-GMP binding protein YcgR controls flagellar motor direction and speed to affect chemotaxis by a ‘backstop brake’ mechanism. Mol Cell. 2010;38:128–139. doi: 10.1016/j.molcel.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilizota T, Brown MT, Leake MC, Branch RW, Berry RM, Armitage JP. A molecular brake, not a clutch, stops the Rhodobacter sphaeroides flagellar motor. Proc Natl Acad Sci USA. 2009;106:11582–11587. doi: 10.1073/pnas.0813164106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt LA, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30:285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- Rubinstein SM, Kolodkin-Gal I, McLoon A, Chai L, Kolter R, Losick R, Weitz DA. Osmotic pressure can regulate matrix gene expression in Bacillus subtilis. Mol Microbiol. 2012;86:426–436. doi: 10.1111/j.1365-2958.2012.08201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzal SM, Sanchez-Rivas C. In Bacillus subtilis DegU-P is a positive regulator of the osmotic response. Curr Microbiol. 1998;37:368–372. doi: 10.1007/s002849900395. [DOI] [PubMed] [Google Scholar]
- Serra DO, Richter AM, Klauck G, Mika F, Hengge R. Microanatomy at cellular resolution and spatial order of physiological differentiation in a bacterial biofilm. mBio. 2013;4:e00103–e00113. doi: 10.1128/mBio.00103-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank EA, Kolter R. Extracellular signaling and multicellularity in Bacillus subtilis. Curr Opin Microbiol. 2011;14:741–747. doi: 10.1016/j.mib.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp LL, Zhou J, Blair DF. Tryptophan-scanning mutagenesis of MotB, an integral membrane protein essential for flagellar rotation in Escherichia coli. Biochemistry. 1995;34:9166–9171. doi: 10.1021/bi00028a028. [DOI] [PubMed] [Google Scholar]
- Srivatsan A, Han Y, Peng J, Tehranchi AK, Gibbs R, Wang JD, Chen R. High-precision, whole-genome sequencing of laboratory strains facilitates genetic studies. PLoS Genet. 2008;4:e1000139. doi: 10.1371/journal.pgen.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley NR, Lazazzera BA. Defining the genetic differences between wild and domestic strains of Bacillus subtilis that affect poly-gamma-dl-glutamic acid production and biofilm formation. Mol Microbiol. 2005;57:1143–1158. doi: 10.1111/j.1365-2958.2005.04746.x. [DOI] [PubMed] [Google Scholar]
- Suaste-Olmos F, Domenzain C, Mireles-Rodriguez JC, Poggio S, Osorio A, Dreyfus G, Camarena L. The flagellar protein FliL is essential for swimming in Rhodobacter sphaeroides. J Bacteriol. 2010;192:6230–6239. doi: 10.1128/JB.00655-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veening JW, Igoshin OA, Eijlander RT, Nijland R, Hamoen LW, Kuipers OP. Transient heterogeneity in extracellular protease production by Bacillus subtilis. Mol Syst Biol. 2008;4:184. doi: 10.1038/msb.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhamme DT, Kiley TB, Stanley-Wall NR. DegU co-ordinates multicellular behaviour exhibited by Bacillus subtilis. Mol Microbiol. 2007;65:554–568. doi: 10.1111/j.1365-2958.2007.05810.x. [DOI] [PubMed] [Google Scholar]
- Verhamme DT, Murray EJ, Stanley-Wall NR. DegU and Spo0A jointly control transcription of two loci required for complex colony development by Bacillus subtilis. J Bacteriol. 2009;191:100–108. doi: 10.1128/JB.01236-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J, Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Vlamakis H, Chai Y, Beauregard P, Losick R, Kolter R. Sticking together: building a biofilm the Bacillus subtilis way. Nat Rev Microbiol. 2013;11:157–168. doi: 10.1038/nrmicro2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Frye JG, McClelland M, Harshey RM. Gene expression patterns during swarming in Salmonella typhimurium: genes specific to surface growth and putative new motility and pathogenicity genes. Mol Microbiol. 2004;52:169–187. doi: 10.1111/j.1365-2958.2003.03977.x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Suzuki A, Mariconda S, Porwollik S, Harshey RM. Sensing wetness: a new role for the bacterial flagellum. EMBO J. 2005;24:2034–2042. doi: 10.1038/sj.emboj.7600668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watnick P, Kolter R. Steps in the development of a Vibrio cholerae El Tor biofilm. Mol Microbiol. 1999;34:586–595. doi: 10.1046/j.1365-2958.1999.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watnick P, Kolter R. Biofilm, city of microbes. J Bacteriol. 2000;182:2675–2679. doi: 10.1128/jb.182.10.2675-2679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PY, Fedor MJ. The ydaO motif is an ATP-sensing riboswitch in Bacillus subtilis. Nat Chem Biol. 2012;8:963–965. doi: 10.1038/nchembio.1095. [DOI] [PubMed] [Google Scholar]
- Wolfe AJ, Chang DE, Walker JD, Seitz-Partridge JE, Vidaurri MD, Lange CF, et al. Evidence that acetyl phosphate functions as a global signal during biofilm development. Mol Microbiol. 2003;48:977–988. doi: 10.1046/j.1365-2958.2003.03457.x. [DOI] [PubMed] [Google Scholar]
- Wolfe AJ, Parikh N, Lima BP, Zemaitaitis B. Signal integration by the two-component signal transduction response regulator CpxR. J Bacteriol. 2008;190:2314–2322. doi: 10.1128/JB.01906-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Lloyd SA, Blair DF. Electrostatic interactions between rotor and stator in the bacterial flagellar motor. Proc Natl Acad Sci USA. 1998a;95:6436–6441. doi: 10.1073/pnas.95.11.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Sharp LL, Tang HL, Lloyd SA, Billings S, Braun TF, Blair DF. Function of protonatable residues in the flagellar motor of Escherichia coli: a critical role for Asp 32 of MotB. J Bacteriol. 1998b;180:2729–2735. doi: 10.1128/jb.180.10.2729-2735.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorraquino V, Garcia B, Latasa C, Echeverz M, Toledo-Arana A, Valle J, et al. Coordinated cyclic-di-GMP repression of Salmonella motility through YcgR and cellulose. J Bacteriol. 2013;195:417–428. doi: 10.1128/JB.01789-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


