Reactive oxygen species (ROS) cause DNA damage. In this work, two SIAMESE/SIAMESE-RELATED (SIM/SMR) genes that encode cyclin-dependent kinase inhibitors are described as being part of a signaling pathway that arrests cell proliferation in response to ROS, revealing a novel cell cycle checkpoint-signaling cascade.
Abstract
Whereas our knowledge about the diverse pathways aiding DNA repair upon genome damage is steadily increasing, little is known about the molecular players that adjust the plant cell cycle in response to DNA stress. By a meta-analysis of DNA stress microarray data sets, three family members of the SIAMESE/SIAMESE-RELATED (SIM/SMR) class of cyclin-dependent kinase inhibitors were discovered that react strongly to genotoxicity. Transcriptional reporter constructs corroborated specific and strong activation of the three SIM/SMR genes in the meristems upon DNA stress, whereas overexpression analysis confirmed their cell cycle inhibitory potential. In agreement with being checkpoint regulators, SMR5 and SMR7 knockout plants displayed an impaired checkpoint in leaf cells upon treatment with the replication inhibitory drug hydroxyurea (HU). Surprisingly, HU-induced SMR5/SMR7 expression depends on ATAXIA TELANGIECTASIA MUTATED (ATM) and SUPPRESSOR OF GAMMA RESPONSE1, rather than on the anticipated replication stress-activated ATM AND RAD3-RELATED kinase. This apparent discrepancy was explained by demonstrating that, in addition to its effect on replication, HU triggers the formation of reactive oxygen species (ROS). ROS-dependent transcriptional activation of the SMR genes was confirmed by different ROS-inducing conditions, including high-light treatment. We conclude that the identified SMR genes are part of a signaling cascade that induces a cell cycle checkpoint in response to ROS-induced DNA damage.
INTRODUCTION
Being sessile, plants are continuously exposed to changing environmental conditions that can impose biotic and abiotic stresses. One of the consequences observed in plants subjected to altered growth conditions is the disruption of reactive oxygen species (ROS) homeostasis (Mittler et al., 2004). Under steady state conditions, ROS are efficiently scavenged by different nonenzymatic and enzymatic antioxidant systems, involving the activity of catalases, peroxidases, and glutathione reductases. However, when stress prevails, the ROS production rate can exceed the scavenging mechanisms, resulting in a cell- or tissue-specific rise in ROS. These oxygen derivatives possess a strong oxidizing potential that can damage a wide diversity of biological molecules, including the electron-rich bases of DNA, which results into single- and double-stranded breaks (DSBs; Amor et al., 1998; Dizdaroglu et al., 2002; Roldán-Arjona and Ariza, 2009). H2O2 is a major ROS compound and is able to transverse cellular membranes, migrating into different compartments. This feature grants H2O2 not only the potential to damage a variety of cellular structures, but also to serve as a signaling molecule, allowing the activation of pathways that modulate developmental, metabolic, and defense pathways (Mittler et al., 2011). One of the signaling effects of H2O2 is the activation of cell division arrest by cell cycle checkpoint activation (Tsukagoshi, 2012); however, the molecular mechanisms involved remain unknown.
Cell cycle checkpoints adjust cellular proliferation to changing growth conditions, arresting it by inhibiting the main cell cycle controllers: the heterodimeric complexes between the cyclin-dependent kinases (CDKs) and the regulatory cyclins (Lee and Nurse, 1987; Norbury and Nurse, 1992). The activators of these checkpoints are the highly conserved ATAXIA TELANGIECTASIA MUTATED (ATM) and ATM AND RAD3-RELATED (ATR) kinases that are recruited in accordance with the type of DNA damage (Zhou and Elledge, 2000; Abraham, 2001; Bartek and Lukas, 2001; Kurz and Lees-Miller, 2004). ATM is activated by DSBs, whereas ATR is activated by single-strand breaks or stalled replication forks, causing inhibition of DNA replication. In mammals, ATM and ATR activation results in the phosphorylation of the Chk2 and Chk1 kinases, respectively. Both kinases subsequently phosphorylate p53, a central transcription factor in the DNA damage response (Chaturvedi et al., 1999; Shieh et al., 2000; Chen and Sanchez, 2004; Rozan and El-Deiry, 2007). Chk1, Chk2, and p53 seemingly appear to have no plant ortholog, although an analogous role for p53 is suggested for the plant-specific SUPPRESSOR OF GAMMA RESPONSE1 (SOG1) transcription factor that is under direct posttranscriptional control of ATM (Yoshiyama et al., 2009, 2013). Another distinct plant feature relates to the inactivation of CDKs in response to DNA stress. CDK activity is in part regulated by its phosphorylation status at the N terminus, determined by the interplay of the CDC25 phosphatase and the antagonistic WEE1 kinase, acting as the “on” and “off” switches of CDK activity, respectively (Francis, 2011). Whereas in mammals and budding yeast the activation of the DNA replication checkpoint, leading to a cell cycle arrest, is predominantly achieved by the inactivation of the CDC25 phosphatase, plant cells respond to replication stress by transcriptional induction of WEE1 (De Schutter et al., 2007). In the absence of WEE1, Arabidopsis thaliana plants become hypersensitive to replication inhibitory drugs, such as hydroxyurea (HU), which causes a depletion of deoxynucleotide triphosphates (dNTPs) by inhibiting the ribonucleotide reductase (RNR) protein. However, WEE1-deficient plants respond similarly as control plants to other types of DNA damage (De Schutter et al., 2007; Dissmeyer et al., 2009). These data suggest the existence of yet to be identified pathways controlling cell cycle progression under DNA stress, operating independently of WEE1.
Potential candidates to operate in checkpoint activation upon DNA stress are CDK inhibitors (CKIs). CKI proteins are mostly low molecular weight proteins that inhibit cell division by their direct interaction with the CDK and/or cyclin subunit (Sherr and Roberts, 1995; De Clercq and Inzé, 2006). The first identified class of plant CKIs was the ICK/KRP (interactors of CDK/Kip-related protein) protein family comprising seven members in Arabidopsis, all sharing a conserved C-terminal domain being similar to the CDK binding domain of the animal CIP/KIP proteins (Wang et al., 1998, 2000; De Veylder et al., 2001). TIC (tissue-specific inhibitors of CDK) is the most recently suggested class of CKIs (DePaoli et al., 2012) and encompasses SCI1 (for STIGMA/STYLE CELL CYCLE INHIBITOR1) in tobacco (Nicotiana tabacum; DePaoli et al., 2011). SCI1 shares no apparent sequence similarity with the other classes of CKIs in plants and has been suggested to connect cell cycle progression and auxin signaling in pistils (DePaoli et al., 2012). The third class of CKIs is the plant-specific SIAMESE/SIAMESE-RELATED (SIM/SMR) gene family. SIM has been identified as a cell cycle inhibitor with a role in trichome development and endocycle control (Churchman et al., 2006). Based on sequence analysis, five additional gene family members have been identified in Arabidopsis and, together with EL2 from rice (Oryza sativa), have been suggested to act as cell cycle inhibitors modulated by biotic and abiotic stresses (Peres et al., 2007). Plants subjected to treatments inducing DSBs showed a rapid and strong induction of specific family members (Culligan et al., 2006; Adachi et al., 2011), suggesting that SIM/SMR proteins might include interesting candidates to complement WEE1 in the global response to DNA stress.
In this work, we identified three SMR genes (SMR4, SMR5, and SMR7) that are transcriptionally activated by DNA damage. Cell cycle inhibitory activity was demonstrated by overexpression analysis, whereas knockout data illustrated that both SMR5 and SMR7 are essential for DNA cell cycle checkpoint activation in leaves of plants grown in the presence of HU. Remarkably, we found that SMR induction mainly depends on ATM and SOG1, rather than ATR, as would be expected for a drug that triggers replication fork defects. Correspondingly, we demonstrate that the HU-dependent activation of SMR genes is triggered by ROS rather than replication problems, linking SMR genes with cell cycle checkpoint activation upon the occurrence of DNA damage-inducing oxidative stress.
RESULTS
Meta-Analysis of DNA Stress Datasets Identifies DNA Damage-Induced SMR Genes
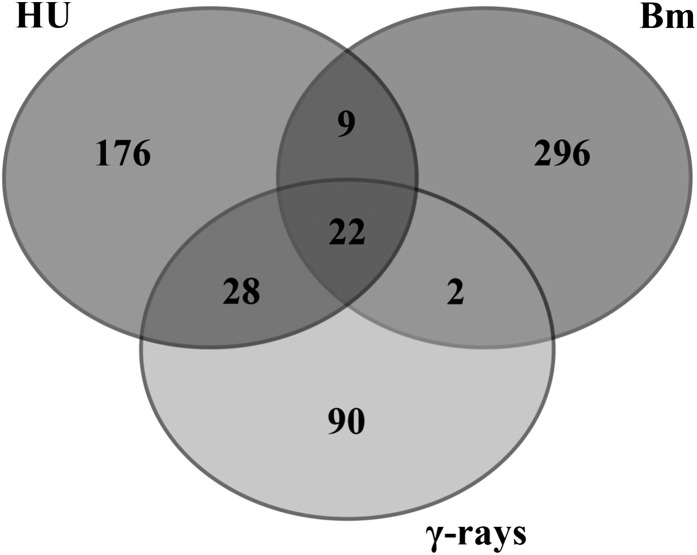
When DNA damage occurs, two global cellular responses are essential for cell survival: activation of the DNA repair machinery and delay or arrest of cell cycle progression. Recently, gene expression inventories have been collected that focus on the transcriptional changes in response to different types of DNA stress (Culligan et al., 2006; Ricaud et al., 2007; Yoshiyama et al., 2009; Cools et al., 2010). To identify novel key signaling components that contribute to cell cycle checkpoint activation, we compared bleomycin-induced genes to those induced by HU treatment (Cools et al., 2010) and γ-radiation (Culligan et al., 2006; Yoshiyama et al., 2009). Twenty-two genes were upregulated in all DNA stress experiments and can be considered as transcriptional hallmarks of the DNA damage response, regardless of the type of DNA stress (Figure 1, Table 1). Within this selection, genes known to be involved in DNA stress and DNA repair are predominantly present, including POLY(ADP-RIBOSE) POLYMERASE2 (PARP2), BREAST CANCER SUSCEPTIBILITY1 (BRCA1), and RAS-ASSOCIATED WITH DIABETES PROTEIN51. In addition, we recognized one member of the SIM/SMR gene family, SMR5. When expanding the selection by considering genes induced in at least two of the three DNA stress experiments, we identified a total of 61 genes (Supplemental Data Set 1). Besides DNA damage response–related genes, this expanded data set included an additional SMR family member (SMR4), which was expressed upon HU treatment and γ-radiation.
Figure 1.
DNA Stress Meta-Analysis.
Venn diagram showing the overlap between transcripts induced by HU, bleomycin (Bm), and γ-radiation (γ-rays). In total, 61 genes were positively regulated in at least two DNA stress experiments and 22 genes accumulated in all DNA stress experiments.
Table 1. Overview of the Transcriptionally Induced Core DNA Damage Genes.
| AGI Locusa | Annotation | HU 24 h/0 hb | γ-Rays 1c | γ-Rays 2d | Bleomycin |
|---|---|---|---|---|---|
| AT4G21070 | Breast cancer susceptibility1 | 10.375 | 581.570 | 57.803 | 2.386 |
| AT5G60250 | Zinc finger (C3HC4-type RING finger) family protein | 8.907 | 34.918 | 40.000 | 2.352 |
| AT1G07500 | Siamese-related 5 | 7.863 | 38.160 | 35.842 | 1.595 |
| AT4G02390 | Poly(ADP-Rib) polymerase | 7.701 | 131.865 | 59.172 | 2.663 |
| AT3G07800 | Thymidine kinase | 7.160 | 46.179 | 20.492 | 2.759 |
| AT5G03780 | TRF-like 10 | 7.111 | 108.316 | 23.474 | 1.600 |
| AT5G64060 | NAC domain containing protein 103 | 5.579 | 28.086 | 13.755 | 2.153 |
| AT2G18600 | Ubiquitin-conjugating enzyme family protein | 5.521 | 21.462 | 11.481 | 1.972 |
| AT4G22960 | Unknown function (DUF544) | 5.315 | 36.380 | 14.451 | 2.282 |
| AT5G48720 | X-ray induced transcript 1 | 5.296 | 285.166 | 65.789 | 2.228 |
| AT5G24280 | γ-Irradiation and mitomycin c induced 1 | 4.823 | 108.578 | 42.918 | 2.584 |
| AT5G20850 | RAS associated with diabetes protein 51 | 4.643 | 186.456 | 31.250 | 1.765 |
| AT3G27060 | Ferritin/ribonucleotide reductase-like family protein | 4.595 | 37.351 | 8.741 | 1.970 |
| AT2G46610 | RNA binding (RRM/RBD/RNP motifs) family protein | 3.593 | 19.913 | 7.331 | 1.546 |
| AT5G40840 | Rad21/Rec8-like family protein | 3.375 | 113.919 | 27.473 | 1.692 |
| AT1G13330 | Hop2 homolog | 2.949 | 17.349 | 13.495 | 1.580 |
| AT5G66130 | RADIATION SENSITIVE17 | 2.888 | 30.411 | 10.384 | 1.627 |
| AT1G17460 | TRF-like 3 | 2.378 | 18.925 | 10.661 | 1.681 |
| AT2G45460 | SMAD/FHA domain-containing protein | 2.378 | 45.673 | 21.053 | 1.575 |
| AT5G49480 | Ca2+ binding protein 1 | 1.952 | 15.106 | 5.851 | 1.580 |
| AT3G25250 | AGC (cAMP-dependent, cGMP-dependent, and protein kinase C) kinase family protein | 1.853 | 12.995 | 17.794 | 1.517 |
| AT5G55490 | Gamete expressed protein 1 | 1.670 | 71.489 | 34.722 | 2.407 |
AGI, Arabidopsis Genome Initiative.
According to Cools et al. (2011).
According to Culligan et al. (2006).
According to Yoshiyama et al. (2009).
The SMR Gene Family Comprises 14 Family Members That Respond to Different Stresses
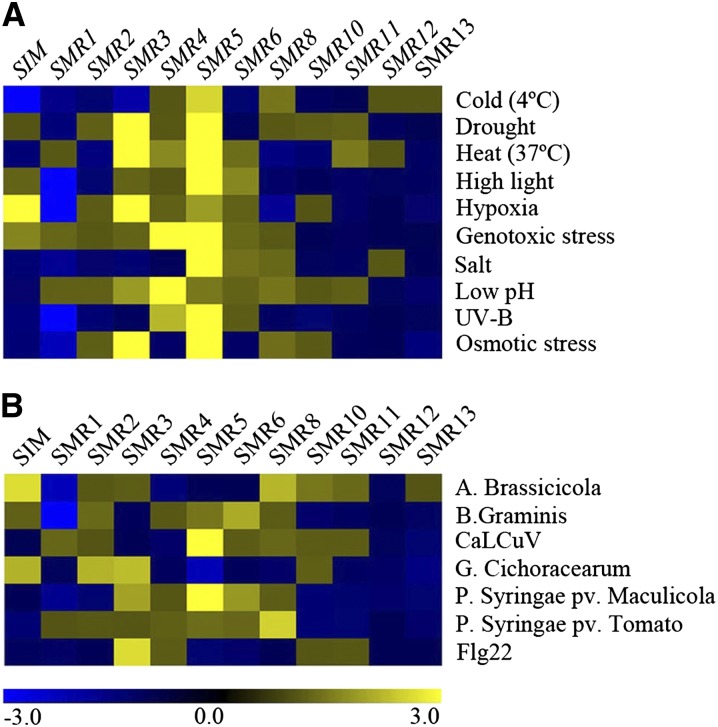
Previously, we reported on the existence of one SIM and five SMR genes (SMR1-SMR5) in the Arabidopsis genome (Peres et al., 2007), whereas protein purification of CDK/cyclin complexes resulted in the identification of two additional family members (SMR6 and SMR8) (Van Leene et al., 2010). With the availability of newly sequenced plant genomes, we reexamined the Arabidopsis genome using iterative BLAST searches for the presence of additional SMR genes, resulting in the identification of six nonannotated family members, named SMR7 to SMR13 (Supplemental Table 1). With the Genevestigator toolbox (Hruz et al., 2008), the expression pattern of the 12 SIM/SMR genes represented on the Affymetrix ATH1 microarray platform was analyzed in response to different biotic and abiotic stress treatments. Distinct family members were induced under various stress conditions, albeit with different specificity (Figure 2). Every SMR gene appeared to be transcriptionally active under at least a number of stress conditions, with SMR5 responding to most diverse types of abiotic stresses. In response to DNA stress (genotoxic stress and UV-B light treatment), two SMR genes responded strongly, namely, SMR4 and SMR5, corresponding with their presence among the DNA stress genes identified by our microarray meta-analysis.
Figure 2.
Hierarchical Average Linkage Clustering of SIM/SMR Genes Induced in Response to Different Stresses.
Arabidopsis plants were exposed to abiotic (A) and biotic (B) stresses.
Data comprise the SIM/SMR represented in publicly available Affymetrix ATH1 microarrays obtained with the Genevestigator toolbox. Blue and yellow indicate down- and upregulation, respectively, whereas black indicates no change in expression. Values indicate fold-change in expression level in stress versus control experiments.
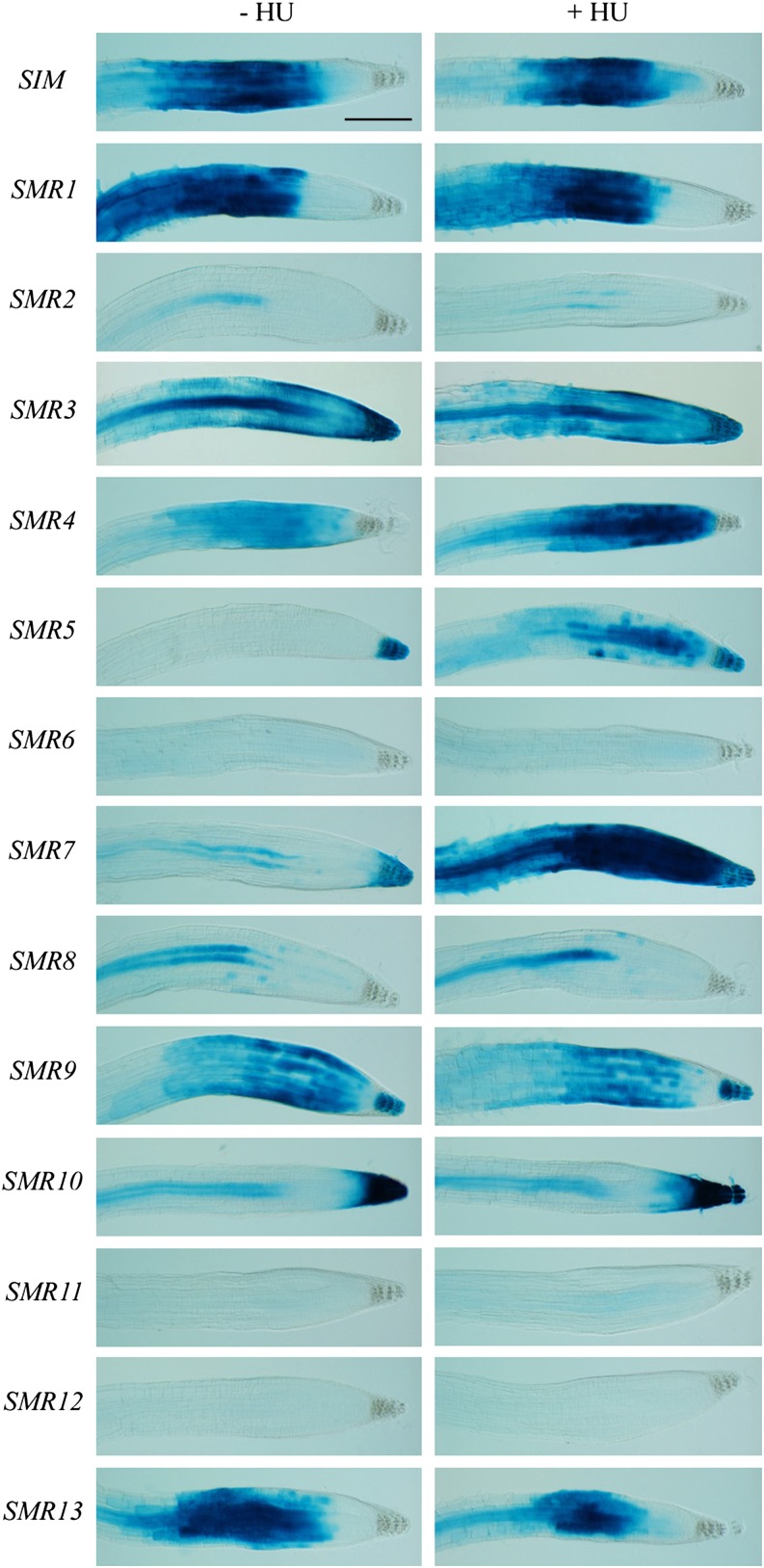
To confirm their involvement in the genotoxic stress response, transcriptional reporter lines containing the putative upstream promoter sequences were constructed for all SIM/SMR genes. After selection of representative reporter lines, 1-week-old seedlings were transferred to control medium or medium supplemented with HU (resulting in stalled replication forks) or bleomycin (causing DSBs). Focusing on the root tips revealed distinct expression patterns (Figure 3; Supplemental Figure 1), with some family members being restricted to the root elongation zone (including SIM and SMR1), while others were confined to vascular tissue (e.g., SMR2 and SMR8) or columella cells (e.g., SMR5). When plants were exposed to HU, three SMR genes showed transcriptional induction in the root meristem, namely, SMR4, SMR5, and SMR7, with the latter two displaying the strongest response (Figure 3). In the presence of bleomycin, an additional weak cell-specific induction of SMR6 was observed (Supplemental Figure 1). Transcriptional induction of SMR4, SMR5, and SMR7 by HU and bleomycin was confirmed by quantitative RT-PCR experiments (Supplemental Figure 2). These data fit the above-described microarray analysis, with the lack of SMR7 being explained by its absence on the ATH1 microarray of the HU and γ-irradiation experiments, although being induced 5.68-fold in the bleomycin experiment performed using the Aragene array. In addition to HU and bleomycin, we confirmed the transcriptional activation of SMR4, SMR5, and SMR7 by γ-irradiation (Supplemental Figure 3).
Figure 3.
SIM/SMR Induction in Response to HU.
One-week-old SMR reporter seedlings (names indicated on the left) were transferred to control (−HU) medium or medium supplemented with 1 mM HU (+HU). GUS assays were performed 24 h after transfer. All images are at the same magnification. Bar = 200 μm.
DNA Stress–Induced SMR Genes Encode Potent Cell Cycle Inhibitors
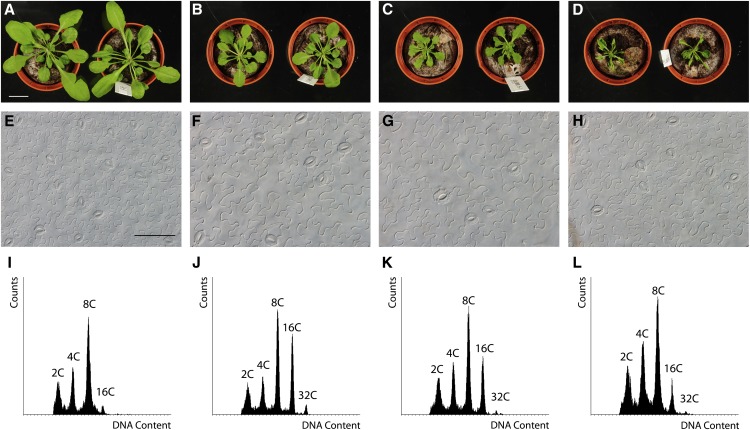
SIM had been proven to encode a potent cell cycle inhibitor, since its ectopic expression results in dwarf plants with fewer cells than control plants (Churchman et al., 2006). To test whether the DNA stress–induced SMR genes encode proteins with cell division inhibitory activity, SMR4-, SMR5-, and SMR7-overexpressing (SMR4OE, SMR5OE, and SMR7OE) plants were generated. For each gene, multiple lines with high transcript levels were isolated, all showing a reduction in rosette size compared with wild-type plants (Figures 4A to 4D). This decrease in leaf size correlated with an increase in cell size (Figures 4E to 4H), indicative of a strong inhibition of cell division. Similar to SIM (Churchman et al., 2006), ectopic expression not only inhibited cell division but also triggered an increase in DNA content by stimulating endoreplication (Figures 4I to 4L; Supplemental Table 2), likely representing a premature onset of cell differentiation. Together with the previously described biochemical interaction between SMR4 and SMR5, and CDKA;1 and D-type cyclins (Van Leene et al., 2010), it can be concluded that the DNA stress–induced SMR genes encode potent cell cycle inhibitors.
Figure 4.
Ectopic SMR4, SMR5, and SMR7 Expression Inhibits Cell Division.
(A) to (D) Four-week-old rosettes of control (A), SMR4OE (B), SMR5OE (C), and SMR7OE (D) plants. All images are at the same magnification. Bar = 2 cm.
(E) to (H) Leaf abaxial epidermal cell images of in vitro–grown 3-week-old control (E), SMR4OE (F), SMR5OE (G), and SMR7OE (H) plants. All images are at the same magnification. Bar = 100 μm.
(I) to (L) Ploidy level distribution of the first leaves of 3-week-old in vitro–grown control (I), SMR4OE (J), SMR5OE (K), and SMR7OE (L) plants.
SMR5 and SMR7 Regulate a HU-Dependent Checkpoint in Leaves
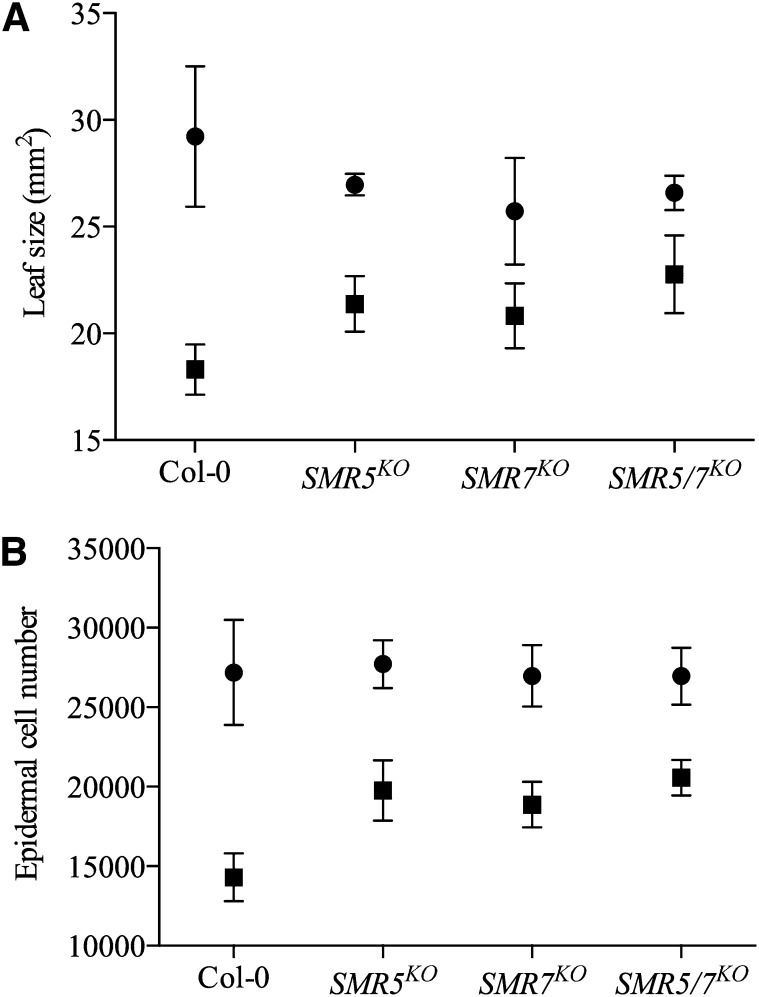
To address the role of the different SMR genes in DNA stress checkpoint regulation, the growth response to HU treatment of plants silenced for SMR5 or SMR7 (Supplemental Figure 4) was compared with that of control plants (Columbia-0 [Col-0]). No significant difference in leaf size was observed for plants grown under standard conditions. By contrast, when comparing plants grown for 3 weeks in the presence of HU, the leaves of the knockout plants SMR5KO and SMR7KO were significantly larger than that of the control plants (Figure 5A). This difference was attributed to a difference in cell number. Control plants responded to the HU treatment with a 47% reduction in epidermal cell number, reflecting an activation of a stringent cell cycle checkpoint. By contrast, in SMR5KO and SMR7KO plants this reduction was restricted to 29 and 30%, respectively (Figure 5B). Within the SMR5KO SMR7KO double mutant, the reduction in leaf size and cell number was even less (Figures 5A and 5B), suggesting that both inhibitors contribute to the cell cycle arrest observed in the control plants by checkpoint activation upon HU stress. A similar role of SMR4 could not be tested due to the lack of an available knockout.
Figure 5.
SMR5 and SMR7 Are Required for an HU-Dependent Cell Cycle Checkpoint.
Leaf size (A) and abaxial epidermal cell number (B) of the first leaves of 3-week-old plants grown on control medium (circles) or medium supplemented with 1 mM HU (squares). Data represent mean with 95% confidence interval (two-way ANOVA, n = 10).
SMR5 and SMR7 Expression Is Triggered by Oxidative Stress
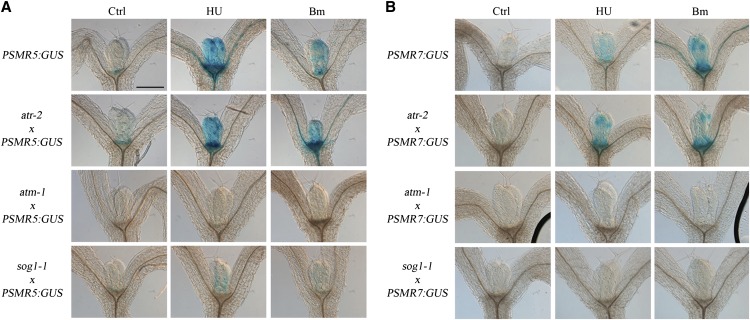
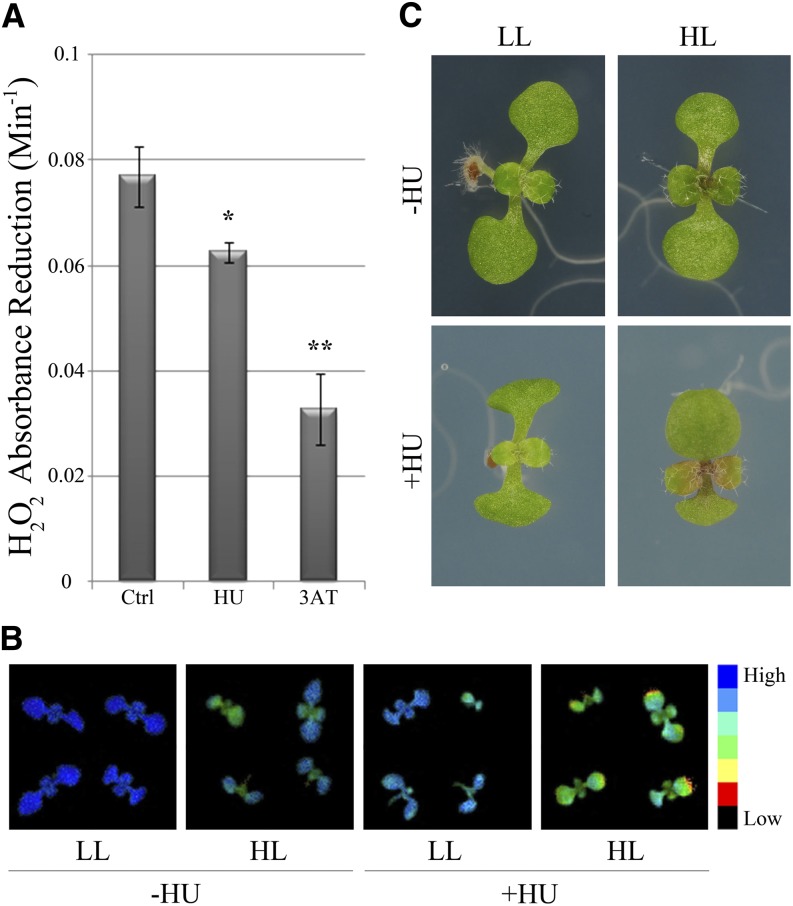
Because of the observed role of SMR5 and SMR7 in DNA stress checkpoint regulation, we analyzed the dependence of their expression on the ATM and ATR signaling kinases and the SOG1 transcription factor by introducing the SMR5 and SMR7 β-glucuronidase (GUS) reporter lines into the atr-2, atm-1, and sog1-1 mutant backgrounds. Both genes were induced in the proliferating leaf upon HU and bleomycin treatment (Figure 6). Moreover, as would be expected for a DSB-inducing agent, the transcriptional activation of SMR5 and SMR7 by bleomycin depended on ATM and SOG1. Surprisingly, the same pattern was observed for HU, whereas one would expect that SMR5/SMR7 induction after arrest of the replication fork would rely on ATR-dependent signaling. These data indicate that the HU-dependent activation of SMR5 and SMR7 might be caused by a genotoxic effect of HU being unrelated to replication stress induced by the depletion of dNTPs. A recent study demonstrated that HU directly inhibits catalase-mediated H2O2 decomposition (Juul et al., 2010). Analogously, in combination with H2O2, HU has been demonstrated to act as a suicide inhibitor of ascorbate peroxidase (Chen and Asada, 1990). Combined, both mechanisms are likely responsible for an increase in the cellular H2O2 concentration, which might trigger DNA damage and, consequently, transcriptional induction of the SMR5 and SMR7 genes. Indeed, extracts of control plants treated with HU displayed a reduced H2O2 decomposition rate (Figure 7A). As catalase and ascorbate peroxidase activity are essential for the scavenging of H2O2 that is generated upon high-light exposure, we subsequently tested the effects of HU treatment on photosystem II (PSII) efficiency in 1-week-old seedlings after transfer from low- to high-light conditions. As illustrated in Figure 7B, transfer for 48 h to high light resulted in a decrease of maximum quantum efficiency of PSII. In the presence of HU, the maximum quantum efficiency of PSII decrease was even more pronounced, which again corroborates the idea that HU might interfere with H2O2 scavenging. Macroscopically, plants grown in the presence of HU showed visible anthocyanin pigmentation in the young leaf tissue within 48 h after transfer, whereas plants grown on control medium showed no effect of the transfer to high light (Figure 7C).
Figure 6.
SMR5 and SMR7 Expression Is ATM and SOG1 Dependent.
PSMR5:GUS (A) and PSMR7:GUS (B) reporter constructs introgressed into atr-2, atm-1, and sog-1 mutant backgrounds were transferred to control medium (Ctrl) or medium supplemented with 2 mM HU or 0.3 μg/mL bleomycin (Bm) for 24 h. All images are at the same magnification. Bar = 250 μm.
Figure 7.
HU Triggers Oxidative Stress.
(A) H2O2 scavenging in extracts from 1-week-old untreated control (Ctrl), HU-treated (1 mM), and 3-amino-1,2,4-triazole (3AT)–treated (6 μM) (positive control) plants. Error bars show se (n = 3 to 4). *P value < 0.05; **P value < 0.01 (two-tailed Student’s t test).
(B) Fluorescence images displaying maximum quantum efficiency of PSII of 6-d-old seedlings grown under low (LL) and high (HL) light for 48 h in the absence (−HU) and presence (+HU) of 1 mM HU.
(C) Light microscope images of plants shown in (B).
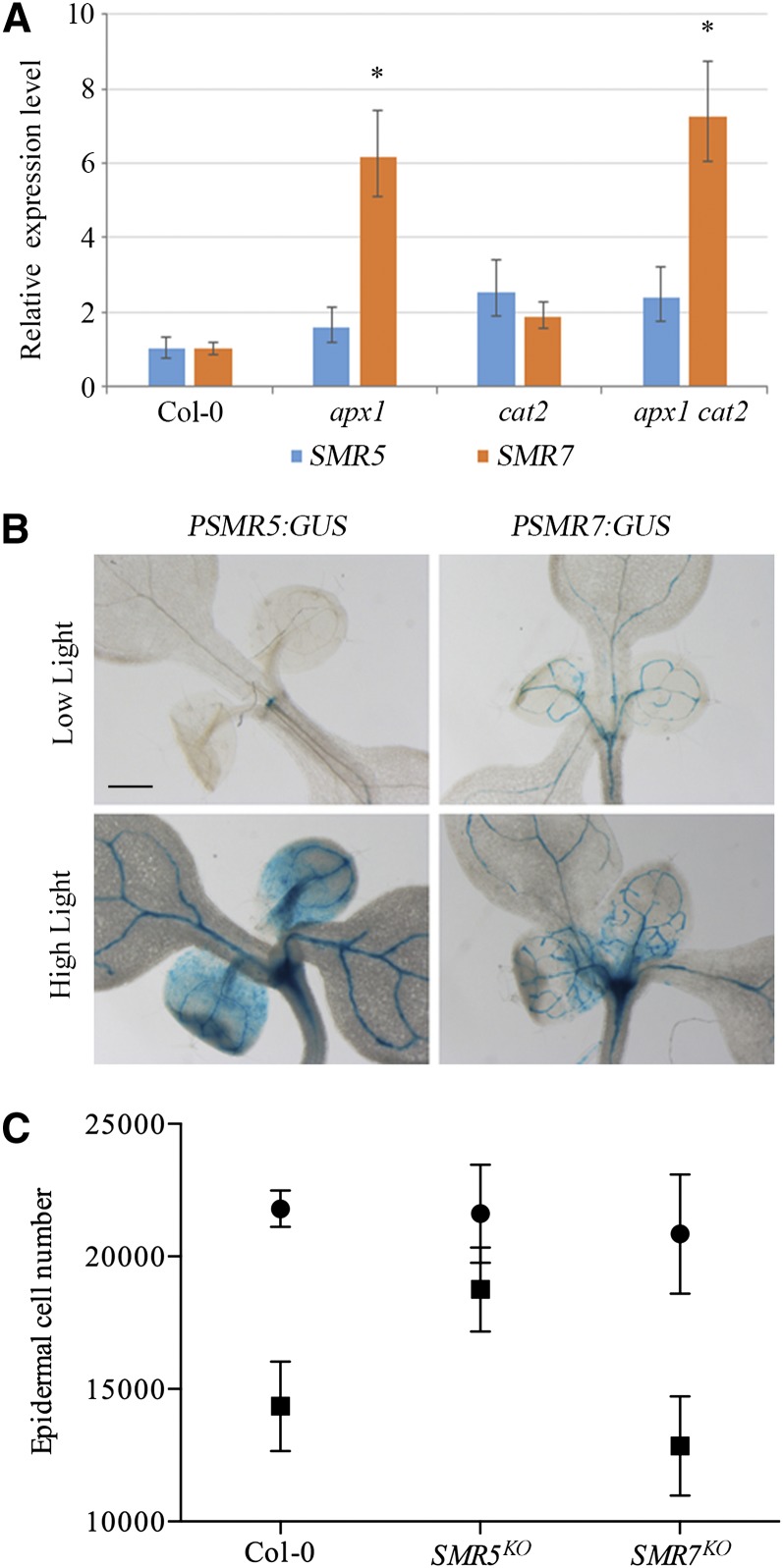
To examine whether an increase in H2O2 might trigger expression of SMR genes, SMR5 and SMR7 expression levels were analyzed in plants that are silenced for CAT2 and/or APX1, encoding two enzymes important for H2O2 scavenging. Whereas SMR5 transcript levels appeared to be stable over all genotypes, SMR7 expression levels were clearly induced in the single apx1 and apx1 cat2 double mutant (Figure 8A). As an independent strategy to induce ROS, SMR5 and SMR7 GUS reporter lines were transferred from control (70 to 80 µmol m–2 s–1) to high light (300 to 400 µmol m–2 s–1) conditions for 2 d. Whereas PSMR7:GUS plants displayed an increase in GUS activity being mainly restricted to the shoot apex, SMR5 promoter activity was strongly stimulated in both the shoot apex and leaf tissue (Figure 8B). SMR5 induction under high light was confirmed by RT-PCR (Supplemental Figure 5). To examine whether this transcriptional induction contributed to a high-light-induced cell cycle checkpoint, we measured epidermal cell numbers in mature first leaves of control (Col-0), SMR5KO, and SMR7KO plants that were transferred for 4 d to high-light conditions at a period when their leaf cells were still undergoing cell division. This high light treatment resulted into a 34 and 38% reduction in cell number in control and SMR7KO plants, respectively (Figure 8C). By contrast, SMR5KO plants displayed only a 13% reduction in cell number, illustrating that SMR5 is essential to activate a high-light-dependent cell cycle checkpoint.
Figure 8.
SMR5 and SMR7 Are Induced by Oxidative Stress–Inducing Stimuli.
(A) Relative SMR5 and SMR7 expression levels in shoots of 6-d-old wild-type (Col-0), apx1, cat2, and apx cat2 mutant plants. Data represent least square means ± se, normalized to wild-type levels that were arbitrary set to one (n = 3, *P value < 0.01).
(B) One-week-old PSMR5:GUS and PSMR7:GUS seedlings grown under low- versus high-light conditions for 48 h. All images are at the same magnification. Bar = 500 μm.
(C) Abaxial epidermal cell number of the first leaves of 3-week-old plants transferred at the age of 8 d for 96 h to control (circles) or high-light (squares) conditions. Data represent mean with 95% confidence interval (two-way ANOVA, n = 8).
SMR5 and SMR7 Are under Direct Regulation of SOG1
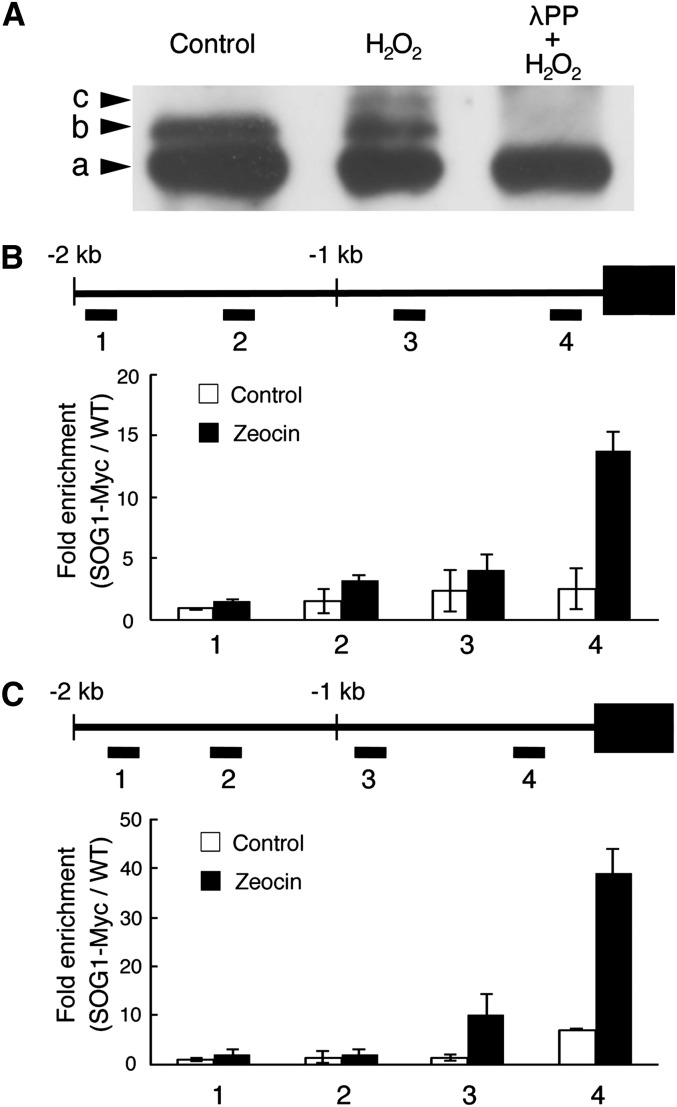
Recently, it was found that the SOG1 transcription factor becomes hyperphosphorylated in an ATM-dependent manner upon the occurrence of DSBs, such as induced by γ-irradiation or treatment with the radiomimetic drug zeocin, and that this phosphorylation is essential for SOG1 activity (Yoshiyama et al., 2013). As SMR5 and SMR7 transcription was found to depend on SOG1 and because both SMR genes respond to oxidative stress, we tested whether SOG1 phosphorylation occurs in response to H2O2 treatment. Lines expressing a Myc-tagged SOG1 under the control of its own promoter (PSOG1:SOG1-Myc) were either transferred to control medium or medium supplemented with H2O2. As described previously, immunoblotting using anti-Myc antibody detected two bands under control conditions (Figure 9A), with the upper band corresponding to SOG1 phosphorylated in a DNA stress–independent manner by a yet to be identified kinase (Yoshiyama et al., 2013). Upon H2O2 treatment, a third slowly migrating band appeared at a similar position as detected by zeocin treatment (Yoshiyama et al., 2013). This band disappeared when protein extracts were treated with the λ protein phosphatase (λPP), indicating that it corresponds to a phosphorylated form of SOG1 (Figure 9A).
Figure 9.
In Vivo Phosphorylation of SOG1 by H2O2 and Its Association with the SMR5 and SMR7 Promoters.
(A) Total protein was immunoblotted with anti-Myc antibody. Plants harboring PSOG1:SOG1-Myc were treated with or without H2O2, and total protein was extracted. Total protein from H2O2-treated plants was incubated with λPP. The phosphorylated forms of SOG1 were separated in an SDS-PAGE gel containing Phos-tag. Nonphosphorylated, phosphorylated, and hyperphosphorylated SOG1-Myc (bands a, b, and c, respectively) are indicated by arrowheads.
(B) and (C) Chromatin bound to the promoter regions of SMR5 (B) and SMR7 (C) was collected by immunoprecipitation with anti-Myc antibodies from PSOG1:SOG1-MYC plants treated with (black bars) and without (white bars) 15 µM zeocin and subjected to qPCR analysis. Fold enrichment for each DNA fragment was determined by dividing the recovery rate with that of wild-type plants (WT = 1). Bar graphs represent the average of two biological replicate ChIP experiments ± se. Positions of PCR amplicons 1 to 4 are also shown.
Subsequently, as SMR5 and SMR7 transcription was found to depend on SOG1 (Figure 6), we tested whether both genes are under the direct control of SOG1. Direct binding of SOG1 to the SMR5 and SMR7 promoters was tested through chromatin immunoprecipitation (ChIP) using PSOG1:SOG1-Myc seedlings that were either transferred to control medium or medium supplemented with the DSB-inducing drug zeocin for 2 h. Promoter scanning revealed that SOG1 binds in a DNA stress–dependent manner to both SMR promoters in close proximity to their transcription start sites (Figures 9B and 9C). These data illustrate that both SMR genes are under direct control of SOG1.
DISCUSSION
At Least Two Different Functional SMR Groups Exist
In this work, we analyzed the SIM/SMR group of CKIs. All share only limited sequence homology, being restricted to short amino acid regions scattered along the protein sequences, among which is a 6–amino acid domain corresponding to a cyclin binding motif (Peres et al., 2007). Although this poor sequence alignment does not allow a clear phylogenetic analysis, biochemically it appears that SIM/SMR proteins fall into at least two different categories. The first category includes the founding members SIM and SMR1 that both have been linked to endocycle onset (Churchman et al., 2006; Roeder et al., 2010), being an alternative cell cycle in which mitosis is repressed in favor of repetitive rounds of DNA replication, resulting in an increase in DNA ploidy level. Through protein purification, these two SMRs were found to copurify with the B-type CDKB1;1 (Van Leene et al., 2010), in agreement with the observation that this particular CDK needs to be inhibited for endocycle onset (Boudolf et al., 2004, 2009). A role in endocycle onset is supported by their expression pattern in the root, showing specific transcription in the cell elongation zone, likely representing the zone of cells in which endocycling begins. In addition to SIM and SMR1, SMR2 also exclusively copurifies with CDKB1;1, suggesting that this particular CKI might also be an SMR family member linked with endocycle onset. As a second category, other SMRs, including SMR4 and SMR5, exclusively copurify with the A-type CDK and D-type cyclins (Van Leene et al., 2010). CDKA;1 is the main driver of S-phase progression (Nowack et al., 2010, 2012), whereas the CYCD/CDKA;1 complex regulates cell cycle onset in response to intrinsic and extrinsic signals (Riou-Khamlichi et al., 2000; Dewitte and Murray, 2003). Therefore, CYCD/CDKA;1 appears to be the most logical CYC/CDK complex to be targeted by those SMRs that aim to link DNA stress signals with cell cycle checkpoint activation.
HU Affects DNA Integrity in Multiple Ways
HU is known for its inhibitory effect on RNR activity, resulting in the depletion of the available dNTPs, causing impaired progression of the replication fork and activation of an ATR-dependent replication checkpoint. However, the observed ATM-dependent induction of SMR5 and SMR7 upon HU treatment suggests that HU affects DNA integrity also in an RNR-independent manner. In particular, our data indicate that ROS might be the primary trigger of SMR5 and SMR7 expression upon HU treatment. A link between HU and oxidative stress has been observed previously in Saccharomyces cerevisiae, where, besides a DNA replication arrest caused by RNR inhibition, exposure to HU results in the activation of the Yap regulon that reacts to oxidative stress and encompasses genes involved in cellular redox homeostasis (Dubacq et al., 2006). In Arabidopsis, Juul et al. (2010) reported a direct interaction between HU and catalase, resulting in a stereoinhibition of the detoxifying capabilities of the catalase protein. Analogously, HU was demonstrated to be a suicide inhibitor of ascorbate peroxidase (Chen and Asada, 1990). In agreement, we demonstrated that HU treatment results in a decrease in the H2O2 scavenging rate. A second source of HU-induced ROS might originate from displacement of the essential cofactor iron from the RNR catalytic site (Nyholm et al., 1993), probably resulting in an increase in the intracellular iron concentration. This increase might contribute to the increase in ROS, as iron catalyzes the production of hydroxyl radicals from H2O2 through the Fenton reaction. Together, the increased H2O2 and iron levels after HU treatment represent a potent source of oxidative stress. The HU-induced oxidative state results in the accumulation of anthocyanin pigments and the reduction in PSII efficiency. The latter is likely due to the deceleration of PSII repair, consequently resulting in further increased levels of intracellular ROS and enhanced photoinhibition (Murata et al., 2012).
Because of its relatively long life and permeability, H2O2 is able to migrate into different cellular compartments. Besides PSII inhibition, H2O2 and hydroxyl radicals are known to affect the DNA in multiple ways, including the oxidation of bases, the creation of DNA interstrand cross-links, and DSBs (Cadet et al., 2012), triggering ATM-dependent signaling. In mammals, oxidation of ATM directly induces its activation (Guo et al., 2010); however, whether a similar mechanism is functional in plants is unknown. In agreement with H2O2 acting as a putative DNA stress–inducing compound, it has been reported that the lack of both catalase and cytosolic ascorbate peroxidase activity results in the transcriptional activation of DNA stress genes, including PARP2 and BRCA1 (Vanderauwera et al., 2011). The fact that within these apx1 cat2 double mutants no detectable rise in ROS levels could be measured suggests that experimentally undetectable levels of H2O2 can already trigger a DNA damage response. Interestingly, the resulting constitutive DNA damage response of the apx1 cat2 plant grants them enhanced tolerance to DNA stress–inducing conditions.
SMR5 and SMR7 Respond to ROS-Induced DNA Damage
Expression analysis under different ROS accumulating conditions strongly indicates that the transcriptional activation of SMR5 and SMR7 in response to HU is primarily mediated through changes in ROS homeostasis rather than by replication stress. Interestingly, SMR5 and SMR7 appear to display a differential transcriptional response toward distinct sources of ROS. Under high-light treatment, which likely generates singlet oxygen rather than H2O2 (Mittler, 2002), it is mainly SMR5 that is induced, in agreement with the observation that a high-light-induced cell cycle checkpoint was only abrogated in the SMR5KO plants. By contrast, SMR7 is the main gene induced in the apx1 and apx1 cat2 mutants. Similar to mature apx1 cat2 double mutant plants, young apx1 mutants display an activated DNA stress response, as supported by the elevated expression of DNA damage reporter genes under control conditions in 8-d-old seedlings (see Supplemental Figure 2 in Vanderauwera et al., 2011). This constitutive DNA damage response likely results from H2O2 leaking from the chloroplast (Davletova et al., 2005) and reaching the nucleus in the absence of cytosolic scavenging by APX1. The mechanisms by which different SMR genes respond to different types of ROS are currently unknown.
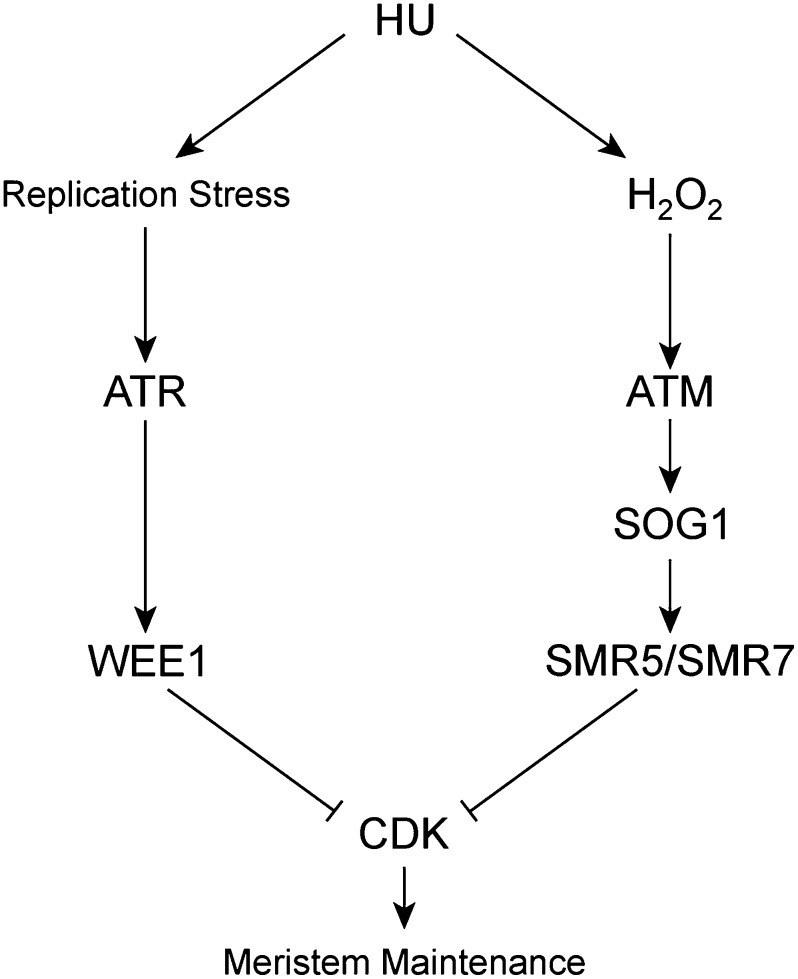
From our data, it can be concluded that HU simultaneously triggers two different cell cycle checkpoint cascades: one related to replication stress and one that responds to H2O2, regulated by ATR and ATM, respectively (Figure 10). Roots of plants silenced for the replication stress checkpoint activators ATR or WEE1 are hypersensitive to HU, indicating that the HU-induced replication defect prevails in roots. By contrast, despite their transcriptional induction, no clear root phenotype was observed for the SMR5KO and SMR7KO plants (Supplemental Figure 6). The restriction of a HU-sensitive phenotype to tissues with photosynthetic activity therefore suggests that the primary response of HU in the shoot tissue might be ROS accumulation (Figure 10). Remarkably, our data indicate that the signaling pathway by which oxidative stress induces SMR5/SMR7 expression is relatively short, with ATM phosphorylating the SOG1 transcription factor that binds directly to the SMR promoters to activate their transcription, as supported by the observation that no SMR5/SMR7 expression is observed in the sog1-1 mutant background. Because SOG1 only associates with the SMR5 and SMR7 promoters in the samples in which DNA stress was induced, we speculate that phosphorylation of SOG1 is a prerequisite for binding to its target genes.
Figure 10.
Model for HU-Dependent Cell Cycle Checkpoint Activation.
HU treatment results in replication stress and an increase in the cellular H2O2 concentration, likely resulting in DNA damage that is sensed by the ATR and ATM signaling cascades, respectively. ATR activates a checkpoint response through transcriptional induction of WEE1, whereas ATM does the same through activation of SMR5 and SMR7. Both pathways allow cells to adapt to the DNA stress and thereby contribute to meristem maintenance.
In addition to being induced by genotoxic stress, SMR5 displays a strong transcriptional response to many different abiotic stress conditions that also involve ROS signaling, including drought, high light, and salt (Figure 2). Therefore, SMR5 might be a general integrator of ROS signaling with cell cycle progression. ROS signaling has previously been linked to cell cycle progression. Treatment of tobacco cells with a ROS-inducing agent impairs the G1-to-S transition, retards the S-phase progression, and delays entry into M-phase, in correlation with the downregulation of CDK activity (Reichheld et al., 1999). Moreover, it has been demonstrated that the G1-to-S transition requires adequate levels of the antioxidant glutathione. Accordingly, the ROOT MERISTEMLESS1 gene, encoding a glutathione biosynthetic enzyme, is required to establish an active meristem (Vernoux et al., 2000). Additionally, recent evidence indicates that the distribution of ROS regulates the transition from proliferation to differentiation: The basic helix-loop-helix transcription factor UPBEAT1 (UPB1) is expressed at the root transition zone and regulates the distribution of ROS by monitoring the expression level of peroxidase genes (Tsukagoshi et al., 2010). Strikingly, the same study revealed that the SIM promoter is bound by the UPB1 protein, which is in agreement with our observation that SIM expression is restricted to the root elongation zone, which is also the site of maximum H2O2 concentration (Dunand et al., 2007). Likewise, ROS signaling has been implicated in pathogen response, whereas the first rice SIM/SMR-like gene (EL2) was described originally as a gene being induced within minutes after addition of the elicitor N-acetylchitoheptaose or purified flagellin protein of the pathogen P. Avenae I (Minami et al., 1996; Che et al., 2000). Moreover, H2O2 has also been detected in root columella cells, root cap cells, and vascular cells (Dunand et al., 2007; Tsukagoshi et al., 2010), to which specific SMR expression patterns can be linked. These data suggest that the transcriptional activation of SIM/SMR genes in response to ROS signals might be a general mechanism linking the oxidative status of a cell with its cell division activity.
METHODS
Plant Materials and Growth Conditions
The smr5 (SALK_100918) and smr7 (SALK_128496) alleles were acquired from the ABRC. Homozygous insertion alleles were checked by genotyping PCR using the primers listed in Supplemental Table 3. The atm-1, atr-2, and sog1-1 mutants have been described previously (Garcia et al., 2003; Preuss and Britt, 2003; Culligan et al., 2004; Yoshiyama et al., 2009). Unless stated otherwise, plants of Arabidopsis thaliana (ecotype Columbia) were grown under long-day conditions (16 h of light/8 h of darkness) at 22°C on half-strength Murashige and Skoog (MS) germination medium (Murashige and Skoog, 1962). Arabidopsis plants were treated with HU as described by Cools et al. (2011). For bleomycin treatments, 5-d-old seedlings were transferred into liquid MS medium supplemented with 0.3 µg/mL bleomycin. For γ-irradiation treatments, 5-d-old in vitro–grown plantlets were irradiated with γ-rays at a dose of 20 Gy. For light treatments, 1-week-old seedlings were transferred to continuous high-light conditions (growth rooms kept at 22°C with 24-h day/0-h night cycles and a light intensity of 300 to 400 µmol m–2 s–1) for 4 d and subsequently retransferred to low-light conditions (70 to 80 µmol m–2 s–1).
DNA and RNA Manipulation
Genomic DNA was extracted from Arabidopsis leaves with the DNeasy plant kit (Qiagen), and RNA was extracted from Arabidopsis tissues with the RNeasy mini kit (Qiagen). After DNase treatment with the RQ1 RNase-Free DNase (Promega), cDNA was synthesized with the iScript cDNA synthesis kit (Bio-Rad). Quantitative RT-PCR was performed using the SYBR Green kit (Roche) with 100 nM primers and 0.125 μL of RT reaction product in a total volume of 5 μL per reaction. Reactions were run and analyzed on the LightCycler 480 (Roche) according to the manufacturer's instructions with the use of the following reference genes for normalization: ACTIN2, EMB2386, PAC1, and RPS26C. Primers used for the RT-PCR are given in Supplemental Table 3. Statistical analysis was executed with the Statistical Analysis Software (SAS Enterprise Guide 5.1; SAS Institute) using the mixed model procedure, and P values were Bonferroni adjusted for multiple measurements.
SIM/SMR promoter sequences were amplified from genomic DNA by PCR using the primers described in Supplemental Table 3. The product fragments were created with the Pfu DNA polymerase kit (Promega) and were cloned into a pDONR P4-P1r entry vector by BP recombination cloning and subsequently transferred into the pMK7S*NFm14GW,0 destination vector by LR cloning, resulting in a transcriptional fusion between the promoter of the SMR genes and the nlsGFP-GUS fusion gene (Karimi et al., 2007). For the overexpression constructs, the SMR coding regions were amplified using primers described in Supplemental Table 3 and cloned into the pDONR221 vector by BP recombination cloning and subsequently transferred into the pK2GW7 destination vector (Karimi et al., 2002) by LR cloning. Based on the available annotation, the amplification of the SMR5 coding sequence yielded in a fragment of smaller size than expected, which suggested sequence misannotation. Further sequencing analysis confirmed the lack of the intronic region. The corrected coding sequencing of SMR5 is represented in Supplemental Figure 4. All constructs were transferred into the Agrobacterium tumefaciens C58C1RifR strain harboring the pMP90 plasmid. The obtained Agrobacterium strains were used to generate stably transformed Arabidopsis lines with the floral dip transformation method (Clough and Bent, 1998). Transgenic plants were selected on 35 mg/L kanamycin-containing medium and later transferred to soil for optimal seed production. All cloning primers are listed in Supplemental Table 3.
GUS Assays
Complete seedlings or tissue cuttings were stained in multiwell plates (Falcon 3043; Becton Dickinson). GUS assays were performed as described by Beeckman and Engler (1994). Samples mounted in lactic acid were observed and photographed with a stereomicroscope (Olympus BX51 microscope) or with a differential interference contrast microscope (Leica).
Microscopy
For leaf measurements, first leaves were harvested at 21 d after sowing on control medium or on medium supplemented with 1 mM HU. Leaves were cleared overnight in ethanol, stored in lactic acid for microscopy, and observed with a microscope fitted with differential interference contrast optics (Leica DMLB). The total (blade) area was determined from images digitized directly with a digital camera mounted on a stereozoom microscope (Stemi SV11; Zeiss). From scanned drawing-tube images of the outlines of at least 30 cells of the abaxial epidermis located between 25 to 75% of the distance between the tip and the base of the leaf, halfway between the midrib and the leaf margin, the following parameters were determined: total area of all cells in the drawing and total numbers of pavement and guard cells, from which the average cell area was calculated. The total number of cells per leaf was estimated by dividing the leaf area by the average cell area (De Veylder et al., 2001). Leaf sizes and epidermal cell numbers in the different lines were analyzed and compared by performing a two-way ANOVA (P value < 0.05). Tukey’s test was used to correct for family-wise error rate. For confocal microscopy, root meristems were analyzed 2 d after transfer using a Zeiss LSM 510 laser scanning microscope and the LSM Browser version 4.2 software (Zeiss). Plant material was incubated for 2 min in a 10 μM propidium iodide solution to stain the cell walls and was visualized with a HeNe laser through excitation at 543 nm. Green fluorescent protein (GFP) was detected with the 488-nm line of an argon laser. GFP and propidium iodide were detected simultaneously by combining the settings indicated above in the sequential scanning facility of the microscope. Acquired images were quantitatively analyzed with ImageJ v1.45s software (http://rsbweb.nih.gov/ij/) and Cell-o-Tape plug-ins (French et al., 2012). Chlorophyll a fluorescence parameters were measured using the Imaging PAM M-Series chlorophyll fluorescence (Walz) and associated software.
Flow Cytometry Analysis
For flow cytometry analysis, root tip tissues were chopped with a razor blade in 300 μL of 45 mM MgCl2, 30 mM sodium citrate, and 20 mM 3-morpholinopropane-1-sulfonic acid, pH 7.0 (Galbraith et al., 1991). One microliter of 4′,6-diamidino-2-phenylindole from a stock of 1 mg/mL was added to the filtered supernatant. Leaf material was chopped in 200 μL of Cystain UV Precise P nuclei extraction buffer (Partec), supplemented with 800 μL of staining buffer. The mix was filtered through a 50-μm green filter and read by the Cyflow MB flow cytometer (Partec). The nuclei were analyzed using Cyflogic software.
Catalase Assay
Plants were germinated on either control medium or medium with 1 mM HU or 6 μM 3-amino-1,2,4-triazole. Leaf tissue of 10 plants was ground in 200 μL extraction buffer (60 mM Tris, pH 6.9, 1 mM phenylmethylsulfonyl fluoride, and 10 mM DTT) on ice. The homogenate was centrifuged at 13,000g for 15 min at 4°C. A total of 45 µg protein extract was mixed with potassium phosphate buffer (50 mM, pH 7.0) (Vandenabeele et al., 2004). After the addition of 11.4 μL H2O2 (7.5%), the absorbance of the sample at 240 nm was measured over a 60-s interval to determine catalase activity (Beers and Sizer, 1952; Vandenabeele et al., 2004).
ChIP
ChIP experiments were performed as described (Gendrel et al., 2005) with minor modifications. Surface-sterile PSOG1:SOG1-Myc (Yoshiyama et al., 2013) seeds were germinated in 100 mL of 0.5× MS medium containing 1.5% Suc, pH 5.7, and cultured under continuous light at 23°C with gentle shaking (50 rpm). After a 14-d culture period, the seedlings were treated with 15 µM zeocin (Invitrogen) or water for 2 h. Wild-type (Col-0), no-treatment seedlings were used as a negative control. Sonicated chromatin solution (corresponding to 0.3 g tissue) was used for immunoprecipitation with anti-Myc antibodies (clone 4A6; Millipore) and an antibody recognizing an invariant domain of histone H3 (AB1791; Abcam). The ChIP products were used for quantitative PCR (qPCR) analysis with the primers listed in Supplemental Table 3. qPCR was performed with the LightCycler system (Roche) and Thunderbird SYBR qPCR Mix (Toyobo) according to the following reaction conditions: 95°C for 1 min; 70 cycles at 95°C for 10 s, at 60°C for 10 s, and at 72°C for 20 s. The signal obtained from ChIP with an anti-Myc antibody was normalized to that obtained from ChIP with an anti-Histone H3 antibody. Finally, each normalized ChIP value was divided by the normalized wild-type ChIP value to calculate the fold enrichment.
Microarray Analysis
Seeds were plated on sterilized membranes and grown under a 16-h/8-h light/dark regime at 21°C. After 2 d of germination and 5 d of growth, the membrane was transferred to MS medium containing 0.3 μg/mL bleomycin for 24 h. Triplicate batches of root meristem material were harvested for total RNA preparation using the RNeasy plant mini kit (Qiagen). Each of the different root tip RNA extracts were hybridized to 12 Affymetrix Arabidopsis Gene 1.0 ST arrays according to the manufacturer’s instructions at the Nucleomics Core Facility. Raw data were processed with the robust multiarray algorithm (Irizarry et al., 2003) using Affymetrix Power Tools and subsequently subjected to a significance analysis of microarray analysis with MultiExperiment Viewer 4 (MeV4) of The Institute for Genome Research (Tusher et al., 2001). The imputation engine was set as 10-nearest neighbor imputer and the number of permutations was 100. Expression values were obtained by log2 transforming the average value of the normalized signal intensities of the triplicate samples. Fold changes were obtained using the expression values of the treatment relative to the control samples. Genes with Q-values < 0.1 and fold change > 1.5 or < 0.666 were retained for further analysis.
Microarray Meta-Analysis
Transcripts induced by bleomycin (Q-value < 0.1 and fold change > 1.5) were compared with different published DNA stress–related data sets. For γ-irradiation, an intersect of the genes with a significant induction (P value < 0.05, Q-value < 0.1, and fold change >1.5) in 5-d-old wild-type seedlings 1.5 h postirradiation (100 Gy) was made of two independent experiments (Culligan et al., 2006; Yoshiyama et al., 2009). For replication stress, genes were selected that showed a significant induction (P value [time] < 0.05, Q-value [time] < 0.1, and fold change >1.5) in 5-d-old wild-type root tips after 24 h of 2 mM HU treatment (Cools et al., 2011). Meta-analysis of the SMR genes during various stress conditions and treatments were obtained using Genevestigator (Hruz et al., 2008). Using the “Response Viewer” tool, the expression profiles of genes following different stimuli were analyzed. Only biotic and abiotic stress treatments with a more than 2-fold change in the transcription level (P value < 0.01) for at least one of the SMR genes were taken into account. Fold-change values were hierarchically clustered for genes and experiments by average linkage in Multiple experiment Viewer from The Arabidopsis Information Resource.
SOG1 Phosphorylation Assay
Plants harboring PSOG1:SOG1-Myc (Yoshiyama et al., 2013) were grown on MS media (1× MS salts including vitamins, 2% [w/v] Suc, and 0.8% [w/v] gellangum, pH 6.0) under continuous light at 23°C. Five-day-old seedlings were transferred onto new MS medium or medium supplemented with 5 mM H2O2 and incubated for 24 h. Total protein was extracted from roots and immunoblotted with anti-Myc antibody (Santa Cruz) as described by Yoshiyama et al. (2013). To detect phosphorylated SOG1 proteins, Phos-tag reagent (NARD Institute) was used for the phoshoprotein mobility shift assay (Kinoshita et al., 2006). λPP (New England Biolabs) was used to dephosphorylate the phosphorylated forms of SOG1.
Accession Numbers
Microarray results have been submitted to MiamExpress (www.ebi.ac.uk/miamexpress) under accession number E-MEXP-3977. Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: SMR4 (At5g02220), SMR5 (At1g07500), SMR7 (At3g27630), ATM (At3g48490), ATR (At5g40820), SOG1 (At1g25580), ACTIN2 (At3g46520), EMB2386 (At1g02780), PAC1 (At3g22110), and RPS26C (At3g56340).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. SIM/SMR Induction in Response to Bleomycin.
Supplemental Figure 2. Transcriptional Induction of SIM/SMR Genes upon HU and Bleomycin Treatment.
Supplemental Figure 3. Transcriptional Induction of SIM/SMR Genes upon γ-Irradiation.
Supplemental Figure 4. Graphical Representation of the SMR5 and SMR7 T-DNA Insertion.
Supplemental Figure 5. SMR5 and SMR7 Expression Levels in Response to High-Light Treatment.
Supplemental Figure 6. Relative Root Growth of SMR5KO, SMR7KO, and SMR5KO SMR7KO Plants upon HU Treatment.
Supplemental Table 1. Annotated Arabidopsis SIM/SMR Genes.
Supplemental Table 2. DNA Ploidy Level Distribution in Transgenic Plants Overexpressing SMR4, SMR5, or SMR7.
Supplemental Table 3. List of Primers Used for Cloning, Genotyping, and RT-PCR.
Supplemental Data Set 1. Meta-Analysis of Genes Induced in Multiple DNA Damage Experiments.
Supplementary Material
Acknowledgments
We thank Annick Bleys for help in preparing the article, Maheshi Dassanayake for pointing out the misannotation of the SMR5 transcript, and Lorin Spruyt and Frank Van Breusegem for use of the high-light infrastructure. This work was supported by Ghent University (Multidisciplinary Research Partnership “Bioinformatics: from nucleotides to networks”), by the Interuniversity Attraction Poles Programme (IUAP P7/29 “MARS”), initiated by the Belgian Science Policy Office, and by a grant from the Research Foundation-Flanders (G.0C72.14N). T.C. is a Postdoctoral Fellow of the Research Foundation-Flanders. D.Y. is indebted to the China Scholarship Council (CSC File 2009685045) for a predoctoral scholarship. M.U. was supported by MEXT KAKENHI Grant 22119009 and JST, Core Research for Evolutional Science and Technology.
AUTHOR CONTRIBUTIONS
D.Y., C.L.A.K., T.C., S.V., A.B., M.U., and L.D.V. conceived and designed the research. D.Y., C.L.A.K., T.C., S.V., N.T., Y.O., T.E., K.O.Y., H.V.d.D., and A.B. performed the experiments. D.Y., C.L.A.K., T.C., S.A., N.T., J.L., A.B., M.U., and L.D.V. analyzed the data and wrote the article. All authors read, revised, and approved the article.
Glossary
- ROS
reactive oxygen species
- CDK
cyclin-dependent kinase
- DSB
double-stranded break
- HU
hydroxyurea
- dNTP
deoxynucleotide triphosphate
- CKI
CDK inhibitor
- Col-0
Columbia-0
- GUS
β-glucuronidase
- PSII
photosystem II
- MS
Murashige and Skoog
- ChIP
chromatin immunoprecipitation
- qPCR
quantitative PCR
Footnotes
Online version contains Web-only data.
References
- Abraham R.T. (2001). Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15: 2177–2196 [DOI] [PubMed] [Google Scholar]
- Adachi S., et al. (2011). Programmed induction of endoreduplication by DNA double-strand breaks in Arabidopsis. Proc. Natl. Acad. Sci. USA 108: 10004–10009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor Y., Babiychuk E., Inzé D., Levine A. (1998). The involvement of poly(ADP-ribose) polymerase in the oxidative stress responses in plants. FEBS Lett. 440: 1–7 [DOI] [PubMed] [Google Scholar]
- Bartek J., Lukas J. (2001). Pathways governing G1/S transition and their response to DNA damage. FEBS Lett. 490: 117–122 [DOI] [PubMed] [Google Scholar]
- Beeckman T., Engler G. (1994). An easy technique for the clearing of histochemically stained plant tissue. Plant Mol. Biol. Rep. 12: 37–42 [Google Scholar]
- Beers R.F., Jr, Sizer I.W. (1952). A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 195: 133–140 [PubMed] [Google Scholar]
- Boudolf V., et al. (2009). CDKB1;1 forms a functional complex with CYCA2;3 to suppress endocycle onset. Plant Physiol. 150: 1482–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudolf V., Vlieghe K., Beemster G.T.S., Magyar Z., Torres Acosta J.A., Maes S., Van Der Schueren E., Inzé D., De Veylder L. (2004). The plant-specific cyclin-dependent kinase CDKB1;1 and transcription factor E2Fa-DPa control the balance of mitotically dividing and endoreduplicating cells in Arabidopsis. Plant Cell 16: 2683–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet J., Douki T., Ravanat J.-L., Wagner J.R. (2012). Measurement of oxidatively generated base damage to nucleic acids in cells: Facts and artifacts. Bioanal. Rev. 4: 55–74 [Google Scholar]
- Chaturvedi P., et al. (1999). Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene 18: 4047–4054 [DOI] [PubMed] [Google Scholar]
- Che F.-S., Nakajima Y., Tanaka N., Iwano M., Yoshida T., Takayama S., Kadota I., Isogai A. (2000). Flagellin from an incompatible strain of Pseudomonas avenae induces a resistance response in cultured rice cells. J. Biol. Chem. 275: 32347–32356 [DOI] [PubMed] [Google Scholar]
- Chen G.X., Asada K. (1990). Hydroxyurea and p-aminophenol are the suicide inhibitors of ascorbate peroxidase. J. Biol. Chem. 265: 2775–2781 [PubMed] [Google Scholar]
- Chen Y., Sanchez Y. (2004). Chk1 in the DNA damage response: Conserved roles from yeasts to mammals. DNA Repair (Amst.) 3: 1025–1032 [DOI] [PubMed] [Google Scholar]
- Churchman M.L., et al. (2006). SIAMESE, a plant-specific cell cycle regulator, controls endoreplication onset in Arabidopsis thaliana. Plant Cell 18: 3145–3157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cools T., Iantcheva A., Maes S., Van den Daele H., De Veylder L. (2010). A replication stress-induced synchronization method for Arabidopsis thaliana root meristems. Plant J. 64: 705–714 [DOI] [PubMed] [Google Scholar]
- Cools T., Iantcheva A., Weimer A.K., Boens S., Takahashi N., Maes S., Van den Daele H., Van Isterdael G., Schnittger A., De Veylder L. (2011). The Arabidopsis thaliana checkpoint kinase WEE1 protects against premature vascular differentiation during replication stress. Plant Cell 23: 1435–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culligan K., Tissier A., Britt A. (2004). ATR regulates a G2-phase cell-cycle checkpoint in Arabidopsis thaliana. Plant Cell 16: 1091–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culligan K.M., Robertson C.E., Foreman J., Doerner P., Britt A.B. (2006). ATR and ATM play both distinct and additive roles in response to ionizing radiation. Plant J. 48: 947–961 [DOI] [PubMed] [Google Scholar]
- De Clercq A., Inzé D. (2006). Cyclin-dependent kinase inhibitors in yeast, animals, and plants: a functional comparison. Crit. Rev. Biochem. Mol. Biol. 41: 293–313 [DOI] [PubMed] [Google Scholar]
- De Schutter K., Joubès J., Cools T., Verkest A., Corellou F., Babiychuk E., Van Der Schueren E., Beeckman T., Kushnir S., Inzé D., De Veylder L. (2007). Arabidopsis WEE1 kinase controls cell cycle arrest in response to activation of the DNA integrity checkpoint. Plant Cell 19: 211–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veylder L., Beeckman T., Beemster G.T.S., Krols L., Terras F., Landrieu I., van der Schueren E., Maes S., Naudts M., Inzé D. (2001). Functional analysis of cyclin-dependent kinase inhibitors of Arabidopsis. Plant Cell 13: 1653–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaoli H.C., Brito M.S., Quiapim A.C., Teixeira S.P., Goldman G.H., Dornelas M.C., Goldman M.H.S. (2011). Stigma/style cell cycle inhibitor 1 (SCI1), a tissue-specific cell cycle regulator that controls upper pistil development. New Phytol. 190: 882–895 [DOI] [PubMed] [Google Scholar]
- DePaoli H.C., Goldman G.H., Goldman M.-H.S. (2012). SCI1, the first member of the tissue-specific inhibitors of CDK (TIC) class, is probably connected to the auxin signaling pathway. Plant Signal. Behav. 7: 53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitte W., Murray J.A.H. (2003). The plant cell cycle. Annu. Rev. Plant Biol. 54: 235–264 [DOI] [PubMed] [Google Scholar]
- Davletova S., Rizhsky L., Liang H., Shengqiang Z., Oliver D.J., Coutu J., Shulaev V., Schlauch K., Mittler R. (2005). Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17: 268–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissmeyer N., Weimer A.K., Pusch S., De Schutter K., Alvim Kamei C.L., Nowack M.K., Novak B., Duan G.-L., Zhu Y.-G., De Veylder L., Schnittger A. (2009). Control of cell proliferation, organ growth, and DNA damage response operate independently of dephosphorylation of the Arabidopsis Cdk1 homolog CDKA;1. Plant Cell 21: 3641–3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizdaroglu M., Jaruga P., Birincioglu M., Rodriguez H. (2002). Free radical-induced damage to DNA: Mechanisms and measurement. Free Radic. Biol. Med. 32: 1102–1115 [DOI] [PubMed] [Google Scholar]
- Dubacq C., Chevalier A., Courbeyrette R., Petat C., Gidrol X., Mann C. (2006). Role of the iron mobilization and oxidative stress regulons in the genomic response of yeast to hydroxyurea. Mol. Genet. Genomics 275: 114–124 [DOI] [PubMed] [Google Scholar]
- Dunand C., Crèvecoeur M., Penel C. (2007). Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: Possible interaction with peroxidases. New Phytol. 174: 332–341 [DOI] [PubMed] [Google Scholar]
- Francis D. (2011). A commentary on the G2/M transition of the plant cell cycle. Ann. Bot. (Lond.) 107: 1065–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French A.P., Wilson M.H., Kenobi K., Dietrich D., Voß U., Ubeda-Tomás S., Pridmore T.P., Wells D.M. (2012). Identifying biological landmarks using a novel cell measuring image analysis tool: Cell-o-Tape. Plant Methods 8: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith D.W., Harkins K.R., Knapp S. (1991). Systemic endopolyploidy in Arabidopsis thaliana. Plant Physiol. 96: 985–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia V., Bruchet H., Camescasse D., Granier F., Bouchez D., Tissier A. (2003). AtATM is essential for meiosis and the somatic response to DNA damage in plants. Plant Cell 15: 119–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendrel A.-V., Lippman Z., Martienssen R., Colot V. (2005). Profiling histone modification patterns in plants using genomic tiling microarrays. Nat. Methods 2: 213–218 [DOI] [PubMed] [Google Scholar]
- Guo Z., Kozlov S., Lavin M.F., Person M.D., Paull T.T. (2010). ATM activation by oxidative stress. Science 330: 517–521 [DOI] [PubMed] [Google Scholar]
- Hruz T., Laule O., Szabo G., Wessendorp F., Bleuler S., Oertle L., Widmayer P., Gruissem W., Zimmermann P. (2008). Genevestigator v3: A reference expression database for the meta-analysis of transcriptomes. Adv. Bioinforma. 2008: 420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry R.A., Hobbs B., Collin F., Beazer-Barclay Y.D., Antonellis K.J., Scherf U., Speed T.P. (2003). Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4: 249–264 [DOI] [PubMed] [Google Scholar]
- Juul T., Malolepszy A., Dybkaer K., Kidmose R., Rasmussen J.T., Andersen G.R., Johnsen H.E., Jørgensen J.-E., Andersen S.U. (2010). The in vivo toxicity of hydroxyurea depends on its direct target catalase. J. Biol. Chem. 285: 21411–21415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M., Bleys A., Vanderhaeghen R., Hilson P. (2007). Building blocks for plant gene assembly. Plant Physiol. 145: 1183–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M., Inzé D., Depicker A. (2002). GATEWAYTM vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 7: 193–195 [DOI] [PubMed] [Google Scholar]
- Kinoshita E., Kinoshita-Kikuta E., Takiyama K., Koike T. (2006). Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol. Cell. Proteomics 5: 749–757 [DOI] [PubMed] [Google Scholar]
- Kurz E.U., Lees-Miller S.P. (2004). DNA damage-induced activation of ATM and ATM-dependent signaling pathways. DNA Repair (Amst.) 3: 889–900 [DOI] [PubMed] [Google Scholar]
- Lee M.G., Nurse P. (1987). Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature 327: 31–35 [DOI] [PubMed] [Google Scholar]
- Minami E., Kuchitsu K., He D.-Y., Kouchi H., Midoh N., Ohtsuki Y., Shibuya N. (1996). Two novel genes rapidly and transiently activated in suspension-cultured rice cells by treatment with N-acetylchitoheptaose, a biotic elicitor for phytoalexin production. Plant Cell Physiol. 37: 563–567 [DOI] [PubMed] [Google Scholar]
- Mittler R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7: 405–410 [DOI] [PubMed] [Google Scholar]
- Mittler R., Vanderauwera S., Gollery M., Van Breusegem F. (2004). Reactive oxygen gene network of plants. Trends Plant Sci. 9: 490–498 [DOI] [PubMed] [Google Scholar]
- Mittler R., Vanderauwera S., Suzuki N., Miller G., Tognetti V.B., Vandepoele K., Gollery M., Shulaev V., Van Breusegem F. (2011). ROS signaling: The new wave? Trends Plant Sci. 16: 300–309 [DOI] [PubMed] [Google Scholar]
- Murashige T., Skoog F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15: 473–497 [Google Scholar]
- Murata N., Allakhverdiev S.I., Nishiyama Y. (2012). The mechanism of photoinhibition in vivo: Re-evaluation of the roles of catalase, α-tocopherol, non-photochemical quenching, and electron transport. Biochim. Biophys. Acta 1817: 1127–1133 [DOI] [PubMed] [Google Scholar]
- Norbury C., Nurse P. (1992). Animal cell cycles and their control. Annu. Rev. Biochem. 61: 441–470 [DOI] [PubMed] [Google Scholar]
- Nowack M.K., Harashima H., Dissmeyer N., Zhao X., Bouyer D., Weimer A.K., De Winter F., Yang F., Schnittger A. (2012). Genetic framework of cyclin-dependent kinase function in Arabidopsis. Dev. Cell 22: 1030–1040 [DOI] [PubMed] [Google Scholar]
- Nowack M.K., Ungru A., Bjerkan K.N., Grini P.E., Schnittger A. (2010). Reproductive cross-talk: Seed development in flowering plants. Biochem. Soc. Trans. 38: 604–612 [DOI] [PubMed] [Google Scholar]
- Nyholm S., Mann G.J., Johansson A.G., Bergeron R.J., Gräslund A., Thelander L. (1993). Role of ribonucleotide reductase in inhibition of mammalian cell growth by potent iron chelators. J. Biol. Chem. 268: 26200–26205 [PubMed] [Google Scholar]
- Peres A., et al. (2007). Novel plant-specific cyclin-dependent kinase inhibitors induced by biotic and abiotic stresses. J. Biol. Chem. 282: 25588–25596 [DOI] [PubMed] [Google Scholar]
- Preuss S.B., Britt A.B. (2003). A DNA-damage-induced cell cycle checkpoint in Arabidopsis. Genetics 164: 323–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichheld J.-P., Vernoux T., Lardon F., Van Montagu M., Inzé D. (1999). Specific checkpoints regulate plant cell cycle progression in response to oxidative stress. Plant J. 17: 647–656 [Google Scholar]
- Ricaud L., Proux C., Renou J.-P., Pichon O., Fochesato S., Ortet P., Montané M.-H. (2007). ATM-mediated transcriptional and developmental responses to γ-rays in Arabidopsis. PLoS ONE 2: e430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou-Khamlichi C., Menges M., Healy J.M.S., Murray J.A.H. (2000). Sugar control of the plant cell cycle: Differential regulation of Arabidopsis D-type cyclin gene expression. Mol. Cell. Biol. 20: 4513–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder A.H.K., Chickarmane V., Cunha A., Obara B., Manjunath B.S., Meyerowitz E.M. (2010). Variability in the control of cell division underlies sepal epidermal patterning in Arabidopsis thaliana. PLoS Biol. 8: e1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldán-Arjona T., Ariza R.R. (2009). Repair and tolerance of oxidative DNA damage in plants. Mutat. Res. 681: 169–179 [DOI] [PubMed] [Google Scholar]
- Rozan L.M., El-Deiry W.S. (2007). p53 downstream target genes and tumor suppression: A classical view in evolution. Cell Death Differ. 14: 3–9 [DOI] [PubMed] [Google Scholar]
- Sherr C.J., Roberts J.M. (1995). Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 9: 1149–1163 [DOI] [PubMed] [Google Scholar]
- Shieh S.-Y., Ahn J., Tamai K., Taya Y., Prives C. (2000). The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 14: 289–300 [PMC free article] [PubMed] [Google Scholar]
- Tsukagoshi H. (2012). Defective root growth triggered by oxidative stress is controlled through the expression of cell cycle-related genes. Plant Sci. 197: 30–39 [DOI] [PubMed] [Google Scholar]
- Tsukagoshi H., Busch W., Benfey P.N. (2010). Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 143: 606–616 [DOI] [PubMed] [Google Scholar]
- Tusher V.G., Tibshirani R., Chu G. (2001). Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 98: 5116–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenabeele S., Vanderauwera S., Vuylsteke M., Rombauts S., Langebartels C., Seidlitz H.K., Zabeau M., Van Montagu M., Inzé D., Van Breusegem F. (2004). Catalase deficiency drastically affects gene expression induced by high light in Arabidopsis thaliana. Plant J. 39: 45–58 [DOI] [PubMed] [Google Scholar]
- Vanderauwera S., Suzuki N., Miller G., van de Cotte B., Morsa S., Ravanat J.-L., Hegie A., Triantaphylidès C., Shulaev V., Van Montagu M.C.E., Van Breusegem F., Mittler R. (2011). Extranuclear protection of chromosomal DNA from oxidative stress. Proc. Natl. Acad. Sci. USA 108: 1711–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leene J., et al. (2010). Targeted interactomics reveals a complex core cell cycle machinery in Arabidopsis thaliana. Mol. Syst. Biol. 6: 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoux T., Wilson R.C., Seeley K.A., Reichheld J.-P., Muroy S., Brown S., Maughan S.C., Cobbett C.S., Van Montagu M., Inzé D., May M.J., Sung Z.R. (2000). The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 12: 97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Qi Q., Schorr P., Cutler A.J., Crosby W.L., Fowke L.C. (1998). ICK1, a cyclin-dependent protein kinase inhibitor from Arabidopsis thaliana interacts with both Cdc2a and CycD3, and its expression is induced by abscisic acid. Plant J. 15: 501–510 [DOI] [PubMed] [Google Scholar]
- Wang H., Zhou Y., Gilmer S., Whitwill S., Fowke L.C. (2000). Expression of the plant cyclin-dependent kinase inhibitor ICK1 affects cell division, plant growth and morphology. Plant J. 24: 613–623 [DOI] [PubMed] [Google Scholar]
- Yoshiyama K., Conklin P.A., Huefner N.D., Britt A.B. (2009). Suppressor of gamma response 1 (SOG1) encodes a putative transcription factor governing multiple responses to DNA damage. Proc. Natl. Acad. Sci. USA 106: 12843–12848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama K.O., Kobayashi J., Ogita N., Ueda M., Kimura S., Maki H., Umeda M. (2013). ATM-mediated phosphorylation of SOG1 is essential for the DNA damage response in Arabidopsis. EMBO Rep. 14: 817–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B.-B., Elledge S.J. (2000). The DNA damage response: Putting checkpoints in perspective. Nature 408: 433–439 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.