Abstract
Phototropism, or the differential cell elongation exhibited by a plant organ in response to directional blue light, provides the plant with a means to optimize photosynthetic light capture in the aerial portion and water and nutrient acquisition in the roots. Tremendous advances have been made in our understanding of the molecular, biochemical, and cellular bases of phototropism in recent years. Six photoreceptors and their associated signaling pathways have been linked to phototropic responses under various conditions. Primary detection of directional light occurs at the plasma membrane, whereas secondary modulatory photoreception occurs in the cytoplasm and nucleus. Intracellular responses to light cues are processed to regulate cell-to-cell movement of auxin to allow establishment of a trans-organ gradient of the hormone. Photosignaling also impinges on the transcriptional regulation response established as a result of changes in local auxin concentrations. Three additional phytohormone signaling pathways have also been shown to influence phototropic responsiveness, and these pathways are influenced by the photoreceptor signaling as well. Here, we will discuss this complex dance of intra- and intercellular responses that are regulated by these many systems to give rise to a rapid and robust adaptation response observed as organ bending.
INTRODUCTION
Plants have evolved a variety of responses to maintain optimal growth and development under ever changing environmental conditions. Photoreceptors and their associated signaling pathways are one way plants cope with changes in their environment, integrating signals of light quality and quantity, to adaptively modify overall growth characteristics from seed germination to reproduction (Kami et al., 2010; Pedmale et al., 2010; Chen and Chory, 2011). Although many photoreceptors, signaling pathways, and processes they regulate have been identified, this review will focus specifically on the components associated with the induction, modulation, and establishment of phototropic responses in higher plants.
Phototropic responses, or a plant’s ability to reorient organ growth toward (positive phototropism) or away (negative phototropism) from a directional light source, has fascinated researchers for well over a century (Holland et al., 2009). Among the historical references to plant phototropism, Darwin’s The Power of Movement of Plants (1880) is arguably the most well known. Therein, Darwin describes a mysterious substance that is transduced from the tip of the seedling, where the light signal is perceived, to lower portions of the seedling, where the signal response can be observed in the form of directional growth changes (Darwin, 1880). It was not until the 1920s that a significant breakthrough occurred when Frits Went (Went, 1926), working on phototropism in the oat (Avena sativa) coleoptile, isolated and identified Darwin’s mysterious substance as the plant hormone auxin. Together with work by Nicolai Cholodny (Cholodny, 1927) on oat root gravitropism (bending response to change in the direction of the gravity vector), these findings formed the basis for the Cholodny-Went hypothesis (Went and Thimann, 1937), which proposes that tropisms result from the lateral redistribution of auxin in response to tropic stimuli. In the case of coleoptile (and stem) phototropism, auxin is redistributed from the lit flank (side closest to the incident light) to the shaded flank (side farthest from the light source) leading to differential auxin-stimulated cell elongation in the shaded versus lit portion of the organ and, thus, curvature toward the light source (Went and Thimann, 1937; Esmon et al., 2006; Holland et al., 2009) (Figure 1). Yet, despite over 100 years of intensive study, the photoreceptor(s) responsible for sensing directional light remained elusive until the 1990s when work in Winslow Briggs’ laboratory at the Carnegie Institution of Washington (Stanford, CA) lead to the identification of the phototropins (Short and Briggs, 1990; Reymond et al., 1992; Liscum and Briggs, 1995; Huala et al., 1997; Christie et al., 1998). Much like the work of Cholodny and Went, the findings of Briggs and colleagues represented a seminal step in our understanding of plant biology. In the following section, we will review our current understanding of phototropin structure and function in phototropic signaling.
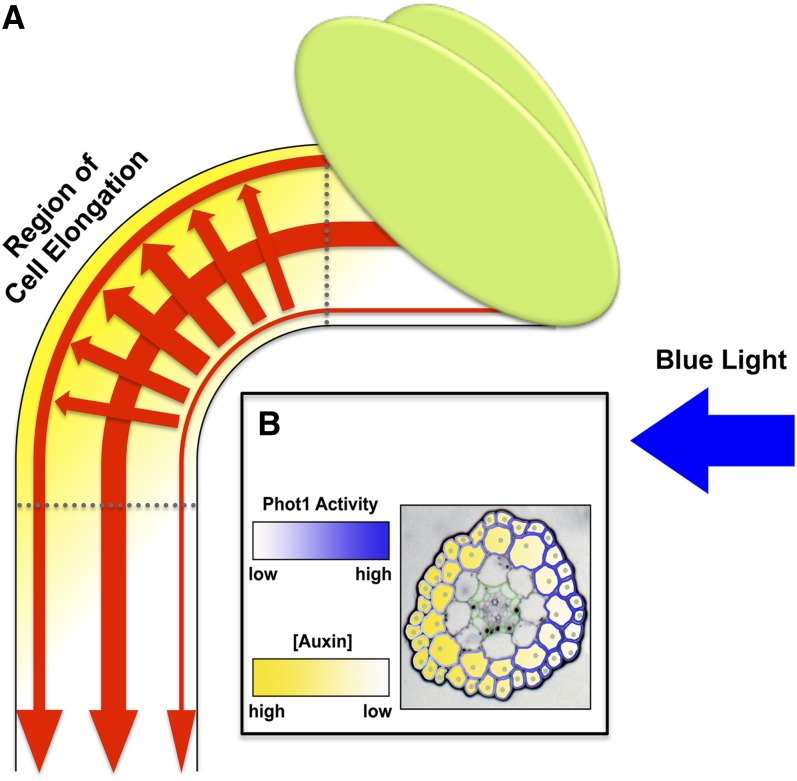
Figure 1.
BL-Induced Phototropism in Higher Plants Requires the Establishment of a Differential Gradient of Auxin.
(A) Diagram of a hypocotyl exhibiting a phototropic response. Auxin synthesized in the apical portions of the stem is polarly transported toward the root predominately through the central vasculature and to a lesser extent via epidermal and subepidermal cell layers (downward pointing red arrows). While in dark-grown seedlings the amount of auxin transported through the outer cell layers is not appreciably different side-to-side across the hypocotyl (data not shown), in seedlings exposed to directional BL, a differential of downward auxin flow is established (see downward pointing outer red arrows). Directional BL also induces a lateral redistribution of auxin from the lit to shaded portion of the hypocotyl (trans-hypocotyl red arrows). Together, these actions result in the differential accumulation of auxin in the elongation zone of shaded versus lit sides of the seedling (yellow shading).
(B) Cross section within the elongation zone of an Arabidopsis seedling hypocotyl illustrating the gradients of phot1 activity (false-colored white to blue) and auxin accumulation (false-colored white to yellow). Gray dots are meant to represent nuclei within the outer two cell layers, regions particularly important to auxin-mediated transcriptional responses (see Figures 3 and 4). Cells outlined in green are endodermal cells.
PHOTOTROPINS
Primary Photoreceptors Modulating Blue Light–Induced Phototropism and Other Responses That Optimize Photosynthetic Capacity
In higher plants, there are two phototropins (phots): phot1 and phot2 (Huala et al., 1997; Jarillo et al., 2001; Suetsugu and Wada, 2013). Under low-intensity blue light (BL) phot1 is the primary receptor controlling phototropism, whereas under moderate to high BL conditions, phot1 and phot2 act redundantly (Liscum and Briggs, 1995; Sakai et al., 2000, 2001). In addition to their roles in phototropism, the phots mediate a number of other BL responses that generally appear to impact photosynthetic capacity in one way or another. For example, phot1 and phot2 are redundantly involved in stomatal opening and photosynthetic gas exchange (Kinoshita et al., 2001). Additionally, both phots positively regulate cotyledon and leaf blade expansion, flattening, and positioning in response to BL (Sakamoto and Briggs, 2002; Ohgishi et al., 2004; Takemiya et al., 2005; Inoue et al., 2008; Han et al., 2013), presumably to increase surface area for photosynthetic light capture. The surface area of chloroplasts within the mesophyll cells also determines efficiency of photosynthetic light absorption: Under low-light conditions, activation of phot1 and phot2 redundantly stimulates chloroplast positioning along the cellular edges perpendicular to the incident light to optimize absorption (Sakai et al., 2001). By contrast, under high-intensity light, phot2 activation causes chloroplasts to move away from the irradiated edge of the cell to avoid photodamage (Jarillo et al., 2001; Kagawa et al., 2001; Sakai et al., 2001; Kasahara et al., 2002).
Structural and Functional Organization of the phot Proteins
All phots share a similar primary amino acid sequence and domain organization: The N-terminal half of the protein contains the photosensory region, while the C-terminal half contains a protein kinase domain (PKD) for signal output (Christie, 2007; Tokutomi et al., 2008; Suetsugu and Wada, 2013) (Figure 2). The photosensory portion of a phot consists of two repeated domains of ∼110 amino acids designated LOV1 (for light, oxygen and voltage) and LOV2 (Huala et al., 1997; Christie et al., 1998), to which a single flavin mononucleotide (FMN) molecule is found associated as a light-absorbing prosthetic group (Christie, 2007). While the FMN molecule is noncovalently associated with the LOV domain in darkness, upon absorption of BL, a reversible photocycle is initiated such that the activated FMN forms a covalent adduct with a nearby Cys residue in the LOV domain (Christie et al., 1999, 2002; Salomon et al., 2000). Although their photocycles are similar, the LOV1 domain is thought primarily to regulate receptor di/multimerization (Salomon et al., 2004; Nakasako et al., 2008; Nakasone et al., 2013), whereas LOV2 appears to regulate the C-terminal PKD of phots through a novel BL-induced derepression mechanism (Christie et al., 2002; Harper et al., 2003, 2004; Jones et al., 2007; Jones and Christie, 2008; Nakasako et al., 2008; Tokutomi et al., 2008). In the absence of light, the LOV2 domain is folded in a way that causes steric inhibition of the PKD (Figure 2A). Upon absorption of BL and formation of the LOV2 cysteinyl-FMN adduct, progressive structural changes occur in the phot, leading to the unfolding of an α-helical region (Jα helix) that resides in the linker domain between LOV2 and the PKD. Unfolding of the Jα helix thereby displaces the LOV2 domain from its dark-state conformation and relieves the steric repression on the PKD, resulting in multisite Ser autophosphorylation of the phot (Inoue et al., 2008, 2011; Sullivan et al., 2008) (Figure 2B). Phosphorylated phot is generally thought to represent the activated version of the photoreceptor (Christie, 2007; Tokutomi et al., 2008).
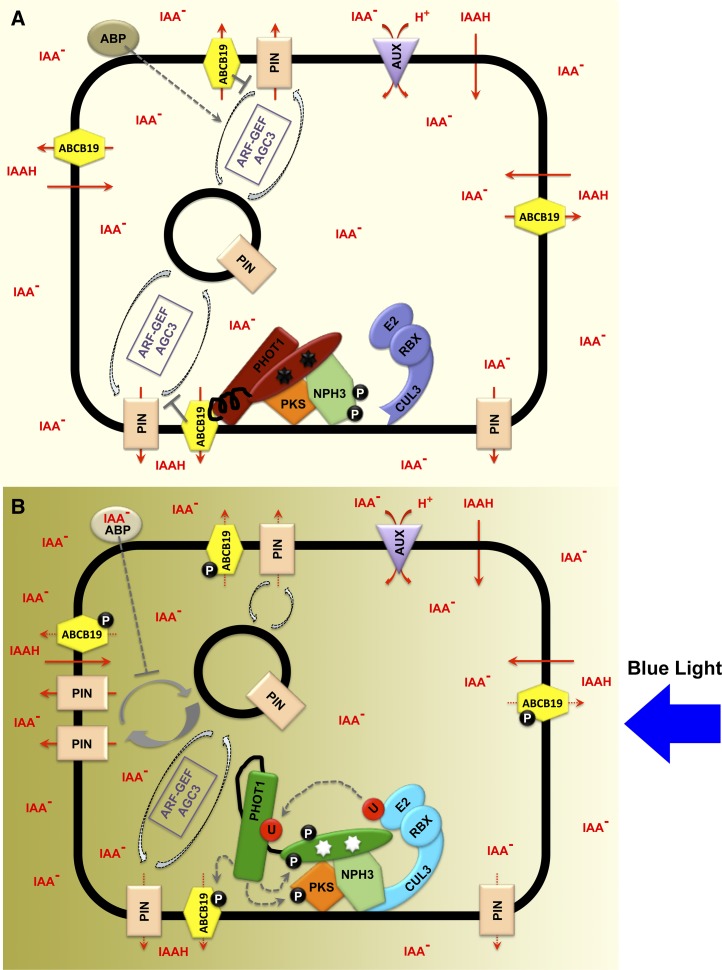
Figure 2.
Early Phototropic Signaling Events Involved in the Regulation of Auxin Transport That Leads to a Differential Gradient of the Hormone.
(A) Hypocotyl cell in darkness. Auxin is moving from the cell wall space to the cytoplasm across the plasma membrane (thick black line) either passively (as IAAH) or via AUX/LAX (purple)–mediated H+-cotransport (as IAA−). Anionic auxin within the cytoplasm can only leave the cell via PIN (light orange) or ABCB (yellow) facilitator–mediated transport. PIN proteins are polarly localized more to basal ends of cells in dark-grown seedlings (thus providing for bulk polar downward flow of auxin depicted in Figure 1), though they also exhibit cycling between the plasma membrane and endosomes through a mechanism regulated by ARF-GEFs and AGC3 kinases (boxed in purple). PIN protein stability at the plasma membrane is enhanced by the presence of ABCB19 (reflected by the gray repression on endocytotic events). By contrast, free ABP not bound to auxin (brown) on the outer surface of the plasma membrane promotes endocytosis of PIN proteins. phot1 (red) is in its dephosphorylated inactive state, as is the Cullin 3-RBX-E3 (CRL) complex (blue-violet). phot1 is shown as its two functional domains; the light-sensing FMN (black stars) binding half, attached to the output PKD via the Jα-helix (black). NPH3 (light green) is present in its phosphorylated inactive state. phot1, NPH3, PKS (dark orange), and ABCB19 are part of a protein complex at the plasma membrane. Auxin is fairly evenly distributed around and within the cell as represented by the consistent light-yellow shading.
(B) Hypocotyl cell after exposure to directional BL (from the right). phot1 (dark green with white stars) is in its active state, promoting phosphorylation (black balls with “P”) of ABCB19, PKS, and phot1 itself. Activation of phot1 also promotes the dephosphorylation of NPH3 (light green) by a presently unidentified type 1 protein phosphatase (data not shown). The CRL complex interacts (light blue) with NPH3 upon photoactivation of phot1, resulting in the ubiquitination (red ball with “U”) of phot1. Phosphorylation (and possibly mono/multiubiquitination) of phot1 further promotes the movement of phot1 away from the plasma membrane. Phosphorylation of ABCB19 suppresses its transport activity and appears to disrupt the stabilizing effect of ABCB19 on PIN protein localization to the plasma membrane. phot1 activation also stimulates the relocalization of PIN proteins to the lateral face of cell, a process that is potentially enhanced by auxin-bound ABP at the cell surface, which can repress endocytosis of PIN proteins. All of these effects lead to a redistribution of auxin from a polar basal flow to a lateral flow away from the light source (graded yellow background).
The PKD of the phots forms the AGC4 subfamily of the AGC-VIII superfamily of protein kinases that appear to share a single common ancestor (Galván-Ampudia and Offringa, 2007). Mutational studies of the phot1 PKD have identified several residues critical for kinase function and signal propagation. For example, a kinase inactive mutant of Arabidopsis thaliana phot1 (phot1D806N) attenuates phot1 signaling and responses (Christie et al., 2002; Inoue et al., 2008). Of the autophosphorylation sites that have been mapped in the phots (Salomon et al., 2003; Inoue et al., 2008; Sullivan et al., 2008), Ser-849 and Ser-851 (position in Arabidopsis phot1) have been shown to be most critical for phot function: Single and double mutations of these residues to Ala result in defective phototropism, stomatal opening, leaf flattening, and chloroplast accumulation responses (Inoue et al., 2008). These data support the hypothesis that phot autophosphorylation is necessary for phot1 signal transduction.
BL Induces the Internalization of phots from the Plasma Membrane
Although phot1 and phot2 are plasma membrane–associated receptors in dark-grown seedlings (Christie, 2007), several studies of fusion proteins with green fluorescent protein (GFP) indicate that both phot1 and phot2 relocalize from the plasma membrane to intracellular locations in response to BL (Sakamoto and Briggs, 2002; Kong et al., 2006, 2007; Aihara et al., 2008; Kong and Nagatani, 2008; Wan et al., 2008). phot2-GFP has been shown to relocalize to the Golgi apparatus in response to BL activation (Kong et al., 2006, 2007; Aihara et al., 2008; Kong and Nagatani, 2008), but the precise movement patterns of phot1-GFP are far from resolved (Sakamoto and Briggs, 2002; Han et al., 2008; Wan et al., 2008). What we do know is that phot1-GFP appears to associate with clathrin (heavy chain) in response to BL activation, suggesting that phot1 is internalized via clathrin-mediated endocytosis (Kaiserli et al., 2009; Roberts et al., 2011). Moreover, the autophosphorylation of phot1 appears to be a prerequisite for internalization of the receptor. For example, phot1-GFP carrying the D806N mutation fails to relocalize in response to BL, whereas a constitutively active kinase mutant (I608E) internalizes in the absence of BL (Kaiserli et al., 2009). It was further shown that Ser-851 acts as the phospho-state regulator of phot1 internalization: phot1S851A-GFP (mutant blocked in autophosphorylation) localized to the plasma membrane independent of light condition, while phot1S851D-GFP (constitutive phosphorylation mimic) was internalized in the absence of light (Kaiserli et al., 2009). Given previous studies showing that phosphorylation at Ser-851 is necessary for phototropic response (Inoue et al., 2008), these latter findings suggest that phot1 internalization is also necessary for phototropic responsiveness. However, a study by Han et al. (2008) suggests that retention of phot1 at the plasma membrane, a response dependent upon prior activation of the red light (RL)/far-red (FR) light photoreceptor phytochrome A, enhances phototropism. Additional studies are necessary to establish how phot localization directly relates to phototropic signal propagation.
In fungal and animal cells, ligand binding often induces mono/multiubiquitination of plasma membrane–localized receptors, resulting in clathrin-dependent endocytosis of the receptor-ligand complex (Haglund and Dikic, 2012). As will be discussed below, recent findings that phot1 is mono/multiubiquitinated in response to low-intensity BL and that this posttranslational modification is also required for normal phototropic responsiveness (Roberts et al., 2011) suggests a similar mechanism for the BL-induced endocytosis of phot1 may be in play.
PHOT-INTERACTING PROTEINS INVOLVED IN PHOTOTROPISM
NPH3: A Substrate Adapter for a Ubiquitin Ligase That Modifies phot1 and Is Essential for Phototropic Responses
NONPHOTOTROPIC HYPOCOTYL3 (NPH3) was originally identified as a phototropic loss-of-function mutant in Arabidopsis (Liscum and Briggs, 1995). NPH3 encodes a protein containing an N-terminal Broad-complex, Tramtrack, and Bric-á-brac (BTB) domain, a central NPH3 domain (Pfam, PF03000), and a C-terminal coiled-coil domain (Motchoulski and Liscum, 1999; Pedmale et al., 2010) (Figure 2). In Arabidopsis, NPH3 is one of 33 members of the NPH3/RPT2-like protein family that is characterized by the presence of a central NPH3 domain (Motchoulski and Liscum, 1999; Inada et al., 2004; Pedmale and Liscum, 2007; Pedmale et al., 2010; Sakai and Haga, 2012). Like phot1, NPH3 associates with the plasma membrane (Motchoulski and Liscum, 1999) apparently through its C-terminal domain (Inada et al., 2004), although it appears to remain membrane associated independent of light condition (Motchoulski and Liscum, 1999). NPH3 is not only necessary for phot1-dependent phototropism under low intensity BL, but also for phot1/phot2-dependent phototropic signaling under high-intensity BL (Motchoulski and Liscum, 1999; Roberts et al., 2011). Given their overlapping intracellular localization and mutant phototropic phenotypes, it should not be surprising that NPH3 physically interacts with phot1 (Motchoulski and Liscum, 1999). This interaction occurs between the N-terminal LOV domain–containing portion of phot1 and the C-terminal coiled-coil region of NPH3 (Motchoulski and Liscum, 1999; Inada et al., 2004).
Pedmale and Liscum (2007) have shown that the phototropic signaling capacity of NPH3 appears to be modulated by its phosphorylation status. In darkness, NPH3 is present as a phosphorylated isoform (NPH3DS) (Figure 2A), but following BL exposure, a dephosphorylated isoform (NPH3LS) predominates (Figure 2B). Conversion of NPH3DS to NPH3LS requires the presence of phot1, and prevention of this conversion through either genetic or pharmacological means abrogates the development of phototropic responses, suggesting that NPH3LS is necessary for normal phototropic signaling. It appears that a type 1 protein phosphatase mediates the dephosphorylation of NPH3DS (Pedmale and Liscum, 2007), while a mitogen-activated protein kinase 3/6 complex may regulate phosphorylation of NPH3 in darkness (Hoehenwarter et al., 2013).
But what is the biochemical function of NPH3? A recent student by Roberts et al. (2011) is starting to illuminate our understanding. It was found that NPH3 interacts with CULLIN3 (CUL3), as a substrate adaptor for a CRL3NPH3 (for Cullin-RING-ubiquitin ligase) complex that targets phot1 for ubiquitination under both low- and high-intensity BL conditions (Figure 2B). Under high BL, phot1 was shown to be both mono/multi- and polyubiquitinated, with polyubiquitination appearing to target the photoreceptor for degradation by the 26S proteasome as a means temper signaling under light-sufficient conditions. By contrast, under low BL, only mono/multiubiquitinated phot1 was detected (Figure 2B), and genetic manipulation indicated that the generation of mono/multiubiquitinated phot1 is necessary for the establishment of phototropic responses (Roberts et al., 2011). Thus, it appears that two BL-dependent posttranslational modifications of phot1 (phosphorylation and ubiquitination) are prerequisites for processing of directional BL cues.
Recently, a protein named ENHANCED BENDING1 (EHB1), with an N-terminal C2/CalB Ca2+ binding domain and a C-terminal ADP-ribosylation factor GTPase-activating protein-like domain, was identified as an NPH3 binding protein (Knauer et al., 2011). Loss-of-function mutations in EHB1 result in enhanced hypocotyl phototropism under moderate intensity BL, implicating EHB1 as a repressor of phot-dependent phototropic signaling (Knauer et al., 2011). Interestingly, ehb1 mutants also exhibited enhanced gravitropic responses (both hypocotyl and root) (Knauer et al., 2011), suggesting that EHB1 function influences a process common to both phototropism and gravitropism (Pedmale et al., 2010), processes to be discussed later. Recent findings that changes in cytosolic Ca2+ are linked to high BL-induced phototropism (Zhao et al., 2013) are compelling in the context of proposed EHB1 function.
RPT2: An NRL Family Member Involved Primarily in High-Intensity BL-Induced Phototropism
ROOT PHOTOTROPISM2 (RPT2) represents the second founding member of the NPH3/RPT2-like protein family (Motchoulski and Liscum, 1999; Inada et al., 2004; Pedmale and Liscum, 2007; Sakai and Haga, 2012). Although originally identified in a screen for Arabidopsis seedlings with defects in root phototropism (Okada and Shimura, 1992), rpt2 mutants have also been shown to be defective in hypocotyl phototropism under high-intensity BL conditions, where both phot1 and phot2 are active, while maintaining normal responsiveness under low BL conditions where phot1 is the predominate photoreceptor (Sakai et al., 2000). The high BL-specific hypocotyl phototropism phenotype of rpt2 mutants is explained in part by the fact that RPT2 transcription is induced by BL and RL in an intensity-dependent fashion (Sakai et al., 2000) through the actions of the BL-absorbing cryptochrome (cry) and RL-absorbing phytochrome (phy) classes of photoreceptors (Tsuchida-Mayama et al., 2010).
Like the phots and NPH3, RPT2 also associates with the plasma membrane and is able to interact physically in planta with both phot1 and NPH3 (Inada et al., 2004). Yeast two-hybrid assays indicate that the RPT2 interaction with the N-terminal LOV domain–containing portion of phot1 is mediated not by the C-terminal portion of RPT2, as occurs with the NPH3-phot1 interaction (Motchoulski and Liscum, 1999), but rather through its N-terminal half (Inada et al., 2004). Two-hybrid assays were also used to demonstrate that the BTB portions of RPT2 and NPH3 mediate their heterodimerization (Inada et al., 2004). While it is currently not known whether RPT2 also interacts with CUL3, it is worth noting that BTB domain–containing proteins are thought to bind to their respective CRL3 complexes as homodi-, heterodi-, or oligomers (Perez-Torrado et al., 2006). Phototropism under low BL might therefore require the action of CRL3NPH3, whereas under high BL, the function of CRL3NPH3/RPT2 might be necessary (Hohm et al., 2013). In this context, it is tantalizing to further speculate that CRL3NPH3 is responsible for the mono/multiubiquitination of phot1 (under both low- and high-intensity BL conditions) and that a separate CRL3NPH3/RPT2 complex mediates the polyubiquitination of phot1 under high BL conditions. These hypotheses remain to be tested.
PKS Proteins: Links to Auxin Transport?
There are four PHYTOCHROME KINASE SUBSTRATE (PKS) proteins in Arabidopsis (de Carbonnel et al., 2010); the first, PKS1, was originally identified in a yeast two-hybrid screen for phyA-interacting proteins (Fankhauser et al., 1999). PKS1, PKS2, and PKS4 have all been implicated in phot1-mediated BL processes, including hypocotyl and root phototropism, leaf flattening, and leaf positioning (Lariguet et al., 2006; Boccalandro et al., 2008; de Carbonnel et al., 2010). PKS1 interacts with both phot1 and NPH3 in planta (Lariguet et al., 2006) (Figure 2). More recently, PKS4 has also been shown to interact with phot1 in planta and represents a phosphorylation substrate of phot1 (Demarsy et al., 2012) (Figure 2B). Although PKS4 does not appear to be essential for phototropic signaling, the light-dependent phosphorylation of PKS4 may play a role in a negative feedback loop that regulates phototropism (Demarsy et al., 2012). Work by de Carbonnel et al. (2010) suggests that the PKS proteins may be involved in modulating light-dependent changes in auxin transport, a key midstream component of phototropism to be discussed shortly.
ABCB19: An Auxin Efflux Carrier Involved in Phototropism
Recently, Christie et al. (2011) reported the in planta interaction of phot1 with ABCB19, a member of ABCB (for ATP binding cassette B) family of transmembrane transporters (Verrier et al., 2008), that appears to represent an auxin efflux carrier (Blakeslee et al., 2007). Moreover, it was shown that ABCB19 is an in vitro phosphorylation substrate of phot1 and that ABCB19-mediated auxin efflux activity in a HeLa cell system can be inhibited by phot1-dependent phosphorylation of the transporter (Christie et al., 2011) (Figure 2B). Interestingly, abcb19 loss-of-function mutants have been shown to exhibit enhanced phototropic responses (Noh et al., 2003), consistent with a model in which phot1-mediated inhibition of ABCB19 activity positively influences the development of phototropic curvatures (Christie and Murphy, 2013; Spalding, 2013). The roles of auxin transporters in phototropism will be discussed in detail in the following section.
AUXIN TRANSPORT AND THE ESTABLISHMENT OF A DIFFERENTIAL GRADIENT OF THE HORMONE
The Cholodny-Went hypothesis (Went and Thimann, 1937) introduced earlier is generally accepted as a sound base on which to build a molecular understanding of how tropic responses are established. Central to this model is the plant hormone auxin and its environmentally regulated differential accumulation. Although de novo synthesis of new auxin, the predominant active form being indole-3-acetic acid (IAA), can occur in most tissues of a plant (Peer et al., 2011; Ljung, 2013), the differential accumulation of auxin in response to tropic stimulation is generally believed to result from alterations in long-range and local (short-range) auxin transport (Christie and Murphy, 2013; Hohm et al., 2013; Ljung, 2013; Spalding, 2013) (Figure 1). Given the ionic nature of IAA, and the typical pH differences between the inside of cells (neutral pH) and their surrounding connective cell wall material (acidic pH), thermodynamic properties dictates that at least the efflux of auxin from cells during intercellular movement must be facilitated (Goldsmith, 1977) (Figure 2). Discovery of three classes of auxin transport facilitators over the past couple of decades has provided molecular structure to such a chemiosmotic model for auxin transport (Spalding, 2013). Here, we will discuss what is known about these transporters and their roles in phototropism.
PIN Proteins: Auxin Efflux Facilitators That Exhibit Environmentally Responsive Asymmetric Cellular Distribution Patterns
There are eight PIN (PIN-FORMED) proteins in Arabidopsis: Five (PIN1 to PIN4 and PIN7) are full-length, or canonical, transporter proteins and appear to facilitate auxin efflux at the plasma membrane where they reside, and three (PIN5, PIN6, and PIN8) are truncated and appear to facilitate intracellular movement (Krecek et al., 2009; Christie and Murphy, 2013). The canonical PINs have been found to exhibit largely asymmetric (polar) cellular localization (Figure 2), with PIN1 and PIN2 apparently functioning as the major auxin efflux carriers in the stem and root, respectively, throughout development from embryogenesis to reproduction and senescence (Grunewald and Friml, 2010; Christie and Murphy, 2013). PIN3, PIN4, and PIN7 exhibit more restricted expression within the plant, and their intracellular localization (whether apolar or polar) appears to be particularly sensitive to developmental and environmental cues (Grunewald and Friml, 2010; Christie and Murphy, 2013). For example, in dark-grown Arabidopsis seedlings, PIN3 is, in general, apolarly distributed in both the hypocotyl and root, whereas phototropic stimulation induces a polar relocalization to lateral sides of specific cells that is itself graded from one side of the seedling to the other (Friml et al., 2002; Ding et al., 2011; Kleine-Vehn et al., 2011; Zhang et al., 2013) (Figure 2).
In the case of hypocotyl phototropism, PIN3 is expressed strongly and apolarly in endodermal cells in darkness but is dramatically reduced in abundance from the outer lateral portion of endodermal cells on the illuminated side of seedlings, apparently through alterations in ADP ribosylation factor–guanine nucleotide exchange factor (ARF-GEF)–dependent endocytic cycling and potentially transcytosis from outer to inner cell side (Ding et al., 2011). The resultant asymmetric distribution of PIN3, both within individual endodermal cells and between lit and shaded flanks of the seedlings is consistent with the establishment of a trans-hypocotyl gradient of auxin in response to unilateral irradiation that can be observed with an auxin responsive reporter (DR5rev:GFP) (Friml et al., 2002; Ding et al., 2011). Interestingly, both the trans-hypocotyl gradient of auxin and PIN3 polarity can be completely abrogated in a phot1 mutant background (Ding et al., 2011).
While these findings suggest that a phot1-dependent PIN3 relocalization could represent the cellular mechanism to explain the Cholodny-Went hypothesis (Went and Thimann, 1937), genetic analyses have shown that pin3 null mutants exhibit only a modest reduction in their phototropic responsiveness of both the hypocotyl (Christie et al., 2011; Ding et al., 2011) and root (Zhang et al., 2013). Therefore, additional auxin transporters must contribute to the lateral auxin gradient established in response to phot activation. Analyses of seedlings mutant for multiple PIN genes suggest that all five of the canonical PINs (PIN1, PIN2, PIN3, PIN4, and PIN7) are able to contribute to phototropic responsiveness under specific conditions (Christie et al., 2011; Ding et al., 2011; Haga and Sakai, 2012; Wan et al., 2012; Willige et al., 2013).
ABCB Proteins: Auxin Efflux Facilitators That Don’t Typically Exhibit Asymmetric Cellular Distributions
As discussed earlier, ABCB19 has been shown to be a phosphorylation substrate for phot1 (Christie et al., 2011). Whereas the PIN proteins all appear to facilitate auxin transport, only three members of the 21-member ABCB family of transporters (Verrier et al., 2008), namely, ABCB1, ABCB4, and ABCB19, have been shown to represent auxin transporters (Peer et al., 2011). The ABCB proteins are also distinct from the PIN proteins in that they do not exhibit strong polar intracellular localization (Cho et al., 2007; Wu et al., 2007) (Figure 2). At present, there is no evidence that either ABCB1 or ABCB4 are involved in phototropism, but analyses of abcb19 mutants have linked the function of ABCB19 to tropic responsiveness. Interestingly, in contrast with the reduced phototropic responses observed in pin mutants, abcb19 mutants actually exhibit enhanced phototropism (Noh et al., 2003; Nagashima et al., 2008). This phenotype and the lack of polar localization of ABCB19 confound the development of a simple mechanistic model for phototropism that incorporates ABCB19 function. However, it has been shown that ABCB19 cooperatively functions with PIN1 to mediate longitudinal auxin transport (Noh et al., 2003), likely through ABCB19-dependent stabilization of PIN membrane localization (Blakeslee et al., 2007; Titapiwatanakun et al., 2009) (Figure 2A). In this context, loss of ABCB19 function, either through mutation (Noh et al., 2003; Nagashima et al., 2008) or phot1-dependent phosphorylation (Christie et al., 2011), could lead to increased auxin concentrations in the hypocotyl because of reduced longitudinal auxin transport. In fact, Christie et al. (2011) have shown that IAA content increases within the upper portion of the hypocotyl above the elongation zone under precisely these conditions. phot1-dependent reduction in longitudinal auxin transport mediated by ABCB19 and PIN1, combined with phot1-induced lateral relocalization of PIN3, would be expected to result in a trans-hypocotyl gradient of IAA, with higher concentrations on the shaded versus lit flank (Christie and Murphy, 2013; Hohm et al., 2013; Spalding, 2013). It would be ideal if this model could explain all phototropic responses, but it does not (Christie et al., 2011; Haga and Sakai, 2012), indicating that additional players and complexities are yet to be uncovered.
AUX/LAX Proteins: H+-IAA Symporters That Facilitate Auxin Influx
While protonated IAA (IAAH) present in the cell wall can passively enter a cell, most of the auxin in the wall (∼85%) is present as the dissociated ionic form (IAA−) and can only enter the cell through facilitated transport (Goldsmith, 1977) (Figure 2). Cloning of AUXIN RESISTANT1 (AUX1) provided the first clear molecular evidence of an auxin influx carrier (Bennett et al., 1996). AUX1 is a member of a four-gene AUX1/LAX (for LIKE-AUX1) family in Arabidopsis that encodes proteins with similarity to amino acid transporters (Swarup and Péret, 2012) and functions as IAA−/H+ symporters to facilitate auxin influx (Yang et al., 2006; Swarup et al., 2008; Péret et al., 2012). In contrast with the obvious alterations in phototropic responses of seedlings carrying dysfunctional alleles of PIN or ABCB19 genes, the phototropic phenotypes of aux/lax mutants are considerably subtler and often conditional. Loss-of-function mutations in AUX1 alone can condition an enhanced phototropic response of roots (Okada and Shimura, 1992) but have no obvious influence on hypocotyl phototropism (Watahiki et al., 1999; Stone et al., 2008). However, when aux1 mutations are combined with both lax2 and lax3 mutations (Christie et al., 2011) or with a null mutation in NPH4/ARF7 (Stone et al., 2008), slight defects in hypocotyl phototropism are revealed. Together, these findings suggest that AUX1/LAX-facilitated auxin influx is not essential for phototropism but can play an important role when auxin is limited or responsiveness to the hormone is compromised (Stone et al., 2008; Hohm et al., 2013).
Posttranslational Modification of Auxin Transporters
A common theme in the regulation of auxin transport has recently emerged, namely, phosphorylation of efflux facilitators by members of the AGC-VIII superfamily of protein kinases (Dhonukshe, 2011; Löfke et al., 2013; Offringa and Huang, 2013). As discussed above, the transport capacity of ABCB19 is suppressed in response to phosphorylation by phot1, an AGC4 (Christie et al., 2011). D6 PROTEIN KINASE (D6PK), a member of the AGC1 subfamily, has been shown to phosphorylate PIN3 in vitro (Zourelidou et al., 2009), and in planta PIN3 phosphorylation is abrogated in d6pk mutants that also exhibited impaired hypocotyl phototropism (Willige et al., 2013). Interestingly, D6PK-mediated phosphorylation of PIN3 is neither BL nor phot dependent, and phot1-dependent PIN3 relocalization in response to phototropic stimulation appears to occur normally in d6pk mutants (Willige et al., 2013). Yet, longitudinal polar auxin transport in the hypocotyl is disrupted in d6pk mutants, suggesting that D6PK phosphorylation of PIN3 (and potentially PIN4 and PIN7) is positively influencing transporter activity independent of phot signaling (Willige et al., 2013).
The AGC3 subfamily, which includes PID (PINOID), WAG1, and WAG2 (Galván-Ampudia and Offringa, 2007), has also been shown to influence auxin transport; but in contrast with the apparent direct affects on efflux facilitator activity through phosphorylation by the AGC1 and AGC4 kinases, the AGC3 kinases appear to operate in a regulatory loop with ARF-GEFs to control the intracellular cycling and polar localization of PIN proteins (Dhonukshe, 2011; Löfke et al., 2013; Offringa and Huang, 2013) (Figure 2). Ding et al. (2011) have shown that PIN3 is a phosphorylation substrate for PID in vitro and that triple mutants lacking PID, WAG1, and WAG2 exhibit suppressed BL-induced lateral relocalization of PIN3 and hypocotyl phototropic responsiveness. Treatment of seedlings with the inhibitor brefeldin A, which targets vesicular trafficking complexes containing ARF-GEFs, results in disrupted PIN3 relocalization in response to phototropic stimulation (Ding et al., 2011). Intriguingly, seedlings carrying a loss-of-function mutation in GNOM, an ARF-GEF involved in PIN trafficking (Dhonukshe, 2011; Löfke et al., 2013) have reduced hypocotyl phototropism (Ding et al., 2011). Together, these results suggest that AGC3 and ARF-GEF activities impact phot1-dependent lateral relocalization of PIN3, which is one of the components in the complex system regulating the trans-hypocotyl redistribution of auxin that is prerequisite for development of phototropic responses (Figure 1). Zhang et al. (2013) have drawn similar conclusions from a study of root phototropism.
Ubiquitination has recently emerged as yet another dynamic means of regulating PIN function (Korbei and Luschnig, 2013; Löfke et al., 2013). To date, no connections have been made between PIN ubiquitination and phototropism, but it is worth noting that PIN2 function in root gravitropism is dramatically impacted by ubiquitination (Leitner et al., 2012a, 2012b). PIN2 exhibits a lateral change in abundance at the plasma membrane from one side of the root to the other in response to gravitropic stimulation (Abas et al., 2006), similar to the dynamic redistribution of PIN3 in phototropically stimulated roots (Zhang et al., 2013). Gravitropic redistribution of PIN2 appears to result from a combination of differential stabilization of the transporter at the plasma membrane and alterations in endocytic sorting that lead to targeting and degradation of PIN2 in lytic vacuoles (Korbei and Luschnig, 2013). Lys-63–linked polyubiquitination of PIN2 has been shown to, at least in part, regulate the latter process (Leitner et al., 2012a, 2012b).
Auxin Transporter Regulation by phot Internalization?
As discussed earlier, phot1 and phot2 are both internalized from the plasma membrane by endocytosis in response to BL activation (Sakamoto and Briggs, 2002; Kong et al., 2006, 2007; Aihara et al., 2008; Kong and Nagatani, 2008; Wan et al., 2008). At least in the case of phot1, this endocytosis event appears to be necessary for phototropic responsiveness (Kaiserli et al., 2009). Internalized phot could represent the phototropically active form of the receptor, as embodied in endosomal signaling models (Contento and Bassham, 2012; Haglund and Dikic, 2012), but it is equally plausible that the mere presence or absence of phot1 in the plasma membrane is critical for signal progression. For example, could relocalization of phot1 alter the membrane abundance or intracellular trafficking of other molecules necessary for phototropic responsiveness, such as PIN or ABCB auxin transporters, thus influencing their activity and establishment of a lateral auxin gradient? This scenario is particularly compelling given the likelihood, by virtue of their various protein–protein interactions, that phot1, NPH3, RPT2, PKS, ABCB, and PIN proteins cluster in unique membrane microdomains (Titapiwatanakun et al., 2009; Yang et al., 2013) (Figure 2). It is also possible that internalization of phot simply releases NPH3, which does not appear to leave the membrane in response to BL (Motchoulski and Liscum, 1999), to mediate the ubiquitination of other target proteins whose localization or activity is thereby modified.
A recent study by Lindeboom et al. (2013) has shown that BL-induces a phot-dependent reorientation of cortical microtubules in hypocotyl cells. This microtubule response requires KATANIN1 (KTN1) and mutants lacking KTN1 exhibit dramatically impaired hypocotyl phototropism (Lindeboom et al., 2013). While the authors did not explore a detailed mechanistic model to connect phots, microtubule arrays, and phototropism, at least two obvious possible models, which are not mutually exclusive, can be proposed: First, phot-dependent BL-induced reorientation of microtubules could be prerequisite for phot internalization and continued signaling. Second, microtubule reorientation could represent the means by which phot activation influences the intracellular distribution of auxin transporters. This latter possibility is certainly in line with recent findings that microtubule array organization is linked to PIN2 polar localization within root cells (Ambrose et al., 2013; Kakar et al., 2013). Although the proposals presented in this section are highly speculative, they are indeed testable.
AUXIN PERCEPTION AND RESPONSE
In the preceding sections, we attempted to pull together the vast array of molecular, biochemical, and cellular data that provide considerable support for the Cholodny-Went hypothesis (Went and Thimann, 1937). However, this has only gotten us as far as establishment of a differential gradient of auxin across a phototropically stimulated organ (Figure 1). In the next section, we address the perception of, and response to, this morphogen gradient.
Auxin Perception through ABP1 and the TIR1/AFB Family of Proteins
Two types of auxin receptor proteins have been identified in plants. First is AUXIN BINDING PROTEIN1 (ABP1) that functions at the endoplasmic reticulum and outer surface of the plasma membrane (Tromas et al., 2010; Sauer and Kleine-Vehn, 2011; Scherer, 2011). Second are the nuclear F-box proteins, S-PHASE KINASE-ASSOCIATED PROTEIN2A (SKP2A), and the members of the TRANSPORT INHIBITOR RESISTANT1/AUXIN BINDING F-BOX (TIR1/AFB) protein family (Peer et al., 2011; Hayashi, 2012). A role for SKP2A in phototropism has not been demonstrated, but both the ABP1 and TIR1/AFB receptors have been genetically linked to phototropic responsiveness. For example, heterozygous ABP1/abp1 insertional mutant seedlings are ∼50% as phototropically responsive as the wild type (Effendi et al., 2011). Extracellular ABP1-IAA (receptor-ligand complex) has been shown to regulate, by mechanisms yet unknown, a number of events at the plasma membrane, including H+ efflux, K+ influx, and clathrin-dependent endocytosis (Sauer and Kleine-Vehn, 2011; Scherer, 2011). Although the first two events are known to promote cell wall loosening (Sampedro and Cosgrove, 2005) and increased cell turgor (Dolan and Davies, 2004), both necessary prerequisites for plant cell elongation, Effendi et al. (2011) proposed that ABP1 influences hypocotyl phototropism through affects on clathrin-dependent endocytosis of PIN efflux carriers (Figure 2). This conclusion is based on the observed similarity in physiological phenotypes and auxin transport defects between the ABP1/abp1 and pin mutant seedlings (Effendi et al., 2011), which the authors argue is consistent with previous studies showing that ABP1 promotes clathrin-dependent endocytosis of PIN proteins while ABP1-IAA inhibits this process (Robert et al., 2010; Chen et al., 2012). Whether the ABP1/abp1 mutants are defective in other events at the plasma membrane, such as H+-ATPase or K+ channel activities, remains to be examined.
Analyses of single and multiple tir1/afb mutants have revealed a clear genetic role for this second type of auxin receptor in the establishment of phototropic responses (Möller et al., 2010). While the relative contribution of each TIR1/AFB receptor in phototropism has not been assessed, a molecular mechanism for how this receptor family could regulate phototropic responsiveness is quite well understood. As will be discussed in the following section, the TIR1/AFB proteins act not only as auxin receptors, but also as substrate adapters in CUL1-based E3 ubiquitin ligases (SCFTIR1/AFB) that regulate transcription in response to changing auxin concentrations (Chapman and Estelle, 2009; Lokerse and Weijers, 2009; Maraschin Fdos et al., 2009; Calderón Villalobos et al., 2012) (Figure 3).
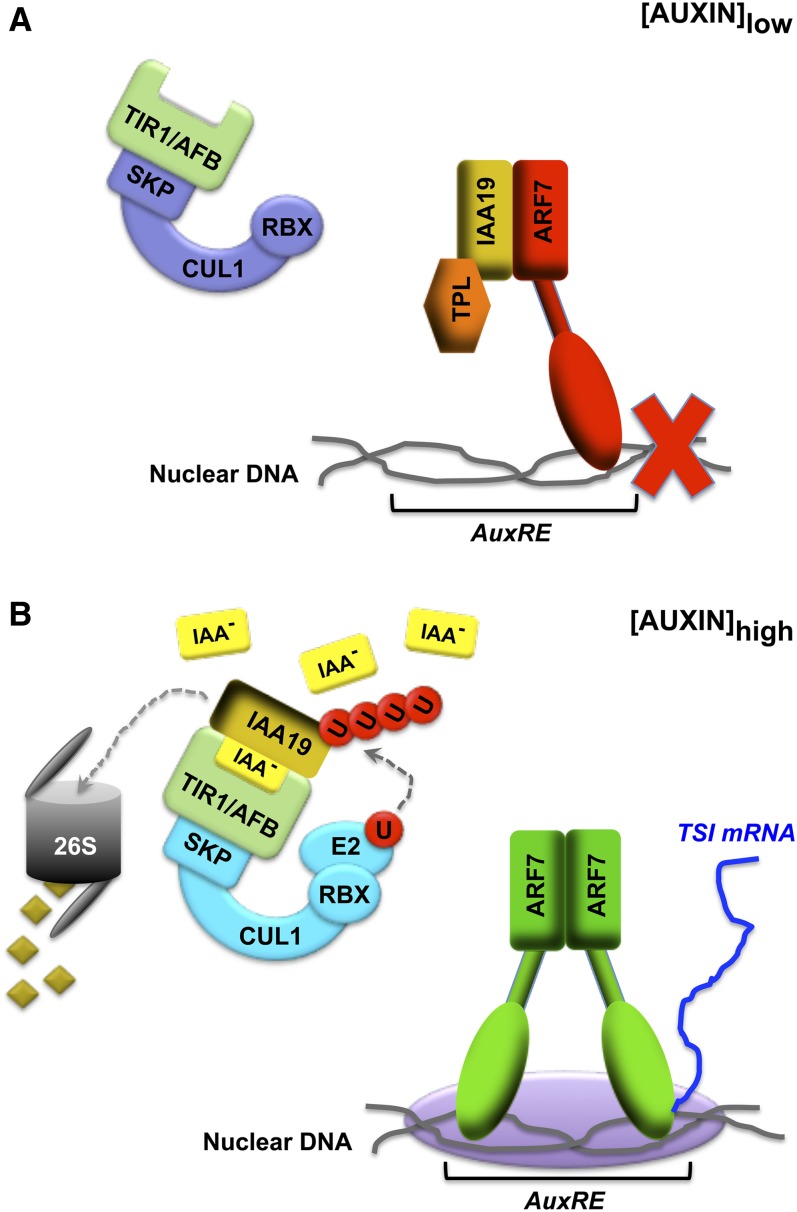
Figure 3.
Model for Regulation of Transcription by Auxin.
(A) A nucleus of a hypocotyl cell in a dark-grown seedling with only basal levels of auxin present. ARF transcription factors, such as NPH4/ARF7 (red), are bound to DNA target sequences (AuxRE) within the nuclear DNA as heteromeric complexes with a dominant transcriptional repressor protein, such as AUX/IAA19 (gold) and a corepressor, such as TPL (orange). This complex is transcriptionally inactive, and, as such, transcription of auxin-regulated genes is repressed (red X). Also present in the nucleus is the SCFTIR1/AFB auxin receptor complex (blue-violet and light green) in its inactive ligand (auxin)-free state.
(B) A nucleus of a hypocotyl cell that is in the shaded portion of a seedling exposed to directional BL where auxin has accumulated. Elevated auxin levels stimulate the binding of AUX/IAA proteins, such as IAA19, to the SCFTIR1/AFB complex (green and light blue), which in turn promotes the polyubiquitination of the AUX/IAA protein and its subsequent degradation by a 26S proteasome (gray). Removal of AUX/IAA proteins releases the corepressor TPL and allows for homodimerization of ARF proteins, which stimulates RNA polymerase core protein activation and transcription of target genes, such as TSI genes.
Auxin-Mediated Changes in Transcription
It has long been recognized that, in addition to its influences on processes at the surface of the cell (Tromas et al., 2010), auxin can induce rapid changes (within minutes) in gene transcription (Key, 1969; Theologis et al., 1985). Over the past decade, an elegant molecular model for auxin-regulated transcription has emerged that can be broken down into six key component parts (Figure 3): (1) DNA target sequences (auxin response elements [AuxREs]), (2) DNA binding transcriptional regulators (ARFs), (3) ARF binding repressor proteins (AUX/IAA proteins), (4) a corepressor protein, such as TOPLESS (TPL), (5) an SCFTIR1/AFB E3 ubiquitin ligase complex, and (6) auxin itself (Chapman and Estelle, 2009; Lokerse and Weijers, 2009; Maraschin Fdos et al., 2009; Calderón Villalobos et al., 2012). Although auxin can either activate or repress transcription depending upon whether the ARF protein contains a transcription activator or repressor domain (Liscum and Reed, 2002), we will confine our discussion below to transcriptional activation (Figure 3).
The molecular model described here has been proposed previously by others (Chapman and Estelle, 2009; Lokerse and Weijers, 2009; Maraschin Fdos et al., 2009; Calderón Villalobos et al., 2012) and represents a simple transcriptional switch that is exquisitely responsive to changes in local auxin concentration. Under low intracellular auxin concentrations, the activator ARF proteins are found associated with AuxREs within specific genes as heterodimeric complexes with repressor AUX/IAA proteins (Figure 3A). An N-terminal DNA binding domain within the ARF protein mediates interaction with the AuxRE, while heterodimerization of ARF and AUX/IAA proteins occurs through interaction of shared C-terminal regions of the two proteins. In this low auxin complex, the AUX/IAA protein can also be found associated with a corepressor protein (e.g., TPL) through an N-terminal domain. The protein interactions occurring within the low auxin complex render the complex transcriptionally repressed, a DNA bound complex in waiting so to speak (Figure 3A). Increases in intracellular auxin concentrations are perceived by a nuclear-localized TIR1/AFB auxin receptor, which itself exists as part of an SCFTIR1/AFB complex (Figure 3B). Auxin binding to TIR1/AFB proteins increases the affinity of these proteins to bind AUX/IAA proteins (Figure 3B). The AUX/IAA proteins are themselves weak auxin binding proteins with variable affinities across the protein family and function as coreceptors with the TIR1/AFB proteins to allow TIR1/AFB-AUX/IAA partner-specific interactions that have different auxin concentration dependencies. Once bound to the SCFTIR1/AFB complex, the AUX/IAA protein is rapidly ubiquitinated and subsequently degraded by the 26S proteasome (Figure 3B). Degradation of the AUX/IAA repressor protein converts the ARF-DNA complex into its transcriptionally active high auxin complex by removing the repressive action of both the AUX/IAA protein and its N-terminally bound corepressor, thus allowing homodimerization of activator ARF proteins (Figure 3B).
The first genetic link between auxin-dependent changes in transcription and phototropism was made with the identification of ARF7 as the affected locus in the nph4 mutants of Arabidopsis (Harper et al., 2000). As the mutant name would imply, the nph4/arf7 mutants were discovered in a screen for nonphototropic seedlings, but unlike phot1 and nph3 mutants (Liscum and Briggs, 1995, 1996; Motchoulski and Liscum, 1999), nph4/arf7 mutants were found to exhibit defects in a number of differential growth responses long associated with auxin (Liscum and Briggs, 1996; Watahiki and Yamamoto, 1997; Stowe-Evans et al., 1998; Watahiki et al., 1999; Harper et al., 2000). Interestingly, dominant gain-of-function mutations in MASSUGU2 (MSG2)/IAA19 that lead to stabilization of the MSG2/IAA19 protein, also condition differential growth phenotypes similar to nph4/arf7 loss-of-function mutants, including a lack of BL-induced hypocotyl phototropism (Tatematsu et al., 2004). The finding that NPH4/ARF7 and MSG2/IAA19 proteins interact is consistent with a direct dominant repression of NPH4/ARF7 activity in the gain-of-function msg2/iaa19 mutants (Tatematsu et al., 2004).
Based on findings of the aforementioned studies and the knowledge that NPH4/ARF7 is an activator-type ARF (Liscum and Reed, 2002), it has been proposed that tropic responses require the activation of particular sets of genes in the portion of the hypocotyl farthest from tropic stimulation that will exhibit an elongation response (i.e., the shaded flank in a phototropically stimulated seedling) (Figure 4). Such gene targets would be expected to encode proteins involved in the regulation of auxin-dependent cell elongation. Using a split hypocotyl system in Brasscia oleracea, Esmon et al. (2006) identified eight TROPIC STIMULUS-INDUCED (TSI) genes that appear to fulfill the predictions of these hypotheses, with each (1) exhibiting increased mRNA accumulation in regions of the hypocotyl where auxin levels increase, whether de novo as a result of response to tropic stimulus-induced auxin facilitator-mediated relocalization or via unilateral exogenous application; (2) exhibiting such increased mRNA accumulations concomitant with, or prior to, development of curvature responses; (3) exhibiting no auxin-induced expression in nph4/arf7 mutants; and (4) encoding a protein whose function fits in the context of auxin-regulated growth. As one example (Figure 4), two of the eight TSI genes, EXP1 and EXP8, are members of the α-EXPANSIN family that encode proteins that mediate cell wall extension at low pH (Sampedro and Cosgrove, 2005), conditions that are promoted by increased auxin levels through ABP1-mediated induction of plasma membrane–localized H+-ATPase activity (Sauer and Kleine-Vehn, 2011; Scherer, 2011). Consistent with a role in promoting cell elongation, mRNAs of both EXP1 and EXP8 accumulate in the region of the hypocotyl that will undergo elongation in response to phototropic stimulation (the shaded flank) well before any visible curvature occurs (Esmon et al., 2006).
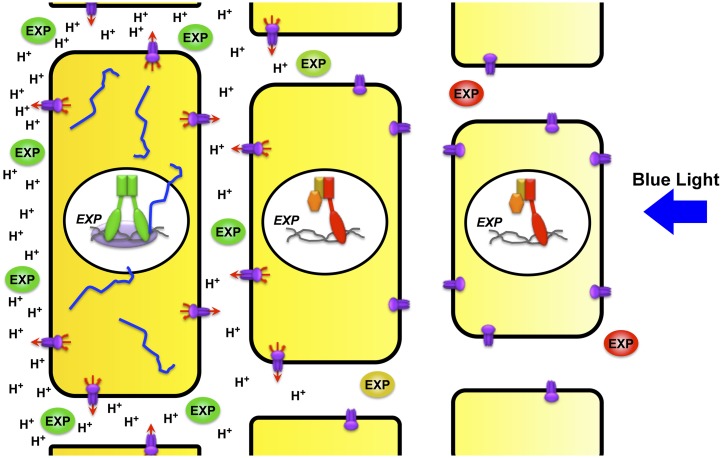
Figure 4.
A Cellular Model for Auxin-Mediated Differential Cell Elongation in Phototropically Stimulated Hypocotyls.
Initial photosensory events in phototropism result in a redistribution of auxin (yellow shading) such that it accumulates in the cells within the elongation zone of the hypocotyl farthest from the incident light (see Figures 1 and 2). Differential accumulation of auxin leads to the homodimerization and activation of NPH4/ARF7 (red and green nuclear proteins) in a graded fashion across the hypocotyl through auxin-mediated degradation of the IAA19 (gold) repressor protein and release of the corepressor TPL (orange) (see Figure 3). This differential in ARF activity thus results in the expression of tropic stimulus-induced genes, such as EXPANSINs (EXP), in the cells where auxin levels have increased beyond a threshold necessary to stimulate AUX/IAA degradation. EXP mRNAs (blue) are then translated into EXP protein, which is then deposited in the cell wall such that EXP protein accumulates beyond its basal level found in unstimulated seedlings (e.g., like that observed in the lit flank; red EXP ball) in regions farthest from the incident light stimulation. Increased auxin levels also stimulate (again in a graded fashion across the hypocotyl) a plasma membrane–localized H+-ATPase (purple) that pumps H+ out of the cell such that the extracellular matrix is acidified, a process that in turn activates the enzymatic activity of the EXP proteins in the wall to promote cell elongation.
Some TSI mRNAs, like GH3.5 (GH representing Glycine max hypocotyl where the gene was first identified; Hagen et al., 1984)/WESO1 (WES1; meaning dwarf stature in Korean; Park et al., 2007a, 2007b), and GH3.6/DWARF IN LIGHT1 (DFL1) (Nakazawa et al., 2001), accumulate not prior to, but coincident with, establishment of curvature, implying that their encoded proteins aren’t likely regulating the initiation of cell elongation (Esmon et al., 2006). Not only do GH3.5/WES1 and GH3.6/DFL1 mRNAs accumulate with a time course similar to the development of phototropic curvature, but both reach maximal levels at about the time the curvature ceases (Esmon et al., 2006). This temporal expression pattern is intriguing since both of these GH3s encode IAA-amido synthetases that conjugate free IAA (active auxin) primarily into an IAA-Asp conjugate (an inactive auxin) (Staswick et al., 2005; Park et al., 2007b). As the protein abundance of GH3.5/WES1 and GH3.6/DFL1 rise, levels of active auxin levels would fall, thus dampening the phototropic response (Esmon et al., 2006; Pedmale et al., 2010). Yet, definitive functional roles for any of the TSI proteins in tropic responsiveness await further analyses.
RESPONSE MODULATION BY SECONDARY PHOTORECEPTORS
Phytochromes
Phytochromes (phys) function as the RL and FR light photoreceptors in plants (although they also absorb BL) and regulate a wide range of responses including seed germination, seedling deetiolation, shade avoidance/neighbor sensing, and flowering (Franklin and Quail, 2010; Kami et al., 2010; Chen and Chory, 2011). Structurally the phys are organized in a general sense like the phots, with the N-terminal half of the protein representing the photosensory domain and the C-terminal portion representing the signal output domain (Sharrock, 2008; Chen and Chory, 2011; Ulijasz and Vierstra, 2011). Unlike the phots and cryptochromes (see below) that use flavin molecules as their light-absorbing cofactors (Christie, 2007; Liu et al., 2011), phys covalently bind a bilin (linear tetrapyrrole) as their cofactor (Montgomery and Lagarias, 2002; Sharrock, 2008). Evolution of this cofactor-polypeptide combination enables the phys to function as a photo-interconvertible switch, shifting between its RL-absorbing (Pr) and FR-absorbing forms (Pfr), thus allowing them to function as photosynthetic light quality sensors (Sharrock, 2008; Franklin and Quail, 2010; Chen and Chory, 2011).
The PHYs form a multigene family in higher plants, and in Arabidopsis, this family encodes five distinct phys, phyA to phyE (Sharrock, 2008). phyA is the dominant species present in dark-grown seedlings and is a type 1, or light-labile phy; phyB to phyE comprise the type 2, or light-stable, phys and are found in much lower abundance than phyA (Sharrock, 2008). Recent analysis of a phyA phyB phyC phyD phyE quintuple mutant lacking all functional phys demonstrates the nonessential nature of the phys with respect to establishment of phototropic responses (Strasser et al., 2010). The quintuple mutant retains only ∼90% of the wild-type hypocotyl phototropic response under moderate BL intensity (5 μmol m−2 s−1), whereas the phyB phyC phy D phyE quadruple mutant retains full responsiveness (Strasser et al., 2010), suggesting that phyA plays a modulatory role in phototropism. This observation is consistent with previous studies of single and double phy mutants under pulse and low-intensity continuous BL conditions where phyA appears to be the dominant modulatory phy influencing phot-dependent phototropism, and phyB, the major light-stable phy, plays a lesser role (Pedmale et al., 2010). While it remains unclear precisely how phys modulate phot-dependent phototropism, a number of potential molecular mechanisms have been proposed, as discussed below.
Phys exhibit dynamic light-dependent intracellular partitioning whereby they are generally cytoplasmically localized in darkness but nuclear localized in response to light absorption (Kevei et al., 2007). Nuclear-localized phy can interact with and regulate the activity of a family of basic helix-loop-helix transcription factors (phytochrome interacting factors [PIFs] and phytochrome interacting factor-like [PIL]), thus providing the plant a direct means of regulating gene expression in response to changing light conditions (Duek and Fankhauser, 2005; Leivar and Quail, 2011). This raises an immediate question: Where in the cell is phy functioning to modulate phot-dependent phototropism? In short, the answer appears to be both the nucleus and cytoplasm.
The expression levels of a number of phy-responsive transcription factors, including PIF4 and PIF5, required for normal phototropism supports the role of nuclear phy function (Kami et al., 2012; Sun et al., 2013). The requirement for phyA and phyB in the BL-induced expression of RPT2 (Tsuchida-Mayama et al., 2010; Kami et al., 2012) and the ability of constitutively expressed RPT2 to suppress the phototropic defects of phyA phyB double mutants (Tsuchida-Mayama et al., 2010) also supports a nuclear role for phy action. At least two auxin transporters, ABCB19 and AUX1, also appear to be transcriptionally responsive to phy signaling under light conditions that are inductive for phototropic enhancement (Nagashima et al., 2008; Stone et al., 2008), although a direct influence of a nuclear-localized phy was not examined. It is also worth noting that nph4/arf7-null mutants, while lacking phot1-dependent hypocotyl phototropism under low intensity BL (Liscum and Briggs, 1995, 1996; Stowe-Evans et al., 1998; Harper et al., 2000), recover a phot1-dependent response when seedlings are preirradiated with RL to activate phyA signaling (Stowe-Evans et al., 2001). Similar RL preirradiation of wild-type seedlings results in a phyA-dependent enhancement of phototropic responsiveness (Parks et al., 1996; Janoudi et al., 1997a, 1997b; Stowe-Evans et al., 2001). Taken together, these results suggested that phyA promotes the function of another, at least partially redundant, ARF system (Stowe-Evans et al., 2001; Stone et al., 2008).
Recently, PIF4 and PIF5 were found likely to act as negative regulators of hypocotyl phototropism through transcriptional activation of IAA19 and IAA29 (Sun et al., 2013). Because PIF4 and PIF5 are known to be targeted for degradation by phy activation and phy-PIF complexing (Shen et al., 2007; Lorrain et al., 2008), we would predict phy activation prior to, or concomitant with, phot activation to result in decreased IAA19 and IAA29 mRNA and protein levels. This in turn would lead to derepression of NPH4/ARF7 and any redundant ARF activities, followed by increased differential expression of TSI genes and enhanced phototropic responsiveness. This prediction fits with the observed phenotypes of wild-type and nph4/arf7 mutants exposed to RL plus BL versus BL alone (Parks et al., 1996; Janoudi et al., 1997a, 1997b; Stowe-Evans et al., 2001).
Rösler et al. (2007) were the first to suggest a cytoplasmic role for phyA in the modulation of phot-dependent phototropism based on their observation that the fhy1 fhl (for far-red elongated hypocotyl1 and fhy1-like) double mutant, which lacks light-induced nuclear translocation of phyA, retains RL enhancement of phot-dependent phototropism. A subsequent more detailed study indicated that the hypocotyl phototropic response of fhy1 fhl double mutants, though still robust, is in fact slowed and dampened in magnitude (Kami et al., 2012). It seems probable that these alterations occur as a result of the loss of nuclear phyA activity since seedlings expressing constitutively nuclear phyA exhibit enhanced phot1-dependent phototropism that is both stronger and faster (Kami et al., 2012).
A recent study in the moss Physcomitrella patens has generated considerable excitement about how phyA might be influencing phot1-dependent phototropism cytoplasmically (Jaedicke et al., 2012). The authors found that phy4, which mediates a RL-induced phototropic response in P. patens (Mittmann et al., 2009), physically interacts with each of the four phots in P. patens and does so at the plasma membrane where the phots reside (Jaedicke et al., 2012). This phot–phy interaction at the membrane appears functionally relevant since RL-induced phy4-mediated phototropism also requires phot function (Jaedicke et al., 2012). Phot–phy cooperative interaction is even more direct in algae and ferns where a chimeric phototropin-phytochrome photoreceptor, named NEOCHROME, has evolved to mediate directional light responses (Kawai et al., 2003; Suetsugu et al., 2005). Relative to the discussion of phyA modulation of phot-dependent phototropism in higher plants, it is particularly compelling to note that Jaedicke et al. (2012) found that Arabidopsis phyA and phot1 interact at the plasma membrane when coexpressed in onion epidermal cells.
While in planta phot1–phyA interaction has not been described in Arabidopsis to date, a potential interaction represents a very attractive model to explain how BL and RL signals absorbed by separate receptors are efficiently integrated. Moreover, several disparate observations suggest such an interaction could be taking place. For example, both phot1 and phys interact with PKS proteins (Fankhauser et al., 1999; Lariguet et al., 2006), who are themselves plasma membrane associated (Lariguet et al., 2006). Also, phyA activation has been shown to repress the BL internalization of phot1 (Han et al., 2008), a response whose characteristics could easily be explained by alterations in phot1-phyA-X protein complex dynamics at the membrane.
Cryptochromes
The cryptochromes (crys) are a second class of BL-specific photoreceptors in plants that regulate, together with the RL/FR-absorbing phys, a wide range of developmental processes throughout the life of a plant (Kami et al., 2010; Chaves et al., 2011; Liu et al., 2011). Crys are members of a larger superfamily of flavin binding proteins that also include the DNA photolyases, enzymes that catalyze light-dependent repair of pyrimidine dimers in damaged DNA (Sancar, 2008; Chaves et al., 2011). Despite their similarity in sequence, structure, and cofactor usage to the photolyases, crys do not mediate DNA repair, but rather transduce perceived BL signals via protein–protein interaction through C-terminal extensions that are not found in any of the photolyases (Chaves et al., 2011; Liu et al., 2011). Several studies of Arabidopsis mutant and transgenic lines expressing altered versions of one or both of the canonical crys, cry1 and cry2, have shown that cry function is required for normal phot1-dependent phototropic responsiveness (Lascève et al., 1999; Whippo and Hangarter, 2003; Ohgishi et al., 2004; Kang et al., 2008; Nagashima et al., 2008; Tsuchida-Mayama et al., 2010). The crys, like the phys with whom they work cooperatively for many light-regulated developmental responses, appear to function in large part through regulation of gene transcription (Kami et al., 2010; Liu et al., 2011). As was observed for phyA and phyB (Tsuchida-Mayama et al., 2010; Kami et al., 2012), cry1 and cry2 appear to regulate the expression of RPT2 in BL (Tsuchida-Mayama et al., 2010). Overexpression of RPT2 suppresses the impaired phototropic response of phyA cry1 cry2 mutants, implying that lack of phy- and cry-dependent BL-induced expression of RPT2 in the triple mutant is what conditions the phototropic defect (Tsuchida-Mayama et al., 2010). BL-induced expression of PKS1 appears to be similarly upregulated by phyA, cry1, and cry2 activation (Lariguet et al., 2006; Kami et al., 2012). Nagashima et al. (2008) reported that mRNA and protein levels of ABCB19 are reduced in the cry1 cry2 mutant background. Whereas RPT2 and PKS function as positive regulators of hypocotyl phototropism (Pedmale et al., 2010; Sakai and Haga, 2012; Hohm et al., 2013), ABCB19 inhibits development of phototropic curvatures (Christie and Murphy, 2013). Thus, repression of ABCB19 expression by phy and cry activity would be expected to promote phototropic responsiveness, a prediction consistent with the observed modulatory influences of phys and crys (Nagashima et al., 2008). In addition to these clear influences on the transcription of known phototropic regulators, the crys also appear to influence phototropism through influences on cell elongation in general, which may or may not be dependent upon transcriptional changes (Whippo and Hangarter, 2003).
INFLUENCE OF OTHER PHYTOHORMONES
Plants have evolved sophisticated and often redundant means to interpret and respond to changes in their environment. Plants growing in nature are exposed to directional light cues and are at the same time processing perhaps hundreds of additional cues. As such, their entire repertoire of signal-response capacities may be engaged (Jaillais and Chory, 2010; Vanstraelen and Benková, 2012). Therefore, it is not surprising that auxin, while a central player in the establishment of phototropism, is not the only plant hormone that impacts phototropic signaling and response.
One of the first clear molecular connections made between different hormones in the regulation of phototropism came with the discovery by Harper et al. (2000) that ethylene can stimulate recovery of phototropic responsiveness in nph4/arf7-null mutants. It was proposed that ethylene exerts its affects on the expression or function of a partially redundant ARF system that can compensate for the absence of NPH4/ARF7, a proposal that is supported by the findings that ethylene stimulates the expression of ARF19 (Li et al., 2006) and arf7 arf19 double mutants lack hypocotyl phototropism even in the presence of ethylene (Stone et al., 2008).
Mutations disrupting brassinosteroid (BR) signal response have also been shown to influence auxin-mediated phot-dependent phototropism. Nakamoto et al. (2006) used a second-site suppressor screen to identify the suppressor of nph4 (snp2) mutation that conditions the recovery of BL-induced phot1-dependent hypocotyl phototropism in the nph4/arf7 mutant background. Since the snp2 mutation is in the DWARF4 (DWF4) gene, which encodes CYP90B1, the rate-limiting cytochrome P450 involved in the biosynthesis of active BR (Kim et al., 2006), it was concluded that BR normally represses phototropism under low BL conditions (Nakamoto et al., 2006). Mutants lacking BRI1-ASSOCIATED RECEPTOR KINASE1 (BAK1)/SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE3, which functions as a BR coreceptor with BRASSINOSTEROID INSENSITIVE1 (Ye et al., 2011), exhibit a normal phototropic response under both low- and high-intensity BL, indicating that BR signaling is not essential for the establishment of phot-dependent hypocotyl phototropism (Whippo and Hangarter, 2005). However, a bak1 mutant allele (elongated) that is hypersensitive to BR exhibits enhanced phototropism under high BL (Whippo and Hangarter, 2005), suggesting that BR, while not essential can certainly act in a modulatory function that is condition dependent. Two pieces of information about the expression of NPH4/ARF7 and MSG2/IAA19 are worth mentioning here: First, BR signaling in dark-grown seedlings stimulates the expression of both MSG2/IAA19 (Nakamura et al., 2003; Sun et al., 2010; Zhou et al., 2013) and NPH4/ARF7 (Zhou et al., 2013). Second, light represses the expression of MSG2/IAA19 (Tatematsu et al., 2004; Sibout et al., 2006; Leivar et al., 2012). Based on these observations, we would expect MSG2/IAA19 mRNA levels to be relatively high under low BL conditions when BR signaling is operating, resulting in an apparent repression of phototropism in response to BR. By contrast, under high BL conditions, MSG2/IAA19 mRNA levels would be low, while BR would promote increased levels of NPH4/ARF7 transcript, thus promoting greater apparent phototropic responsiveness.
As appears to be the case with both ethylene and BR influences on phototropism, gibberellic acid (GA) effects on phototropism may involve the regulation of NPH4/ARF7-dependent transcription. Tsuchida-Mayama et al. (2010) showed that quintuple mutants lacking each of the five DELLA transcriptional repressors (GIBBERELLIN INSENSITIVE [GAI], REPRESSOR OF GA1-3 [RGA], and RGA-LIKE1 [RGL1], RGL2, and RGL3) have slightly reduced hypocotyl gravitropic and phototropic responses. Analogous to the AUX/IAA proteins in the auxin-regulated ARF transcriptional system (Figure 3), the DELLA proteins generally function as strong trans-acting transcriptional repressors in the absence of GA but are degraded in the presence of GA to allow transcriptional activation through derepression (Davière and Achard, 2013). The similarities between the auxin and GA signaling systems don’t end there: Binding of DELLA proteins to the SCFSLY/GID2 complex that targets them for polyubiquitination is stimulated by the prior high-affinity interaction of DELLAs with the nuclear hormone ligand-receptor complex, GA-GID1 (for GA-INSENSITIVE DWARF1). The DELLAs repress the expression of a number of target genes, one of which is MSG2/IAA19 (Gallego-Bartolomé et al., 2011a, 2011b). In this context, the observed phototropic impairment in the gai rga rgl1 rgl2 rgl3 quintuple mutant (Tsuchida-Mayama et al., 2010) makes sense since MSG2/IAA19 protein levels would be elevated due to increased MSG2/IAA19 transcription (via lack of DELLA-dependent repression), thus resulting in increased repression of NPH4/ARF7-dependent transcription and reduced tropic growth. It seems likely that at least part of the positive influence of crys on phototropism occurs through BL-induced cry-mediated decreases in the major bioactive gibberellin in plants, GA4 (Zhao et al., 2007; Tsuchida-Mayama et al., 2010). It is interesting to note that GA signaling can affect the intracellular trafficking of PIN2 to positively influence root gravitropism (Willige et al., 2011; Löfke et al., 2013), although such a mechanism has not been addressed relative to phototropic responsiveness.
CONCLUSIONS AND FUTURE PROSPECTS
We have come a long way since the initial identification of Darwin’s “substance” (auxin) as a major regulator of phototropic responsiveness. Despite its seeming physiological simplicity, phototropism continues to amaze us with its complexity. For example, processing of light cues to derive proper adaptive phototropic responses appear to involve (1) at least six photoreceptors (phot1, phot2, phyA, phyB, cry1, and cry2); (2) a primary directional BL receptor-signaling complex comprised of multiple proteins (e.g., phot1/phot2, NPH3, RPT2, an SCF complex, one or more PKS proteins, and ABCB19); (3) at least nine auxin transporters (PIN1, PIN2, PIN3, PIN4, PIN7, ABCB19, AUX1, LAX2, and LAX3), each with its own complex regulation of activity and intracellular localization; (4) as many as seven auxin receptors, both extracellular (ABP1) and intracellular/intranuclear (TIR1 and AFB1-5); (5) gene expression that involves both multiple transcriptional regulators (e.g., NPH4/ARF7, ARF19, MSG2/IAA19, IAA29, PIF4, PIF5, GAI, RGA, and RGLs1-3) and target genes (e.g., EXP1, EXP8, GH3.5/WES1, and GH3.6/DFL1); and (6) integration of at least four phytohormone signaling pathways (auxin, ethylene, BR, and gibberellin). Although the list of associated components continues to grow, as does our understanding of the functions and interactions of such components, a complete all-encompassing temporal-spatial model for phototropism remains to be realized. Fortunately, the phototropic path from before Darwin to date has been, and continues to be, populated with amazingly talented and creative individuals. As such, it is just a matter of time before the phototropic response puzzle yields all its pieces and reveals the entire picture.
Acknowledgments
We thank Winslow R. Briggs, who at 85 years young is still active in research and continues to inspire new generations of scientists. His impact has indeed been great; nearly everyone who currently works on phototropism (or phototropins) can probably claim three or fewer degrees of separation from him. S.K.A. was supported by a National Institutes of Health–Initiative for Maximizing Student Diversity Fellowship. D.L.L. was supported by a National Science Foundation–Graduate STEM Fellows in K–12 Education Fellowship. D.R.C. was supported by MU Interdisciplinary Plant Group, Department of Education Graduate Assistance in Areas of National Need, and National Institutes of General Medical Sciences fellowships (T34-GM008396). We gratefully acknowledge support from the National Science Foundation (IOS-1146142 to E.L.).
AUTHOR CONTRIBUTIONS
All authors contributed to writing the article.
Footnotes
Articles can be viewed online without a subscription.
References
- Abas L., Benjamins R., Malenica N., Paciorek T., Wiśniewska J., Moulinier-Anzola J.C., Sieberer T., Friml J., Luschnig C. (2006). Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 8: 249–256 Erratum. Nat. Cell Biol. 8: 424. [DOI] [PubMed] [Google Scholar]
- Aihara Y., Tabata R., Suzuki T., Shimazaki K., Nagatani A. (2008). Molecular basis of the functional specificities of phototropin 1 and 2. Plant J. 56: 364–375 [DOI] [PubMed] [Google Scholar]
- Ambrose C., Ruan Y., Gardiner J., Tamblyn L.M., Catching A., Kirik V., Marc J., Overall R., Wasteneys G.O. (2013). CLASP interacts with sorting nexin 1 to link microtubules and auxin transport via PIN2 recycling in Arabidopsis thaliana. Dev. Cell 24: 649–659 [DOI] [PubMed] [Google Scholar]
- Bennett M.J., Marchant A., Green H.G., May S.T., Ward S.P., Millner P.A., Walker A.R., Schulz B., Feldmann K.A. (1996). Arabidopsis AUX1 gene: A permease-like regulator of root gravitropism. Science 273: 948–950 [DOI] [PubMed] [Google Scholar]
- Blakeslee J.J., et al. (2007). Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell 19: 131–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccalandro H.E., De Simone S.N., Bergmann-Honsberger A., Schepens I., Fankhauser C., Casal J.J. (2008). PHYTOCHROME KINASE SUBSTRATE1 regulates root phototropism and gravitropism. Plant Physiol. 146: 108–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón Villalobos L.I., et al. (2012). A combinatorial TIR1/AFB-Aux/IAA co-receptor system for differential sensing of auxin. Nat. Chem. Biol. 8: 477–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman E.J., Estelle M. (2009). Mechanism of auxin-regulated gene expression in plants. Annu. Rev. Genet. 43: 265–285 [DOI] [PubMed] [Google Scholar]
- Chaves I., Pokorny R., Byrdin M., Hoang N., Ritz T., Brettel K., Essen L.O., van der Horst G.T., Batschauer A., Ahmad M. (2011). The cryptochromes: Blue light photoreceptors in plants and animals. Annu. Rev. Plant Biol. 62: 335–364 [DOI] [PubMed] [Google Scholar]
- Chen M., Chory J. (2011). Phytochrome signaling mechanisms and the control of plant development. Trends Cell Biol. 21: 664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Naramoto S., Robert S., Tejos R., Löfke C., Lin D., Yang Z., Friml J. (2012). ABP1 and ROP6 GTPase signaling regulate clathrin-mediated endocytosis in Arabidopsis roots. Curr. Biol. 22: 1326–1332 [DOI] [PubMed] [Google Scholar]
- Cho M., Lee S.H., Cho H.T. (2007). P-glycoprotein4 displays auxin efflux transporter-like action in Arabidopsis root hair cells and tobacco cells. Plant Cell 19: 3930–3943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholodny N. (1927). Wuchshormone und tropismem bei den planzen. Biol. Zentralbl. 47: 604–626 [Google Scholar]
- Christie J.M. (2007). Phototropin blue-light receptors. Annu. Rev. Plant Biol. 58: 21–45 [DOI] [PubMed] [Google Scholar]
- Christie J.M., Murphy A.S. (2013). Shoot phototropism in higher plants: New light through old concepts. Am. J. Bot. 100: 35–46 [DOI] [PubMed] [Google Scholar]
- Christie J.M., Reymond P., Powell G.K., Bernasconi P., Raibekas A.A., Liscum E., Briggs W.R. (1998). Arabidopsis NPH1: A flavoprotein with the properties of a photoreceptor for phototropism. Science 282: 1698–1701 [DOI] [PubMed] [Google Scholar]
- Christie J.M., Salomon M., Nozue K., Wada M., Briggs W.R. (1999). LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1): Binding sites for the chromophore flavin mononucleotide. Proc. Natl. Acad. Sci. USA 96: 8779–8783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie J.M., Swartz T.E., Bogomolni R.A., Briggs W.R. (2002). Phototropin LOV domains exhibit distinct roles in regulating photoreceptor function. Plant J. 32: 205–219 [DOI] [PubMed] [Google Scholar]
- Christie J.M., et al. (2011). phot1 inhibition of ABCB19 primes lateral auxin fluxes in the shoot apex required for phototropism. PLoS Biol. 9: e1001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contento A.L., Bassham D.C. (2012). Structure and function of endosomes in plant cells. J. Cell Sci. 125: 3511–3518 [DOI] [PubMed] [Google Scholar]
- Darwin, C. (1880). The Power of Movement in Plants. (London: John Murray Publishers). [Google Scholar]
- Davière J.M., Achard P. (2013). Gibberellin signaling in plants. Development 140: 1147–1151 [DOI] [PubMed] [Google Scholar]
- de Carbonnel M., Davis P., Roelfsema M.R.G., Inoue S., Schepens I., Lariguet P., Geisler M., Shimazaki K., Hangarter R., Fankhauser C. (2010). The Arabidopsis PHYTOCHROME KINASE SUBSTRATE2 protein is a phototropin signaling element that regulates leaf flattening and leaf positioning. Plant Physiol. 152: 1391–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarsy E., Schepens I., Okajima K., Hersch M., Bergmann S., Christie J.M., Shimazaki K., Tokutomi S., Fankhauser C. (2012). Phytochrome Kinase Substrate 4 is phosphorylated by the phototropin 1 photoreceptor. EMBO J. 31: 3457–3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P. (2011). PIN polarity regulation by AGC-3 kinases and ARF-GEF: A recurrent theme with context dependent modifications for plant development and response. Plant Signal. Behav. 6: 1333–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z., Galván-Ampudia C.S., Demarsy E., Łangowski Ł., Kleine-Vehn J., Fan Y., Morita M.T., Tasaka M., Fankhauser C., Offringa R., Friml J. (2011). Light-mediated polarization of the PIN3 auxin transporter for the phototropic response in Arabidopsis. Nat. Cell Biol. 13: 447–452 [DOI] [PubMed] [Google Scholar]
- Dolan L., Davies J. (2004). Cell expansion in roots. Curr. Opin. Plant Biol. 7: 33–39 [DOI] [PubMed] [Google Scholar]
- Duek P.D., Fankhauser C. (2005). bHLH class transcription factors take centre stage in phytochrome signalling. Trends Plant Sci. 10: 51–54 [DOI] [PubMed] [Google Scholar]
- Effendi Y., Rietz S., Fischer U., Scherer G.F. (2011). The heterozygous abp1/ABP1 insertional mutant has defects in functions requiring polar auxin transport and in regulation of early auxin-regulated genes. Plant J. 65: 282–294 [DOI] [PubMed] [Google Scholar]
- Esmon C.A., Tinsley A.G., Ljung K., Sandberg G., Hearne L.B., Liscum E. (2006). A gradient of auxin and auxin-dependent transcription precedes tropic growth responses. Proc. Natl. Acad. Sci. USA 103: 236–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C., Yeh K.C., Lagarias J.C., Zhang H., Elich T.D., Chory J. (1999). PKS1, a substrate phosphorylated by phytochrome that modulates light signaling in Arabidopsis. Science 284: 1539–1541 [DOI] [PubMed] [Google Scholar]
- Franklin K.A., Quail P.H. (2010). Phytochrome functions in Arabidopsis development. J. Exp. Bot. 61: 11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J., Wiśniewska J., Benková E., Mendgen K., Palme K. (2002). Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809 [DOI] [PubMed] [Google Scholar]
- Gallego-Bartolomé J., Alabadí D., Blázquez M.A. (2011a). DELLA-induced early transcriptional changes during etiolated development in Arabidopsis thaliana. PLoS ONE 6: e23918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego-Bartolomé J., Kami C., Fankhauser C., Alabadí D., Blázquez M.A. (2011b). A hormonal regulatory module that provides flexibility to tropic responses. Plant Physiol. 156: 1819–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván-Ampudia C.S., Offringa R. (2007). Plant evolution: AGC kinases tell the auxin tale. Trends Plant Sci. 12: 541–547 [DOI] [PubMed] [Google Scholar]
- Goldsmith M.H.M. (1977). The polar transport of auxin. Annu. Rev. Plant Physiol. 28: 439–478 [Google Scholar]
- Grunewald W., Friml J. (2010). The march of the PINs: Developmental plasticity by dynamic polar targeting in plant cells. EMBO J. 29: 2700–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga K., Sakai T. (2012). PIN auxin efflux carriers are necessary for pulse-induced but not continuous light-induced phototropism in Arabidopsis. Plant Physiol. 160: 763–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen G., Kleinschmidt A.J., Guilfoyle T.J. (1984). Auxin-regulated gene expression in intact soybean hypocotyl and excised hypocotyl sections. Planta 162: 147–153 [DOI] [PubMed] [Google Scholar]
- Haglund K., Dikic I. (2012). The role of ubiquitylation in receptor endocytosis and endosomal sorting. J. Cell Sci. 125: 265–275 [DOI] [PubMed] [Google Scholar]
- Han I.S., Cho H.Y., Moni A., Lee A.Y., Briggs W.R. (2013). Investigations on the photoregulation of chloroplast movement and leaf positioning in Arabidopsis. Plant Cell Physiol. 54: 48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han I.S., Tseng T.S., Eisinger W., Briggs W.R. (2008). Phytochrome A regulates the intracellular distribution of phototropin 1-green fluorescent protein in Arabidopsis thaliana. Plant Cell 20: 2835–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper R.M., Stowe-Evans E.L., Luesse D.R., Muto H., Tatematsu K., Watahiki M.K., Yamamoto K., Liscum E. (2000). The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissue. Plant Cell 12: 757–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper S.M., Christie J.M., Gardner K.H. (2004). Disruption of the LOV-Jalpha helix interaction activates phototropin kinase activity. Biochemistry 43: 16184–16192 [DOI] [PubMed] [Google Scholar]
- Harper S.M., Neil L.C., Gardner K.H. (2003). Structural basis of a phototropin light switch. Science 301: 1541–1544 [DOI] [PubMed] [Google Scholar]
- Hayashi K. (2012). The interaction and integration of auxin signaling components. Plant Cell Physiol. 53: 965–975 [DOI] [PubMed] [Google Scholar]
- Hoehenwarter W., Thomas M., Nukarinen E., Egelhofer V., Röhrig H., Weckwerth W., Conrath U., Beckers G.J. (2013). Identification of novel in vivo MAP kinase substrates in Arabidopsis thaliana through use of tandem metal oxide affinity chromatography. Mol. Cell. Proteomics 12: 369–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohm T., Preuten T., Fankhauser C. (2013). Phototropism: Translating light into directional growth. Am. J. Bot. 100: 47–59 [DOI] [PubMed] [Google Scholar]
- Holland J.J., Roberts D., Liscum E. (2009). Understanding phototropism: From Darwin to today. J. Exp. Bot. 60: 1969–1978 [DOI] [PubMed] [Google Scholar]
- Huala E., Oeller P.W., Liscum E., Han I.S., Larsen E., Briggs W.R. (1997). Arabidopsis NPH1: A protein kinase with a putative redox-sensing domain. Science 278: 2120–2123 [DOI] [PubMed] [Google Scholar]
- Inada S., Ohgishi M., Mayama T., Okada K., Sakai T. (2004). RPT2 is a signal transducer involved in phototropic response and stomatal opening by association with phototropin 1 in Arabidopsis thaliana. Plant Cell 16: 887–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S., Kinoshita T., Matsumoto M., Nakayama K.I., Doi M., Shimazaki K. (2008). Blue light-induced autophosphorylation of phototropin is a primary step for signaling. Proc. Natl. Acad. Sci. USA 105: 5626–5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S., Matsushita T., Tomokiyo Y., Matsumoto M., Nakayama K.I., Kinoshita T., Shimazaki K. (2011). Functional analyses of the activation loop of phototropin2 in Arabidopsis. Plant Physiol. 156: 117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaedicke K., Lichtenthäler A.L., Meyberg R., Zeidler M., Hughes J. (2012). A phytochrome-phototropin light signaling complex at the plasma membrane. Proc. Natl. Acad. Sci. USA 109: 12231–12236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais Y., Chory J. (2010). Unraveling the paradoxes of plant hormone signaling integration. Nat. Struct. Mol. Biol. 17: 642–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoudi A.K., Gordon W.R., Wagner D., Quail P., Poff K.L. (1997a). Multiple phytochromes are involved in red-light-induced enhancement of first-positive phototropism in Arabidopsis thaliana. Plant Physiol. 113: 975–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoudi A.K., Whitelam G.C., Gordon W.R., Poff K.L. (1997b). Both phytochrome A and phytochrome B are required for the normal expression of phototropism in Arabidopsis thaliana seedlings. Physiol. Plant. 101: 278–282 [Google Scholar]
- Jarillo J.A., Gabrys H., Capel J., Alonso J.M., Ecker J.R., Cashmore A.R. (2001). Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature 410: 952–954 [DOI] [PubMed] [Google Scholar]
- Jones M.A., Christie J.M. (2008). Phototropin receptor kinase activation by blue light. Plant Signal. Behav. 3: 44–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M.A., Feeney K.A., Kelly S.M., Christie J.M. (2007). Mutational analysis of phototropin 1 provides insights into the mechanism underlying LOV2 signal transmission. J. Biol. Chem. 282: 6405–6414 [DOI] [PubMed] [Google Scholar]
- Kagawa T., Sakai T., Suetsugu N., Oikawa K., Ishiguro S., Kato T., Tabata S., Okada K., Wada M. (2001). Arabidopsis NPL1: A phototropin homolog controlling the chloroplast high-light avoidance response. Science 291: 2138–2141 [DOI] [PubMed] [Google Scholar]
- Kaiserli E., Sullivan S., Jones M.A., Feeney K.A., Christie J.M. (2009). Domain swapping to assess the mechanistic basis of Arabidopsis phototropin 1 receptor kinase activation and endocytosis by blue light. Plant Cell 21: 3226–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakar K., Zhang H., Scheres B., Dhonukshe P. (2013). CLASP-mediated cortical microtubule organization guides PIN polarization axis. Nature 495: 529–533 [DOI] [PubMed] [Google Scholar]
- Kami C., Hersch M., Trevisan M., Genoud T., Hiltbrunner A., Bergmann S., Fankhauser C. (2012). Nuclear phytochrome A signaling promotes phototropism in Arabidopsis. Plant Cell 24: 566–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kami C., Lorrain S., Hornitschek P., Fankhauser C. (2010). Light-regulated plant growth and development. Curr. Top. Dev. Biol. 91: 29–66 [DOI] [PubMed] [Google Scholar]
- Kang B., Grancher N., Koyffmann V., Lardemer D., Burney S., Ahmad M. (2008). Multiple interactions between cryptochrome and phototropin blue-light signalling pathways in Arabidopsis thaliana. Planta 227: 1091–1099 [DOI] [PubMed] [Google Scholar]
- Kasahara M., Kagawa T., Oikawa K., Suetsugu N., Miyao M., Wada M. (2002). Chloroplast avoidance movement reduces photodamage in plants. Nature 420: 829–832 [DOI] [PubMed] [Google Scholar]
- Kawai H., Kanegae T., Christensen S., Kiyosue T., Sato Y., Imaizumi T., Kadota A., Wada M. (2003). Responses of ferns to red light are mediated by an unconventional photoreceptor. Nature 421: 287–290 [DOI] [PubMed] [Google Scholar]
- Kevei E., Schafer E., Nagy F. (2007). Light-regulated nucleo-cytoplasmic partitioning of phytochromes. J. Exp. Bot. 58: 3113–3124 [DOI] [PubMed] [Google Scholar]
- Key J.L. (1969). Hormones and nucleic acid metabolism. Annu. Rev. Plant Physiol. 20: 449–474 [Google Scholar]
- Kim H.B., Kwon M., Ryu H., Fujioka S., Takatsuto S., Yoshida S., An C.S., Lee I., Hwang I., Choe S. (2006). The regulation of DWARF4 expression is likely a critical mechanism in maintaining the homeostasis of bioactive brassinosteroids in Arabidopsis. Plant Physiol. 140: 548–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Doi M., Suetsugu N., Kagawa T., Wada M., Shimazaki K. (2001). Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414: 656–660 [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J., et al. (2011). Recycling, clustering, and endocytosis jointly maintain PIN auxin carrier polarity at the plasma membrane. Mol. Syst. Biol. 7: 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauer T., Dümmer M., Landgraf F., Forreiter C. (2011). A negative effector of blue light-induced and gravitropic bending in Arabidopsis. Plant Physiol. 156: 439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong S.G., Kinoshita T., Shimazaki K., Mochizuki N., Suzuki T., Nagatani A. (2007). The C-terminal kinase fragment of Arabidopsis phototropin 2 triggers constitutive phototropin responses. Plant J. 51: 862–873 [DOI] [PubMed] [Google Scholar]
- Kong S.G., Nagatani A. (2008). Where and how does phototropin transduce light signals in the cell? Plant Signal. Behav. 3: 275–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong S.G., Suzuki T., Tamura K., Mochizuki N., Hara-Nishimura I., Nagatani A. (2006). Blue light-induced association of phototropin 2 with the Golgi apparatus. Plant J. 45: 994–1005 [DOI] [PubMed] [Google Scholar]
- Korbei B., Luschnig C. (2013). Plasma membrane protein ubiquitylation and degradation as determinants of positional growth in plants. J. Integr. Plant Biol. 55: 809–823 [DOI] [PubMed] [Google Scholar]
- Krecek P., Skupa P., Libus J., Naramoto S., Tejos R., Friml J., Zazímalová E. (2009). The PIN-FORMED (PIN) protein family of auxin transporters. Genome Biol. 10: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariguet P., Schepens I., Hodgson D., Pedmale U.V., Trevisan M., Kami C., de Carbonnel M., Alonso J.M., Ecker J.R., Liscum E., Fankhauser C. (2006). PHYTOCHROME KINASE SUBSTRATE 1 is a phototropin 1 binding protein required for phototropism. Proc. Natl. Acad. Sci. USA 103: 10134–10139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascève G., Leymarie J., Olney M.A., Liscum E., Christie J.M., Vavasseur A., Briggs W.R. (1999). Arabidopsis contains at least four independent blue-light-activated signal transduction pathways. Plant Physiol. 120: 605–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner J., Petrášek J., Tomanov K., Retzer K., Pařezová M., Korbei B., Bachmair A., Zažímalová E., Luschnig C. (2012b). Lysine63-linked ubiquitylation of PIN2 auxin carrier protein governs hormonally controlled adaptation of Arabidopsis root growth. Proc. Natl. Acad. Sci. USA 109: 8322–8327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner J., Retzer K., Korbei B., Luschnig C. (2012a). Dynamics in PIN2 auxin carrier ubiquitylation in gravity-responding Arabidopsis roots. Plant Signal. Behav. 7: 1271–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P., Quail P.H. (2011). PIFs: Pivotal components in a cellular signaling hub. Trends Plant Sci. 16: 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P., Tepperman J.M., Cohn M.M., Monte E., Al-Sady B., Erickson E., Quail P.H. (2012). Dynamic antagonism between phytochromes and PIF family basic helix-loop-helix factors induces selective reciprocal responses to light and shade in a rapidly responsive transcriptional network in Arabidopsis. Plant Cell 24: 1398–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Dai X., Zhao Y. (2006). A role for auxin response factor 19 in auxin and ethylene signaling in Arabidopsis. Plant Physiol. 140: 899–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeboom J.J., Nakamura M., Hibbel A., Shundyak K., Gutierrez R., Ketelaar T., Emons A.M.C., Mulder B.M., Kirik V., Ehrhardt D.W. (2013). A mechanism for reorientation of cortical microtubule arrays driven by microtubule severing. Science 342: 1245533. [DOI] [PubMed] [Google Scholar]
- Liscum E., Briggs W.R. (1995). Mutations in the NPH1 locus of Arabidopsis disrupt the perception of phototropic stimuli. Plant Cell 7: 473–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E., Briggs W.R. (1996). Mutations of Arabidopsis in potential transduction and response components of the phototropic signaling pathway. Plant Physiol. 112: 291–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E., Reed J.W. (2002). Genetics of Aux/IAA and ARF action in plant growth and development. Plant Mol. Biol. 49: 387–400 [PubMed] [Google Scholar]
- Liu H., Liu B., Zhao C., Pepper M., Lin C. (2011). The action mechanisms of plant cryptochromes. Trends Plant Sci. 16: 684–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung K. (2013). Auxin metabolism and homeostasis during plant development. Development 140: 943–950 [DOI] [PubMed] [Google Scholar]
- Löfke C., Luschnig C., Kleine-Vehn J. (2013). Posttranslational modification and trafficking of PIN auxin efflux carriers. Mech. Dev. 130: 82–94 [DOI] [PubMed] [Google Scholar]
- Lokerse A.S., Weijers D. (2009). Auxin enters the matrix—Assembly of response machineries for specific outputs. Curr. Opin. Plant Biol. 12: 520–526 [DOI] [PubMed] [Google Scholar]
- Lorrain S., Allen T., Duek P.D., Whitelam G.C., Fankhauser C. (2008). Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J. 53: 312–323 [DOI] [PubMed] [Google Scholar]
- Maraschin Fdos, S., Memelink J., Offringa R. (2009). Auxin-induced, SCF(TIR1)-mediated poly-ubiquitination marks AUX/IAA proteins for degradation. Plant J. 59: 100–109 [DOI] [PubMed] [Google Scholar]
- Mittmann F., Dienstbach S., Weisert A., Forreiter C. (2009). Analysis of the phytochrome gene family in Ceratodon purpureus by gene targeting reveals the primary phytochrome responsible for photo- and polarotropism. Planta 230: 27–37 [DOI] [PubMed] [Google Scholar]
- Möller B., Schenck D., Lüthen H. (2010). Exploring the link between auxin receptors, rapid cell elongation and organ tropisms. Plant Signal. Behav. 5: 601–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery B.L., Lagarias J.C. (2002). Phytochrome ancestry: Sensors of bilins and light. Trends Plant Sci. 7: 357–366 [DOI] [PubMed] [Google Scholar]
- Motchoulski A., Liscum E. (1999). Arabidopsis NPH3: A NPH1 photoreceptor-interacting protein essential for phototropism. Science 286: 961–964 [DOI] [PubMed] [Google Scholar]
- Nagashima A., et al. (2008). Phytochromes and cryptochromes regulate the differential growth of Arabidopsis hypocotyls in both a PGP19-dependent and a PGP19-independent manner. Plant J. 53: 516–529 [DOI] [PubMed] [Google Scholar]
- Nakamoto D., Ikeura A., Asami T., Yamamoto K.T. (2006). Inhibition of brassinosteroid biosynthesis by either a dwarf4 mutation or a brassinosteroid biosynthesis inhibitor rescues defects in tropic responses of hypocotyls in the arabidopsis mutant nonphototropic hypocotyl 4. Plant Physiol. 141: 456–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A., Higuchi K., Goda H., Fujiwara M.T., Sawa S., Koshiba T., Shimada Y., Yoshida S. (2003). Brassinolide induces IAA5, IAA19, and DR5, a synthetic auxin response element in Arabidopsis, implying a cross talk point of brassinosteroid and auxin signaling. Plant Physiol. 133: 1843–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakasako M., Zikihara K., Matsuoka D., Katsura H., Tokutomi S. (2008). Structural basis of the LOV1 dimerization of Arabidopsis phototropins 1 and 2. J. Mol. Biol. 381: 718–733 [DOI] [PubMed] [Google Scholar]
- Nakasone Y., Zikihara K., Tokutomi S., Terazima M. (2013). Photochemistry of Arabidopsis phototropin 1 LOV1: Transient tetramerization. Photochem. Photobiol. Sci. 12: 1171–1179 [DOI] [PubMed] [Google Scholar]
- Nakazawa M., Yabe N., Ichikawa T., Yamamoto Y.Y., Yoshizumi T., Hasunuma K., Matsui M. (2001). DFL1, an auxin-responsive GH3 gene homologue, negatively regulates shoot cell elongation and lateral root formation, and positively regulates the light response of hypocotyl length. Plant J. 25: 213–221 [DOI] [PubMed] [Google Scholar]
- Noh B., Bandyopadhyay A., Peer W.A., Spalding E.P., Murphy A.S. (2003). Enhanced gravi- and phototropism in plant mdr mutants mislocalizing the auxin efflux protein PIN1. Nature 423: 999–1002 [DOI] [PubMed] [Google Scholar]
- Offringa R., Huang F. (2013). Phosphorylation-dependent trafficking of plasma membrane proteins in animal and plant cells. J. Integr. Plant Biol. 55: 789–808 [DOI] [PubMed] [Google Scholar]
- Ohgishi M., Saji K., Okada K., Sakai T. (2004). Functional analysis of each blue light receptor, cry1, cry2, phot1, and phot2, by using combinatorial multiple mutants in Arabidopsis. Proc. Natl. Acad. Sci. USA 101: 2223–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K., Shimura Y. (1992). Aspects of recent developments in mutational studies of plant signaling pathways. Cell 70: 369–372 [DOI] [PubMed] [Google Scholar]
- Park J.E., Park J.Y., Kim Y.S., Staswick P.E., Jeon J., Yun J., Kim S.Y., Kim J., Lee Y.H., Park C.M. (2007b). GH3-mediated auxin homeostasis links growth regulation with stress adaptation response in Arabidopsis. J. Biol. Chem. 282: 10036–10046 [DOI] [PubMed] [Google Scholar]
- Park J.E., Seo P.J., Lee A.K., Jung J.H., Kim Y.S., Park C.M. (2007a). An Arabidopsis GH3 gene, encoding an auxin-conjugating enzyme, mediates phytochrome B-regulated light signals in hypocotyl growth. Plant Cell Physiol. 48: 1236–1241 [DOI] [PubMed] [Google Scholar]
- Parks B.M., Quail P.H., Hangarter R.P. (1996). Phytochrome A regulates red-light induction of phototropic enhancement in Arabidopsis. Plant Physiol. 110: 155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedmale U.V., Celaya R.B., Liscum E. (2010). Phototropism: Mechanisms and outcomes. The Arabidopsis Book 8: e0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedmale U.V., Liscum E. (2007). Regulation of phototropic signaling in Arabidopsis via phosphorylation state changes in the phototropin 1-interacting protein NPH3. J. Biol. Chem. 282: 19992–20001 [DOI] [PubMed] [Google Scholar]
- Peer W.A., Blakeslee J.J., Yang H., Murphy A.S. (2011). Seven things we think we know about auxin transport. Mol. Plant 4: 487–504 [DOI] [PubMed] [Google Scholar]
- Péret B., et al. (2012). AUX/LAX genes encode a family of auxin influx transporters that perform distinct functions during Arabidopsis development. Plant Cell 24: 2874–2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Torrado R., Yamada D., Defossez P.A. (2006). Born to bind: The BTB protein-protein interaction domain. Bioessays 28: 1194–1202 [DOI] [PubMed] [Google Scholar]
- Reymond P., Short T.W., Briggs W.R., Poff K.L. (1992). Light-induced phosphorylation of a membrane protein plays an early role in signal transduction for phototropism in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 89: 4718–4721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert S., et al. (2010). ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell 143: 111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D., Pedmale U.V., Morrow J., Sachdev S., Lechner E., Tang X., Zheng N., Hannink M., Genschik P., Liscum E. (2011). Modulation of phototropic responsiveness in Arabidopsis through ubiquitination of phototropin 1 by the CUL3-Ring E3 ubiquitin ligase CRL3NPH3. Plant Cell 23: 3627–3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösler J., Klein I., Zeidler M. (2007). Arabidopsis fhl/fhy1 double mutant reveals a distinct cytoplasmic action of phytochrome A. Proc. Natl. Acad. Sci. USA 104: 10737–10742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Haga K. (2012). Molecular genetic analysis of phototropism in Arabidopsis. Plant Cell Physiol. 53: 1517–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Kagawa T., Kasahara M., Swartz T.E., Christie J.M., Briggs W.R., Wada M., Okada K. (2001). Arabidopsis nph1 and npl1: Blue light receptors that mediate both phototropism and chloroplast relocation. Proc. Natl. Acad. Sci. USA 98: 6969–6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Wada T., Ishiguro S., Okada K. (2000). RPT2. A signal transducer of the phototropic response in Arabidopsis. Plant Cell 12: 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K., Briggs W.R. (2002). Cellular and subcellular localization of phototropin 1. Plant Cell 14: 1723–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon M., Christie J.M., Knieb E., Lempert U., Briggs W.R. (2000). Photochemical and mutational analysis of the FMN-binding domains of the plant blue light receptor, phototropin. Biochemistry 39: 9401–9410 [DOI] [PubMed] [Google Scholar]
- Salomon M., Knieb E., von Zeppelin T., Rüdiger W. (2003). Mapping of low- and high-fluence autophosphorylation sites in phototropin 1. Biochemistry 42: 4217–4225 [DOI] [PubMed] [Google Scholar]
- Salomon M., Lempert U., Rüdiger W. (2004). Dimerization of the plant photoreceptor phototropin is probably mediated by the LOV1 domain. FEBS Lett. 572: 8–10 [DOI] [PubMed] [Google Scholar]
- Sampedro J., Cosgrove D.J. (2005). The expansin superfamily. Genome Biol. 6: 242, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A. (2008). Structure and function of photolyase and in vivo enzymology: 50th anniversary. J. Biol. Chem. 283: 32153–32157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer M., Kleine-Vehn J. (2011). AUXIN BINDING PROTEIN1: The outsider. Plant Cell 23: 2033–2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer G.F. (2011). AUXIN-BINDING-PROTEIN1, the second auxin receptor: what is the significance of a two-receptor concept in plant signal transduction? J. Exp. Bot. 62: 3339–3357 [DOI] [PubMed] [Google Scholar]
- Sharrock R.A. (2008). The phytochrome red/far-red photoreceptor superfamily. Genome Biol. 9: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Khanna R., Carle C.M., Quail P.H. (2007). Phytochrome induces rapid PIF5 phosphorylation and degradation in response to red-light activation. Plant Physiol. 145: 1043–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short T.W., Briggs W.R. (1990). Characterization of a rapid, blue light-mediated change in detectable phosphorylation of a plasma membrane protein from etiolated pea (Pisum sativum L.) seedlings. Plant Physiol. 92: 179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibout R., Sukumar P., Hettiarachchi C., Holm M., Muday G.K., Hardtke C.S. (2006). Opposite root growth phenotypes of hy5 versus hy5 hyh mutants correlate with increased constitutive auxin signaling. PLoS Genet. 2: e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding E.P. (2013). Diverting the downhill flow of auxin to steer growth during tropisms. Am. J. Bot. 100: 203–214 [DOI] [PubMed] [Google Scholar]
- Staswick P.E., Serban B., Rowe M., Tiryaki I., Maldonado M.T., Maldonado M.C., Suza W. (2005). Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 17: 616–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone B.B., Stowe-Evans E.L., Harper R.M., Celaya R.B., Ljung K., Sandberg G., Liscum E. (2008). Disruptions in AUX1-dependent auxin influx alter hypocotyl phototropism in Arabidopsis. Mol. Plant 1: 129–144 [DOI] [PubMed] [Google Scholar]
- Stowe-Evans E.L., Harper R.M., Motchoulski A.V., Liscum E. (1998). NPH4, a conditional modulator of auxin-dependent differential growth responses in Arabidopsis. Plant Physiol. 118: 1265–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe-Evans E.L., Luesse D.R., Liscum E. (2001). The enhancement of phototropin-induced phototropic curvature in Arabidopsis occurs via a photoreversible phytochrome A-dependent modulation of auxin responsiveness. Plant Physiol. 126: 826–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser B., Sánchez-Lamas M., Yanovsky M.J., Casal J.J., Cerdán P.D. (2010). Arabidopsis thaliana life without phytochromes. Proc. Natl. Acad. Sci. USA 107: 4776–4781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu N., Mittmann F., Wagner G., Hughes J., Wada M. (2005). A chimeric photoreceptor gene, NEOCHROME, has arisen twice during plant evolution. Proc. Natl. Acad. Sci. USA 102: 13705–13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu N., Wada M. (2013). Evolution of three LOV blue light receptor families in green plants and photosynthetic stramenopiles: phototropin, ZTL/FKF1/LKP2 and aureochrome. Plant Cell Physiol. 54: 8–23 [DOI] [PubMed] [Google Scholar]
- Sullivan S., Thomson C.E., Lamont D.J., Jones M.A., Christie J.M. (2008). In vivo phosphorylation site mapping and functional characterization of Arabidopsis phototropin 1. Mol. Plant 1: 178–194 [DOI] [PubMed] [Google Scholar]
- Sun J., Qi L., Li Y., Zhai Q., Li C. (2013). PIF4 and PIF5 transcription factors link blue light and auxin to regulate the phototropic response in Arabidopsis. Plant Cell 25: 2102–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., et al. (2010). Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev. Cell 19: 765–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup K., et al. (2008). The auxin influx carrier LAX3 promotes lateral root emergence. Nat. Cell Biol. 10: 946–954 [DOI] [PubMed] [Google Scholar]
- Swarup R., Péret B. (2012). AUX/LAX family of auxin influx carriers-an overview. Front. Plant Sci. 3: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemiya A., Inoue S., Doi M., Kinoshita T., Shimazaki K. (2005). Phototropins promote plant growth in response to blue light in low light environments. Plant Cell 17: 1120–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatematsu K., Kumagai S., Muto H., Sato A., Watahiki M.K., Harper R.M., Liscum E., Yamamoto K.T. (2004). MASSUGU2 encodes Aux/IAA19, an auxin-regulated protein that functions together with the transcriptional activator NPH4/ARF7 to regulate differential growth responses of hypocotyl and formation of lateral roots in Arabidopsis thaliana. Plant Cell 16: 379–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Huynh T.V., Davis R.W. (1985). Rapid induction of specific mRNAs by auxin in pea epicotyl tissue. J. Mol. Biol. 183: 53–68 [DOI] [PubMed] [Google Scholar]
- Titapiwatanakun B., et al. (2009). ABCB19/PGP19 stabilises PIN1 in membrane microdomains in Arabidopsis. Plant J. 57: 27–44 [DOI] [PubMed] [Google Scholar]
- Tokutomi S., Matsuoka D., Zikihara K. (2008). Molecular structure and regulation of phototropin kinase by blue light. Biochim. Biophys. Acta 1784: 133–142 [DOI] [PubMed] [Google Scholar]
- Tromas A., Paponov I., Perrot-Rechenmann C. (2010). AUXIN BINDING PROTEIN 1: Functional and evolutionary aspects. Trends Plant Sci. 15: 436–446 [DOI] [PubMed] [Google Scholar]
- Tsuchida-Mayama T., Sakai T., Hanada A., Uehara Y., Asami T., Yamaguchi S. (2010). Role of the phytochrome and cryptochrome signaling pathways in hypocotyl phototropism. Plant J. 62: 653–662 [DOI] [PubMed] [Google Scholar]
- Ulijasz A.T., Vierstra R.D. (2011). Phytochrome structure and photochemistry: Recent advances toward a complete molecular picture. Curr. Opin. Plant Biol. 14: 498–506 [DOI] [PubMed] [Google Scholar]
- Vanstraelen M., Benková E. (2012). Hormonal interactions in the regulation of plant development. Annu. Rev. Cell Dev. Biol. 28: 463–487 [DOI] [PubMed] [Google Scholar]
- Verrier P.J., et al. (2008). Plant ABC proteins—A unified nomenclature and updated inventory. Trends Plant Sci. 13: 151–159 [DOI] [PubMed] [Google Scholar]
- Wan Y., Jasik J., Wang L., Hao H., Volkmann D., Menzel D., Mancuso S., Baluška F., Lin J. (2012). The signal transducer NPH3 integrates the phototropin1 photosensor with PIN2-based polar auxin transport in Arabidopsis root phototropism. Plant Cell 24: 551–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y.L., Eisinger W., Ehrhardt D., Kubitscheck U., Baluska F., Briggs W. (2008). The subcellular localization and blue-light-induced movement of phototropin 1-GFP in etiolated seedlings of Arabidopsis thaliana. Mol. Plant 1: 103–117 [DOI] [PubMed] [Google Scholar]
- Watahiki M.K., Tatematsu K., Fujihira K., Yamamoto M., Yamamoto K.T. (1999). The MSG1 and AXR1 genes of Arabidopsis are likely to act independently in growth-curvature responses of hypocotyls. Planta 207: 362–369 [DOI] [PubMed] [Google Scholar]
- Watahiki M.K., Yamamoto K.T. (1997). The massugu1 mutation of Arabidopsis identified with failure of auxin-induced growth curvature of hypocotyl confers auxin insensitivity to hypocotyl and leaf. Plant Physiol. 115: 419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Went, F.W. (1926). On growth accelerating substances in the coleoptile of Avena sativa Proc. Kon. Ned. Akad. Wet. 30: 10–19. [Google Scholar]
- Went, F.W., and Thimann, K.V. (1937). Phytohormones. (New York: Macmillan Company). [Google Scholar]
- Whippo C.W., Hangarter R.P. (2003). Second positive phototropism results from coordinated co-action of the phototropins and cryptochromes. Plant Physiol. 132: 1499–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whippo C.W., Hangarter R.P. (2005). A brassinosteroid-hypersensitive mutant of BAK1 indicates that a convergence of photomorphogenic and hormonal signaling modulates phototropism. Plant Physiol. 139: 448–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willige B.C., Ahlers S., Zourelidou M., Barbosa I.C., Demarsy E., Trevisan M., Davis P.A., Roelfsema M.R., Hangarter R., Fankhauser C., Schwechheimer C. (2013). D6PK AGCVIII kinases are required for auxin transport and phototropic hypocotyl bending in Arabidopsis. Plant Cell 25: 1674–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willige B.C., Isono E., Richter R., Zourelidou M., Schwechheimer C. (2011). Gibberellin regulates PIN-FORMED abundance and is required for auxin transport-dependent growth and development in Arabidopsis thaliana. Plant Cell 23: 2184–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Lewis D.R., Spalding E.P. (2007). Mutations in Arabidopsis multidrug resistance-like ABC transporters separate the roles of acropetal and basipetal auxin transport in lateral root development. Plant Cell 19: 1826–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Richter G.L., Wang X., Młodzińska E., Carraro N., Ma G., Jenness M., Chao D.Y., Peer W.A., Murphy A.S. (2013). Sterols and sphingolipids differentially function in trafficking of the Arabidopsis ABCB19 auxin transporter. Plant J. 74: 37–47 [DOI] [PubMed] [Google Scholar]
- Yang Y., Hammes U.Z., Taylor C.G., Schachtman D.P., Nielsen E. (2006). High-affinity auxin transport by the AUX1 influx carrier protein. Curr. Biol. 16: 1123–1127 [DOI] [PubMed] [Google Scholar]
- Ye H., Li L., Yin Y. (2011). Recent advances in the regulation of brassinosteroid signaling and biosynthesis pathways. J. Integr. Plant Biol. 53: 455–468 [DOI] [PubMed] [Google Scholar]
- Zhang K.X., Xu H.H., Yuan T.T., Zhang L., Lu Y.T. (2013). Blue-light-induced PIN3 polarization for root negative phototropic response in Arabidopsis. Plant J. 76: 308–321 [DOI] [PubMed] [Google Scholar]
- Zhao X., Wang Y.-L., Qiao X.-R., Wang J., Wang L.-D., Xu C.-S., Zhang X. (2013). Phototropins function in high-intensity blue light-induced hypocotyl phototropism in Arabidopsis by altering cytosolic calcium. Plant Physiol. 162: 1539–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Yu X., Foo E., Symons G.M., Lopez J., Bendehakkalu K.T., Xiang J., Weller J.L., Liu X., Reid J.B., Lin C. (2007). A study of gibberellin homeostasis and cryptochrome-mediated blue light inhibition of hypocotyl elongation. Plant Physiol. 145: 106–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.Y., Song L., Xue H.W. (2013). Brassinosteroids regulate the differential growth of Arabidopsis hypocotyls through auxin signaling components IAA19 and ARF7. Mol. Plant 6: 887–904 [DOI] [PubMed] [Google Scholar]
- Zourelidou M., Müller I., Willige B.C., Nill C., Jikumaru Y., Li H., Schwechheimer C. (2009). The polarly localized D6 PROTEIN KINASE is required for efficient auxin transport in Arabidopsis thaliana. Development 136: 627–636 [DOI] [PubMed] [Google Scholar]