Abstract
Designing of biologically active scaffolds with optimal characteristics is one of the key factors for successful tissue engineering. Recently, hydrogels have received a considerable interest as leading candidates for engineered tissue scaffolds due to their unique compositional and structural similarities to the natural extracellular matrix, in addition to their desirable framework for cellular proliferation and survival. More recently, the ability to control the shape, porosity, surface morphology, and size of hydrogel scaffolds has created new opportunities to overcome various challenges in tissue engineering such as vascularization, tissue architecture and simultaneous seeding of multiple cells. This review provides an overview of the different types of hydrogels, the approaches that can be used to fabricate hydrogel matrices with specific features and the recent applications of hydrogels in tissue engineering. Special attention was given to the various design considerations for an efficient hydrogel scaffold in tissue engineering. Also, the challenges associated with the use of hydrogel scaffolds were described.
Keywords: hydrogels; scaffolds; biodegradability; bioadhesion; biocompatibility, tissue engineering
Introduction
Tissue engineering is a rapidly expanding interdisciplinary field involving biomaterials science, cell biology, cell-material interactions and surface characterization. Research in this field aims to restore, preserve, or enhance tissue functions. It also aims to replace diseased or damaged organs, or tissues that are defective or have been lost as a result of accidents or disease. Tissue engineering typically involves four key components as illustrated in (Figure 1); (a) selected and isolated cells (progenitor or stem cells from different origins), (b) biomaterial scaffolds which may be natural or synthetic, to provide a platform for cell function, adhesion and transplantation, (c) signaling molecules such as proteins and growth factors deriving the cellular functions of interest, and (d) bioreactors that support a biologically active environment for cell expansion and differentiation such as cell culture.
Figure 1. .
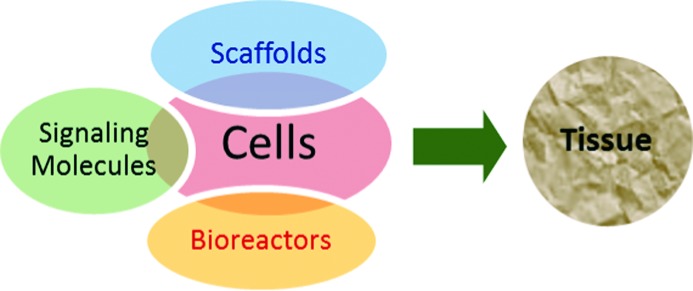
A schematic illustration of the four key components of tissue engineering.
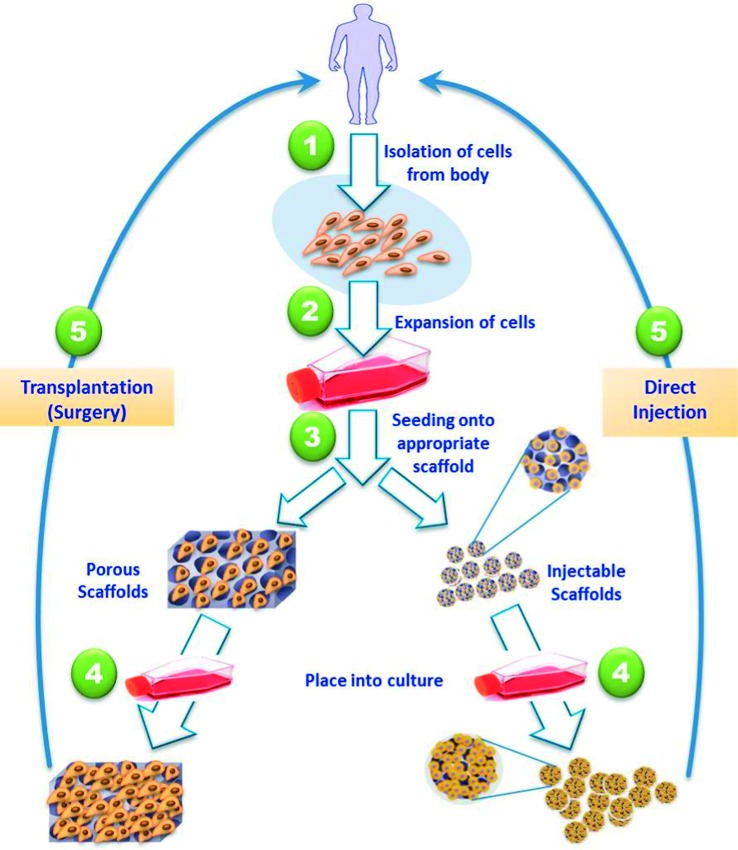
Tissues or organs can be potentially developed via a number of approaches. The most common approach (Figure 2) involves isolation of tissue-specific cells from the patient's small tissue biopsy and harvested in vitro. The isolated cells are then expanded and seeded into three-dimensional scaffold that mimic the natural extracellular matrices (ECM) of the targeted tissues. The key functions of these scaffolds are to (a) deliver the seeded cells to the desired site in the patient's body, (b) encourage cell-biomaterial interactions, (c) promote cell adhesion, (d) permit adequate transport of gases, nutrients and growth factors to ensure cell survival, proliferation, and differentiation, (e) confer a negligible inflammation extent or toxicity in vivo, and (f) control the structure and function of the engineered tissue.1 The cell-loaded scaffolds are subsequently transplanted into the patient either through direct injection with the aid of a needle or other minimally invasive delivery technique, or through implantation of the fabricated tissue at the desired site in the patient's body using surgery.2
Figure 2. .
Schematic illustration of the most common tissue engineering approaches. Tissue-specific cells are isolated from a small biopsy from the patient, expanded in vitro, seeded into a well-designed scaffold and transplanted into the patient either through injection, or via implantation at the desired site using surgery.
Designing a scaffold with optimal characteristics is, as mentioned above, one of the main key components for successful tissue engineering. Over the last decade, hydrogel scaffolds have received a considerable attention due to their unique compositional and structural similarities to the natural ECM in addition to their desirable framework for cellular proliferation and survival.
Hydrogels, an overview
Hydrogels are three-dimensional networks composed of hydrophilic polymers crosslinked either through covalent bonds or held together via physical intramolecular and intermolecular attractions. Hydrogels can absorb huge amounts of water or biological fluids, up to several thousand %, and swell readily without dissolving. The high hydrophilicity of hydrogels is particularly due to the presence of hydrophilic moieties such as carboxyl, amide, amino, and hydroxyl groups distributed along the backbone of polymeric chains. In the swollen state, hydrogels are soft and rubbery, resembling to a great extent the living tissues. In addition, many hydrogels, such as chitosan and alginate-based hydrogels show desirable biocompatibility.3
The appearance of hydrogels dates back more than fifty years, when Wichterle et al. (1955–1960)4 developed and investigated a poly(2-hydroxyethyl methacrylate)-based hydrogel for contact lens applications. Since then, the research in the field of hydrogels has expanded dramatically particularly in the last two decades. In addition, the uses of hydrogels have extended to cover a wide range of applications that include, but are not limited to, drug delivery, wound healing, ophthalmic materials and tissue engineering.5,6
Hydrogels usually reach their equilibrium swelling when a balance occurs between osmotic driving forces, which encourage the entrance of water or biological fluids into the hydrophilic hydrogel matrix, and the cohesive forces exerted by the polymer strands within the hydrogel. These cohesive forces resist the hydrogel expansion and the extent of these forces depends particularly on the hydrogel crosslinking density.7,8 In general, the more hydrophilic the polymer forming the hydrogel, the higher the total water amount absorbed by the hydrogel. Equally, the higher the crosslinking extent of a particular hydrogel, the lower the extent of the gel swelling. Hydrogels in their dried forms are usually called “xerogels”, whereas, dry porous hydrogels resulting from the use of drying techniques such as freeze-drying or solvent extraction are referred to as “aerogels”.7
Hydrogels as compared to gels
One of the common misconceptions in polymer science is the use of the concept “gel” instead of “hydrogel” and vice verse. Gels are semi-solid materials made of hydrophilic polymers comprising small amounts of solids, dispersed in relatively large amounts of liquid. However, gels may appear more solid-like than liquid-like.9 Hydrogels are also made of hydrophilic polymer chains but they are crosslinked and that enables them to swell while retaining their three-dimensional structure without dissolving.10 Hence, the principle feature of hydrogels, that differentiates them from gels, is their inherent crosslinking. However, gels can also get a low level of virtual crosslinking under the influence of sheer forces, but this type of crosslinking is very weak and reversible.
Classifications of hydrogels
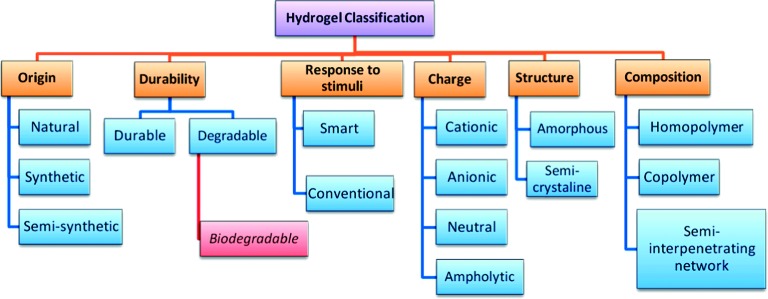
Numerous classifications have been applied to hydrogels as summarized in (Figure 3). Some of these classifications are discussed below11:
Figure 3. .
Schematic diagram showing the most common classes of hydrogels.
According to hydrogel origin
Hydrogels can be classified into natural, synthetic and semi-synthetic according to their origin. Most of the synthetic hydrogels are synthesized by traditional polymerization of vinyl or vinyl-activated monomers. The equilibrium swelling values of these synthetic hydrogels vary widely according to the hydrophilicity of the monomers and the crosslinking density. A bi-functional monomer is usually added to carry out an in situ crosslinking reaction. Natural hydrogels are made of natural polymers including polynucleotides, polypeptides, and polysaccharides. These natural polymers are obtained from various natural origins. For instance, collagen is obtained from mammals whereas chitosan is obtained from shellfish exoskeletons.
According to hydrogel durability
Hydrogels can be either durable (such as most polyacrylate-based hydrogels) or biodegradable (such as polysaccharides-based hydrogels), depending on their stability characteristics in a physiological environment. Recently, a significant body of research has focused on the fabrication and utilization of new biodegradable hydrogels. Applications of these biodegradable hydrogels are now covering many areas, including both biomedical and non-biomedical uses.12 The degradable polymers inside the hydrogel matrices undergo chain scission to form oligomers of low molecular weight. Then, the resulting oligomers are either eliminated by body or undergo further degradation.
According to hydrogel response to environmental stimuli
The past decade has witnessed vast advances in preparation and investigating a unique category of hydrogels called “smart hydrogels”. In spite of the similarities with conventional types in preparation methods and characterization techniques, smart hydrogels can, however exhibit unusual changes in their swelling behavior, network structure and/or mechanical characteristics in response to various environmental stimuli such as pH, temperature, light, ionic strength or electric field.13–15 These changes occur to smart hydrogels, in response to any of these environmental stimuli, usually disappear upon removal of the stimulus and consequently the hydrogels restore their original state and so on in a reversible manner. (Figure 4) illustrates, as example, the significant change in swelling extent of a smart hydrogel in response to various environmental stimuli.
Figure 4. .
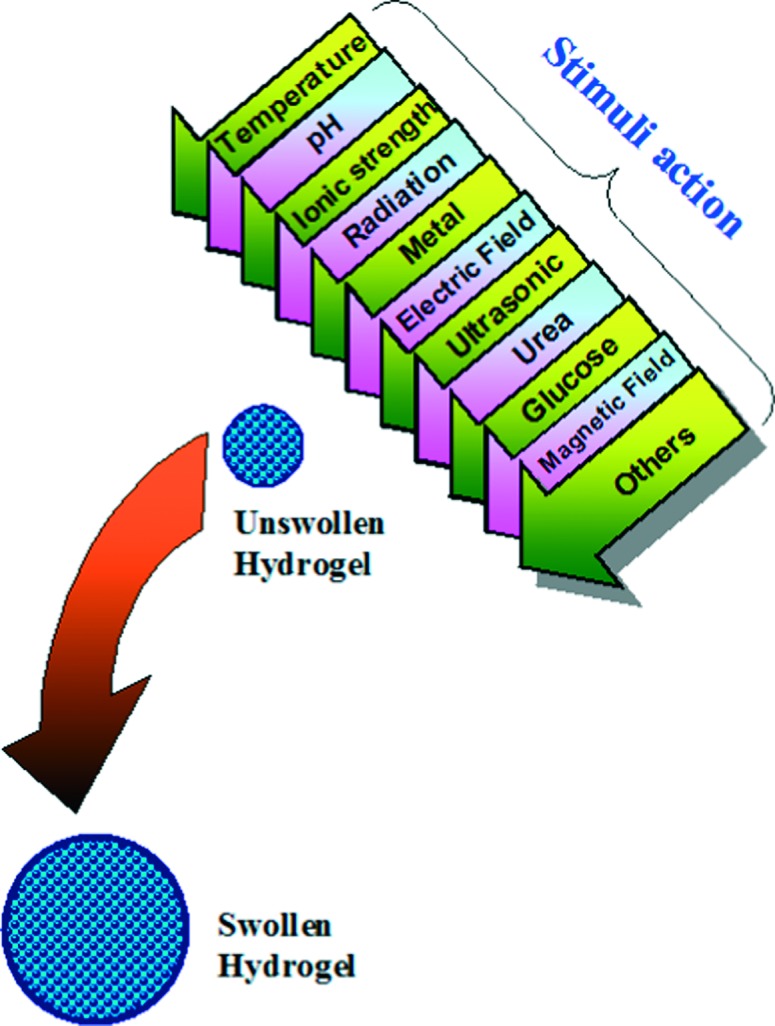
Stimuli-responsive swelling of a smart hydrogel.
Methods of preparation of hydrogels
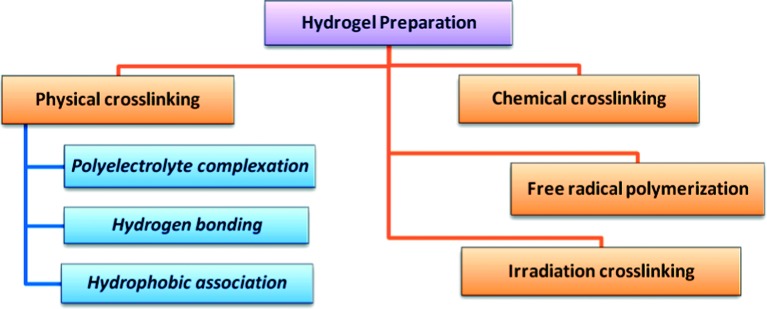
Hydrogels can be prepared by various methods depending on the designed structure and the desired application. Some of these methods are discussed below and summarized in (Figure 5).
Figure 5. .
Schematic diagram showing the most common methods of preparation of hydrogels.
Free radical polymerization
Conventional free radical polymerization is the preferred technique for preparation of hydrogels based on some monomers such as acrylates, amides and vinyl lactams.16,17 It can also be used for development of natural polymers-based hydrogels provided that these polymers have suitable functional groups or have been functionalized with radically polymerizable groups. For instance, this method has been used to develop various chitosan-based hydrogels.18,19 Preparation of hydrogels using this technique involves the typical free radical polymerizations steps; initiation, propagation, chain transfer and termination. In the initiation step a wide variety of visible, thermal, ultraviolet and red-ox initiators can be used for radical generation. These radicals then react with monomers converting them into active forms which react with more monomers and so on in the propagation step. The resulting long chain radicals undergo termination either through chain transfer or through radical combination forming polymeric matrices.
This method of hydrogel preparation can be performed either in solution or neat (bulk). Solution polymerization is desirable during synthesis of large quantities of hydrogels and in this case, water is the most commonly used solvent. Bulk polymerization, however is faster than solution polymerization and does not need a solvent removal, which is time-consuming in many cases.
Irradiation crosslinking of hydrogel polymeric precursors
Ionizing-radiation techniques, especially if combined with a simultaneous sterilization process, are very effective methods for synthesis of hydrogels. Ionizing radiations, such as electron beam and γ–rays, have high energy enough to ionize simple molecules either in air or water.11 During irradiation of a polymer solution, many reactive sites are generated along the polymer strands. Then, the combination of these radicals leads to formation of a large number of crosslinks. Formation of hydrogels using this approach can be performed via irradiation of the polymers either in bulk or in solution. However, irradiation of a polymer solution is the favored due to the less energy required for formation of macroradicals. Besides, in solution the efficiency of radicals is high due to the reduced viscosity of reaction mixture.
Applying irradiation to hydrogel development offers many advantages over other preparation methods where, during the irradiation process, no catalysts or additives are needed to initiate the reaction. Also, irradiation methods are simple and the crosslinking extent can be controlled easily by varying the irradiation dose.20,21 Due to these advantages, this technique has been used for developing a wide range of hydrogels for many biomedical applications, where even the slightest contamination is undesirable. For example, it has been used efficiently to prepare acrylic acid hydrogels20 and poly(ethylene glycol)/carboxymethyl chitosan-based pH-responsive hydrogels.22 However, this technique is not recommended for preparation of hydrogels from some polymers that can degrade under the ionizing irradiation.
Chemical crosslinking of hydrogel polymeric precursors
Chemical crosslinking of hydrophilic polymers is one of the main methods of hydrogel preparation. In this technique, a bi-functional crosslinking agent is added to a dilute solution of a hydrophilic polymer and the polymer should have a suitable functionality to react with the crosslinking agent. This method is suitable for preparation of hydrogels from both natural and synthetic hydrophilic polymers. For instance, albumin and gelatin–based hydrogels were developed using dialdehyde or formaldehyde as crosslinking agents.23,24 Also hydrogels of high water content based on crosslinking of functionalized polyethylene glycol and a lysine-containing polypeptide have been developed by this method.24
Physical crosslinking of hydrogel polymeric precursors
Crosslinking of polymers through physical interactions is one of the common approaches for hydrogel formation. This physical crosslinking includes interactions such as polyelectrolyte complexation, hydrogen bonding and hydrophobic association, and the hydrogels developed by this technique are usually prepared under mild conditions.
Polyelectrolyte complexation (Ionic interactions)
In this approach, hydrogels are prepared through formation of polyelectrolyte complexes, where links are formed between pairs of charged sites along the polymer backbones. The formed electrolytic links vary in their stabilities according to the pH of the system. An example of hydrogels developed by this method are those resulting from the polyelectrolyte complexation of the carboxylate groups of sodium alginate with the amino groups distributed along chitosan chains.25
Hydrogen bonding
Hydrogen bonding between polymer chains can also participate in hydrogel formation, for instance, in developing gelatin-based hydrogel.24 A hydrogen bond is formed through the association of an electron deficient hydrogen atom and a functional group of high electronegativity. Hydrogels developed by this technique are affected by many factors, such as polymer concentration, molar ratio of each polymer, type of solvent, solution temperature, and the degree of association between the polymer functionalities.
Hydrophobic association
A further methodology for obtaining Hydrogels is through hydrophobic interactions.26 Polymers and copolymers, such as graft and block copolymers, usually form structures separated by hydrophobic micro-domains. These hydrophobic domains act as associated crosslinking points in the entire polymeric structure, and are surrounded by hydrophilic water absorbing regions. This approach has been utilized to develop a hydrogel based on a graft-type copolymer composed of hydrophilic poly(hydroxyethyl methacrylate) (PHEMA) as a backbone and a small amount of hydrophobic poly(methylmethacrylate) (PMMA) as a long branch.26 In general, the mechanical characteristics of these hydrophobically combined polymers are poor due to the poor interfacial adhesion. However, this approach for hydrogel preparation has some advantages such as the low cost of the system.
Potential applications of hydrogels in tissue engineering
A significant body of research has focused recently on the utilization of hydrogels for various applications in tissue engineering. For instance, hydrogels have been used as scaffolds that mimic the extracellular matrices, to provide the structural integrity and bulk for cellular organization and morphogenic guidance, to encapsulate and deliver cells, to act as tissue barriers and bioadhesives, to serve as depots for drugs, and to deliver bioactive moieties that encourage the natural reparative process.
Hydrogels as carriers for cell transplantation
Hydrogels can be ultimately beneficial in cell transplantation due to their unique ability to offer immunoisolation while still allowing nutrients, oxygen, and metabolic products to diffuse easily into their matrices. For instance, photo-polymerized poly(ethylene glycol) diacrylate, (PEG diacrylate)-based hydrogels have been developed to transplant islets of Langerhans.27 In this study, the islet cells were suspended in the photo-polymerizable polymer solution and the solution was then used to formulate PEG-based microspheres that incorporated the islets. These PEG-based hydrogel microspheres showed adequate immunoisolation but the diffusion ability of nutrients to the entrapped cells was relatively limited. This shortcoming has been overcome through reducing the thickness of the interfacially photo-polymerized hydrogel microspheres and consequently, the encapsulated islets remained viable for prolonged periods and the hydrogel particles retained their immunoisolation function.27 In another study,28 the in vitro potential of a metalloproteinase (MMP)-responsive PEG-based hydrogel was investigated as bioactive co-encapsulation system for vascular cells and a small bioactive peptide, thymosin beta4. The study demonstrated that incorporation of thymosin beta4 in the matrix creates a three-dimensional environment favorable for human umbilical vein endothelial cell (HUVEC) adhesion, survival, migration and organization. In addition, thymosin beta4 has improved the HUVEC attachment and induced vascular-like network formation within the PEG-hydrogels. These developed MMP-responsive PEG-hydrogels may thus be tailored to serve as controlled co-encapsulation system of vascular cells and bioactive agents for in situ regeneration of ischemic tissues. In a recent study, Nichol et al.29 have developed gelatin methacrylate (GelMA) as an inexpensive, cell-responsive hydrogel platform for creating cell-laden microtissues. The investigated cells readily bound to, proliferated, elongated and migrated when encapsulated in the developed microfabricated GelMA hydrogels.
Hydrogels as scaffolds
Hydrogels can be used in tissue engineering either directly after their preparation (with or without cell entrapment) or after formulation as scaffolds. Hydrogel-based scaffolds are a very important class of scaffolds due to the ability to tailor their mechanical characteristics to mimic those of natural tissues. Hydrogel scaffolds are used in particular to provide bulk and mechanical structures to a tissue construct, whether cells are suspended within or adhered to the 3D hydrogel framework. When the cellular-hydrogel adhesion is preferred over the suspension within the scaffold, inclusion of appropriate peptide moieties on the surface or throughout the bulk of the hydrogel scaffold can significantly increase the extent of cellular attachment. For instance, one of the most successful approaches to facilitate cellular attachment is the incorporation of the RGD (arginine–glycine–aspartic acid) adhesion peptide sequence. Inclusion of these RGD domains in hydrogels has shown improved cellular migration, proliferation, growth, and organization in tissue regeneration applications.30,31 In addition, a variety of cells have been shown to favorably bind to the RGD-modified hydrogel scaffolds. These cells include endothelial cells (ECs), fibroblasts, smooth muscle cells (SMCs), chondrocytes and osteoblasts.
Hydrogels as barrier against restenosis
Hydrogels can be used to enhance the healing response following a tissue injury where they can be used as barriers in order to avoid restenosis or thrombosis due to post-operative adhesion formation.32,33 It has been found that forming a thin hydrogel film intravascularly through interfacial photo-polymerization can inhibit restenosis by reducing the intimal thickening and thrombosis.32,33 The reduction in the intimal thickening is due to acting of the hydrogel thin film as a barrier preventing platelets, plasma proteins and coagulation factors from being in direct contact with the vascular walls where the contact of these factors with vessel walls would encourage smooth muscle cell proliferation, migration, and matrix synthesis and consequently cause restenosis. Hydrogels based on poly(ethylene glycol-co-lactic acid) diacrylate, for instance, were developed through bulk photo-polymerization on intraperitoneal surfaces where they were able to prevent fibrin deposition and fibroblast attachment at the tissue surface.34,35
Hydrogels as drug depots
One of the most common applications of hydrogels is their use as localized drug depots. This is attributed to their highly hydrophilic nature, biocompatibility and the ability to control and trigger the drug release in a smart manner from them through the interaction with bio-molecular stimuli.36–38 Hydrophilic macromolecular therapeutic agents, such as some proteins or oligonucleotides are innately compatible with hydrogel matrices. In addition, the delivery kinetics can be managed according to the desired drug release schedule via controlling the swelling degree, crosslinking extent and the biodegradation rate of hydrogels. Photo-polymerizable hydrogels are especially attractive for localized drug delivery due to their ability to adhere and conform to targeted tissue when formed in situ. Moreover, the drug delivery ability of hydrogels can be used simultaneously with their functioning as barrier layer, as described earlier, to deliver drugs locally and at the same time inhibit any post-operative adhesion formation. In one example, biodegradable photo-polymerized hydrogel layers have been formed on intraperitoneal tissues to locally release urokinase plasminogen activator, tissue plasminogen activator and ancrod.34,39 These developed formulations demonstrated a significant reduction in adhesion formation as compared to using intraperitoneal injections or hydrogel barriers alone. In another example, mono- and multilayer hydrogels formed on the inner surface of blood vessels through interfacial photo-polymerization have been utilized for intravascular drug delivery.39,40
Design criteria for hydrogel scaffolds in tissue engineering
The extracellular matrix (ECM) is the extracellular component of natural tissue that provides structural support to the cells as well as performing various other important functions. Hence, the ECM is one of the most significant guides in the design of scaffolds for tissue engineering. The ECM is a hydrophilic 3D micro-matrix with two major solid structures; collagen fibers and the proteoglycan filaments. The collagen fibers present as bundles and extend through the interstitium, providing the tensile strength and durability for the surrounding tissue. The proteoglycan filaments are coiled structures and made from protein and hyaluronic acid. Together with the entrapped interstitial fluid, which is a plasma-like fluid but of a lower protein concentration, ECM demonstrates a gel-like uniformity.41
Hydrogels in tissue engineering must meet a number of design criteria to mimic the ECM and consequently to function appropriately and promote new tissue formation. These hydrogel scaffolds for instance, should provide a 3D architecture for cell growth. This architecture better mimics the natural tissues and allows for morphology and gene expression that cannot be attained in 2D structures. The design criteria should also include both classical mechanical and physicochemical parameters (such as biodegradation, porosity and proper surface chemistry), and biological performance parameters (such as biocompatibility and cell adhesion), as well as demonstrating enhanced vascularization. In addition, parameters such as accessibility and commercial feasibility should be considered upon developing hydrogel scaffolds for tissue engineering purposes.
Biodegradation
A basic requirement of a scaffold for tissue engineering is to maintain cellular proliferation and desired cellular distribution during the anticipated life of the scaffold. In many cases, the scaffold's life would be until degradation is complete. Therefore the rate and extent of biodegradation are critical design considerations for hydrogels in tissue engineering. The significance of scaffold degradation in tissue culture has been evaluated by examining the cellular viability in non degradable scaffolds. For instance, PEG and PEG-dimethacrylate (PEGDMA) have been photo-polymerized to produce hydrogel scaffolds encapsulating bovine and ovine chondrocytes for cartilage regeneration.42,43 After the photo-polymerization, cells within the scaffold were able to maintain their viability and were evenly dispersed, but because of the non biodegradability of these PEG-based scaffolds, the cell counts decreased significantly over time. In a contrary situation, a biodegradable hydrogel scaffold was developed via photo-polymerization of poly(propylene fumarate-co-ethylene glycol) and used to encapsulate endothelial cells for vascular cell growth.44 This study showed that the cells were distributed throughout the hydrogel scaffold and were actively proliferating. Additionally, cells were found to spread and migrate in proteolytically degradable hydrogel scaffolds, but they were grouped in clusters in non degradable hydrogel scaffolds. It was also demonstrated that in proteolytically degradable hydrogel scaffolds, cells attained an increase in their proliferation rate and ECM production over cells in non degradable hydrogel scaffolds.39
In spite of the significance of biodegradation as a key factor for hydrogel scaffolds in tissue engineering, some tissue engineering applications may not require complete scaffold degradation, such as with corneal replacement or articular cartilage. For these types of tissues, semi-permanent or permanent scaffolds may be the best choice to replace the basic function of lost or damaged tissue.
In general, degradable hydrogel scaffolds are developed via incorporating cleavable crosslinks and/or cleavable moieties into the polymer backbone. In the case of biodegradable hydrogel scaffolds, an important class of degradable scaffolds, degradation is achieved through biological processes, mainly enzymatic digestion.45 Biodegradable hydrogel scaffolds can also be made by incorporating naturally biodegradable ECM components, such hyaluronic acid, laminin, fibronectin and collagen. Moreover, cell-driven degradation of hydrogel scaffolds has been reported by Hubbell et al.46,47
Biocompatibility
Biocompatibility, “the ability of a material to perform with an appropriate host response in a specific application”,48 is a key design factor for engineered tissue constructs. In other words, biocompatibility means that no or very limited harmful immunological, toxic, or foreign body responses should take place as a result of regenerative medical intervention. This definition of biocompatibility is particularly relevant in tissue engineering because the nature of tissue constructs is to continuously interact with the body during the healing and cellular regeneration process, and also during the scaffold degradation. If this design parameter is not considered, the resulting hydrogel matrix can be fouled or there may be scarring to the connected tissues, whether these tissues are directly adjacent or linked through vasculature.
One of the main challenges for in vivo biocompatibility of hydrogel scaffolds is the toxic moieties and chemicals that may be used in the polymerization of synthetic hydrogels or in the crosslinking of natural polymer hydrogel precursors, especially if reaction conversion is less than 100%. Ingredients such as unreacted monomers, stabilizers, initiators, organic solvents and emulsifiers which are used in hydrogel preparation may also be harmful, if they leak to the seeded cells or tissues. For instance, Irgacure, a commonly used free radical photo-initiator has been found to decrease cell viability even at limited concentrations.49 Therefore, hydrogel scaffolds developed for tissue engineering should typically be purified from remaining unreacted hazardous chemicals before use. This purification can be performed using various techniques such as dialysis or extensive solvent washing. In some cases, purification of hydrogel scaffolds is more challenging, or even not viable, such as in the case of hydrogels that are prepared through in situ gelation. This is because the reactants required to synthesize the hydrogel are injected into the body while still in a pre-polymer solution. Hence, upon using such in situ gelation techniques, particular caution should be considered to ensure all ingredients are non toxic and reasonably safe.
Pore size and porosity extent
Hydrogel scaffolds designed for tissue engineering must be highly porous with an open interconnected geometry, to allow a large surface area relative to the scaffold's volume. This high, interconnected porosity will encourage cell ingrowth, uniform cell distribution and assist the neovascularization of the matrix.50 Not only is the porosity extent important, but many other parameters such as pore size, pore volume, pore size distribution, pore throat size, pore shape, pore wall roughness and the pore interconnectivity are equally significant considerations when designing a hydrogel scaffold for tissue engineering purposes. For instance, pore interconnectivity is critical to ensuring that all cells are within 200 μm from the blood supply in order to provide for mass transfer of nutrients and oxygen.51,52 Pore size is also a very important parameter because if the developed pores were too small, pore blocking by the cells would occur, inhibiting cellular penetration, ECM production, and neovascularization of the inner areas of the scaffold. The effect of scaffold pore size on tissue regeneration, and optimum pore sizes for different purposes have been reported in many recent studies. For instance, it has been demonstrated that the optimum pore size for neovascularization is 5 μm,53 5–15 μm for ingrowth of fibroblast,54 20 μm for hepatocytes ingrowth,51 20–125 μm for regeneration of adult mammalian skin,55 and 200–350 μm for osteoconduction.56
Mechanical characteristics
The mechanical characteristics of hydrogels as scaffolds for tissue engineering can have a significant effect on either attached or encapsulated cells. It is well established that the ECM has a certain level of isometric tension between the cells in a given tissue, which varies according to tissue type and can be changed in disease processes. Also, the response of individual cells to changes in these tensions and stresses can vary from morphological alterations to changes in gene expression.57 For this reason, hydrogel scaffolds may need to be designed with tissue specific mechanical characteristics. For instance, it was reported that hydrogel stiffness can be used to control the differentiation of mesenchymal stem cells.58 One of the main parameters that control the mechanical compliance of hydrogel scaffolds is the crosslinking density, which can also be used to affect cells encapsulated within hydrogel networks. For instance, it has been reported that changes in the crosslinking density of PEG-based hydrogel caused changes in cell growth and morphology.59,60
Surface characteristics
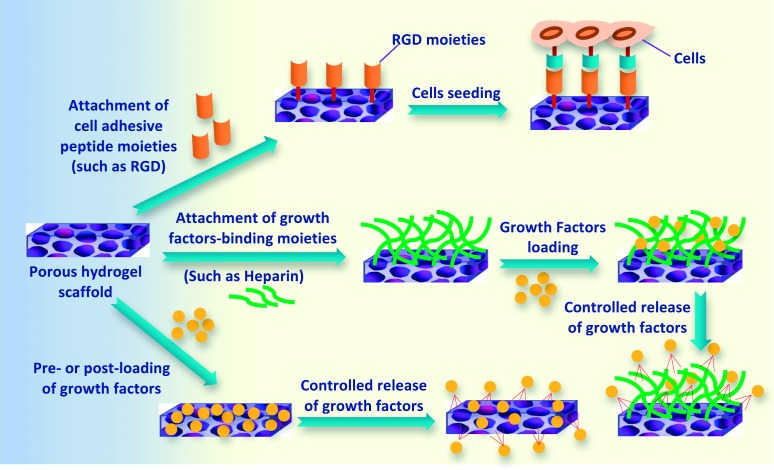
The surface of hydrogel scaffolds is the initial and primary site of interaction with surrounding cells and tissues. Therefore, both physicochemical and topographical surface characteristics of scaffolds are vital parameters in controlling and affecting cellular adhesion and proliferation.61 As most cells used in the engineered tissues are anchorage-dependent, the hydrogel scaffolds should be designed in such a way to facilitate their attachment. For this reason, hydrogel scaffolds with relatively large and accessible surface area are advantageous in order to accommodate the number of cells required to replace or reinstate tissue or organ functions. Surface characteristics of hydrogel scaffolds can be selectively improved by various approaches including thin film deposition and immobilizations of adhesive biomoieties such as RGD peptides, growth factors (like bFGF, EGF), insulin, fibronectin and collagen (Figure 6). This modification can enhance the biocompatibility of the hydrogel scaffold and consequently, cells can specifically recognize the scaffold. The adhesive biomoieties can either be covalently linked, electrostatically absorbed, or self-assembled on the surface of hydrogel scaffolds.62
Figure 6. .
Some approaches for selective enhancement of surface characteristics of hydrogel scaffolds toward increasing surface-cells attachment and controlled release of regulatory growth factors.
Vascularization
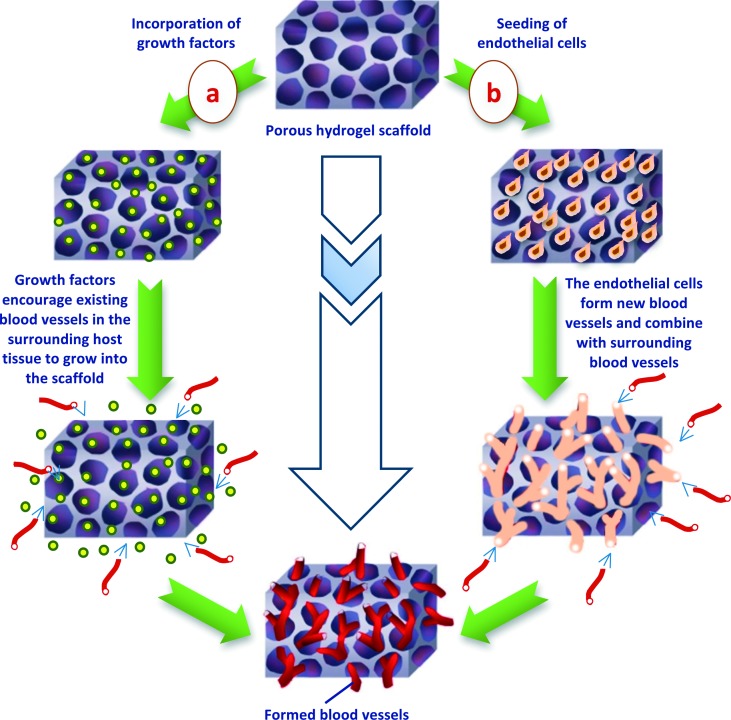
Vascularization is essential to provide a conduit for nutrient exchange and the elimination of waste products through perfusion. Formation of new blood vessels in adult tissue (Neovascularization) is therefore a key design factor for most tissue engineering initiatives. However, designing the appropriate scaffolds that allow and encourage new blood vessels to grow is a big challenge. Vascularizable scaffolds should have high porosity extent, desirable pore sizes and make allowances for vascular remodeling to take place as tissues mature. In certain tissue engineering applications, hydrogels have been efficient as vascularizable scaffolds. For instance, alginate-based hydrogel scaffolds were found to be very successful in vivo in recreation of vascularized bone.63 In general, there are two main approaches to encourage the vascularization of a tissue engineered scaffold as illustrated in (Figure 7). In the first approach, vasculogenic growth factors are incorporated into the hydrogel scaffold to motivate the vasculature from surrounding host tissues to grow into the scaffold. The second approach involves seeding the hydrogel scaffold with endothelial cells (ECs). Using the first approach, for instance, gelatin-,64 alginate-,65,66 PEG-,67 and hyaluronic acid-based68,69 hydrogels loaded with vasculogenic growth factors have been shown to successfully induce the microvessel growth following implantation process.
Figure 7. .
Schematic illustration of blood vessels formation encouraged by either (a) incorporating of regulatory growth factors or (b) via seeding of endothelial cells into the porous hydrogel scaffold.
One of the other investigated approaches for encouraging vascularization of tissue engineering scaffolds is based on recruiting endothelial progenitor cells (EPCs). However, relying on the EPCs and the native surrounding vasculature to invade the implanted scaffolds is a process dependent upon presence of sufficient amounts of circulating EPCs and can take long time (up to days) to take place. Generally, cells cannot survive more than few hundred micrometers from blood vessels, so if other types of cells were incorporated within the hydrogel scaffold, they may subject to necrosis while waiting for vascular ingrowth. In addition, although EPCs represent a limitless source of cells for in vitro prevascularization, their differentiation needs to be controlled. In a relatively recent study, Gerecht et al.70 found that hyaluronic acid-based hydrogels could maintain ESCs in their undifferentiated state until vascular differentiation was attained.
Natural polymers-based hydrogels for tissue engineering
Several hydrogels have been developed from natural polymers for tissue engineering applications. Some of these natural polymer-based hydrogels are described in Table 1. These natural polymers include for instance, polynucleotides, polypeptides, and different polysaccharides. They are obtained from a variety of natural origins; for example chitosan is obtained from shellfish exoskeletons whereas, collagen is obtained from mammals.
Table 1 .
Some natural polymer-based hydrogels for tissue engineering applications.
| Hydrogel pre-polymer | Used cells | Developed tissue | Ref. |
| Hyaluronic acid (HA) | Fibroblasts | Connective Tissue | [71] |
| – | Eye | [72] | |
| – | Facial | [73] | |
| – | Intraperitoneal | [74] | |
| Fibroblasts | Skin | [75] | |
| – | Vascular | Tabata et al. and Peattie et al.[64,68] | |
| h-ESCs | Vascular | [70] | |
| Peptide amphiphile–Ti composite | Osteoblasts | Bone | [76] |
| HA, Chondroitin Sulfate, Gelatin | Fibroblasts | ECM | [77] |
| Fibrin | Chondrocytes | Cartilage | [78] |
| Bone marrow cells | Cardiovascular | [79] | |
| – | Skin | [80] | |
| Chondrocytes | Cartilage | [81] | |
| Alginate | Chondrocytes | Cartilage | [82] |
| Chondrocytes | Facial | [83] | |
| – | Vascular | [65,66] | |
| Alginate, HA | – | Cartilage/Bone | [63] |
| Collagen | Chondrocytes | Cartilage | [84] |
| – | Skin | [85] | |
| – | Neural | [86] | |
| Astroglial cells | Spinal cord | [87] | |
| Collagen, Alginate | – | Vocal Cord | [88] |
| Collagen, HA | Chondrocytes | Cartilage | [89] |
| PLLA, Agar, Gelatin | Chondrocytes | Cartilage | [90] |
| HA, Collagen | Chondrocytes | Cartilage | [91] |
| HA, Alginate, Carboxymethylcellulose | Hepatocytes | Cardiovascular | [92] |
| HA–Gelatin | – | Vocal Cord | [93] |
| Gelatin | – | Vascular | [64] |
| Dextran | h-ESCs | Vascular | [94] |
| Chondroitin sulfate, HA | – | Skin | [95] |
| Chitin/hydroxyapatite | COS-7 cells | Bone | [96] |
| HA derivatives | NIH3T3 cells | - | [97] |
| Agarose carbomer | Glial cells | Neural | [98] |
Collagen hydrogel fibers are one of the most popular natural polymer-based hydrogel scaffolds in tissue engineering applications. As shown in (Figure 8), these collagen hydrogel fibers are formed particularly through self-aggregation and crosslinking (through pyridinium crosslinks) of collagen molecules in a hydrated environment. Collagen molecules are composed of tropocollagen triple helixes, where each triple helix results from the self-arranging of three polypeptides strands.
Figure 8. .
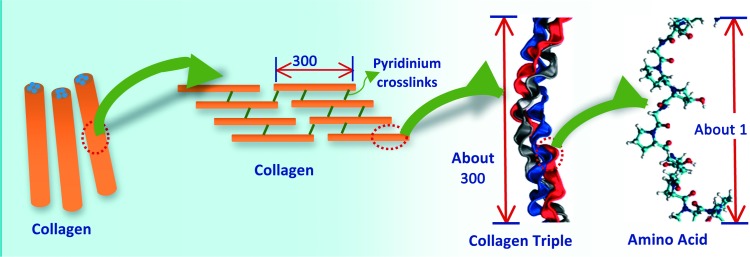
Schematic illustration showing the basic structure of collagen hydrogel fibers. Images were adapted with modification from Buehler.99
In general, hydrogels based on polymers from natural origins such as collagen are advantageous in tissue engineering applications due to their intrinsic characteristics of biological recognition, including presentation of receptor-binding ligands and the susceptibility to cell-triggered proteolytic remodeling and degradation. However, the use of natural component-based hydrogels has shown some drawbacks, which involve the complexities associated with purification, immunogenicity and pathogen transmission.
Synthetic polymer-based hydrogels for tissue engineering
Hydrogels made from various synthetic polymers were developed for different tissue engineering applications. Table 2 shows some of these synthetic polymer-based hydrogels.
Table 2 .
Some synthetic polymers-based hydrogels for tissue engineering applications.
| Hydrogel pre-polymer | Used cells | Developed tissue | Ref. |
| PEG–PLA | Osteoblasts | Bone | [100,101] |
| PEG | Fibroblasts | Bone | [102] |
| Embryonic carcinoma | Cardiovascular | [103] | |
| Chondrocytes | Cartilage | [104] | |
| ESCs | Cartilage | [105–108] | |
| Chondrocytes | Cartilage | [109] | |
| Chondrocytes | Cartilage | [110] | |
| Chondrocytes | Cartilage | [110] | |
| Mesenchymal (MSCs) | Cartilage | [111] | |
| Chondrocytes, MSCs | Cartilage | [112,113] | |
| – | Intraperitoneal | [114] | |
| Islet of Langerhans | Pancreatic | [115] | |
| – | Vascular | [32,33] | |
| – | Vascular | [67] | |
| MSCs, Primary smooth muscle | Vascular | [116] | |
| Smooth muscle cells | Vascular | [117] | |
| Endothelial cells | Vascular | [118] | |
| PEGDA | – | Vascular | [119] |
| Osteoarthritic chondrocytes | Cartilage | [120] | |
| PEG–PLA | Chondrocytes | Cartilage | [121,122] |
| Islet of Langerhans | Pancreatic | [123] | |
| PEG–PLA–PVA | Chondrocytes | Cartilage | [124] |
| PEO Semi-IPN | Chondrocytes | Cartilage | [42,43] |
| PVA | Chondrocytes | Cartilage | [49] |
| PEG, PEG/PLA | – | Intraperitoneal | [34,35] |
| PHEMA–MMA | – | Neural | [125] |
| PHEMA | – | Eye | [126] |
| Myoblasts | Skeletal Muscle | [127] | |
| – | Spinal cord | [128] | |
| Dex-MA-LA & Gel-MA | ECs & SMCs | Vascular | [129] |
PEG: poly(ethylene glycol), PEGDA: poly(ethylene glycol) diacrylate, PLA: poly(lactic acid), PEO: poly(ethylene oxide), PVA: poly(vinyl alcohol), PHEMA: poly(hydroxyl-ethyl methacrylate), IPN: interpenetrating polymeric network, SMC: smooth muscle cell, Dex-MA-LA: methacrylated dextran-graft-lysine, Gel-MA: methacrylamide-modified gelatin
As described in section 2.6., hydrogels based on natural polymers have demonstrated many shortcomings including the difficulty of purification, immunogenicity and pathogen transmission. Although some of these shortcomings can be overcome, greater control over material characteristics and tissue responses are achievable when using hydrogels based on synthetic analogs.
Self-assembled peptides (SAPs)-based hydrogels for tissue engineering
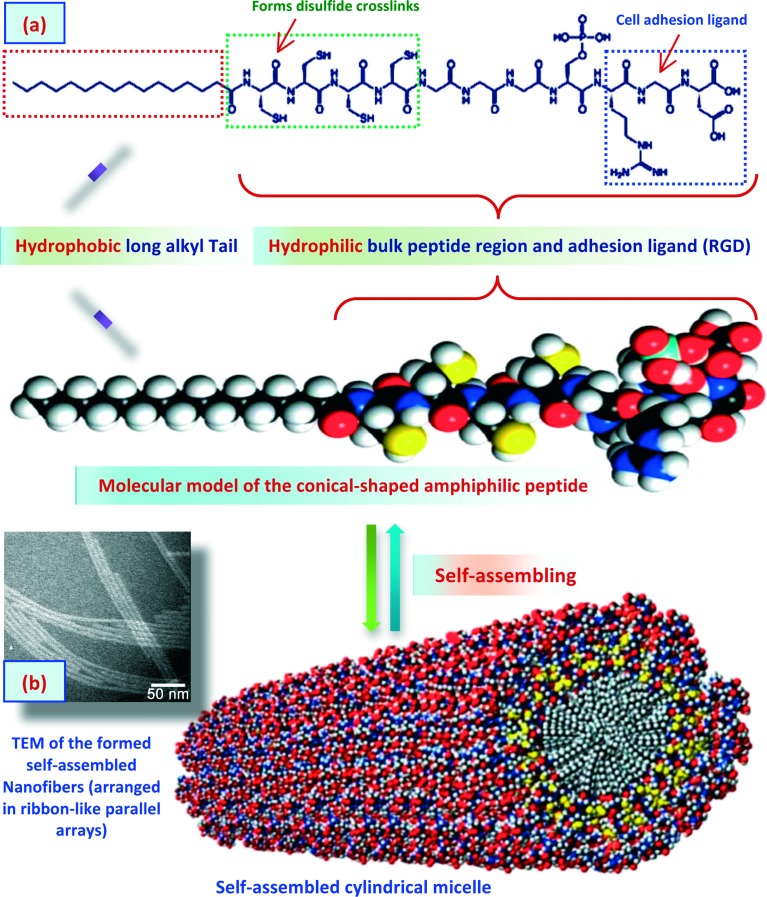
Hydrogel scaffolds based on self-assembled peptides (SAPs) are one of the main classes in tissue engineering applications. SAPs are polypeptides that undergo self-assembly under specific conditions, typically a hydrophilic environment, to form fibers or other types of nanostructures.13,130–133Figure 9 shows, for instance, a schematic illustration of the self-assembling of amphiphilic peptide molecules. These amphiphilic molecules comprise a polypeptide linked to a long chain alkyl tail and also functionalized with cell adhesion ligand (RGD). The polypeptide represents the hydrophilic region of the amphiphilic molecule whereas the long chain alkyl part represents the hydrophobic region. These peptide-based amphiphilic molecules undergo self-assembly into a fibrous crosslinked hydrogel scaffold (arranged in ribbon-like parallel arrays). A variety of amphiphilic SAPs-based hydrogels have been used in various tissue engineering applications.130,134,135 These SAPs-based hydrogels can also be used to incorporate bioactive molecules and allow their controlled release. SAPs-based hydrogels can also be chemically conjugated to different moieties to allow signaling to cell surface receptors and to enhance cellular adhesion. For instance, SAPs-based hydrogels have been attached to fibronectin and laminin peptide domains.135 A further class of SAPs-based hydrogels has been developed by Zhang et al.136,137 In this class, the synthesized functionalized-peptides were self-assembled into beta sheets, which subsequently converted into hydrogels. The results of the studies showed that these developed SAPs-based hydrogels are very promising in generating 3D environments for cell culture and tissue engineering applications.
Figure 9. .
Schematic illustration of the self-assembled peptide-amphiphiles (SAPs) functionalized with cell adhesion ligand (RGD) into fibrous crosslinked hydrogel scaffold for bone tissue engineering applications. Images were adapted with modification from Hartgerink et al.134
In spite of the many superior advantages of using SAPs, such as their effectiveness in forming tissue-like hydrogels, the absence of cross-linking agents to remove and the relatively easy functionalization, unfortunately they demonstrate poor mechanical characteristics. Consequently, they cannot be used for tissue engineering applications that require scaffolds with high mechanical integrity.
Fabrication of hydrogel scaffolds for tissue engineering
Emulsification
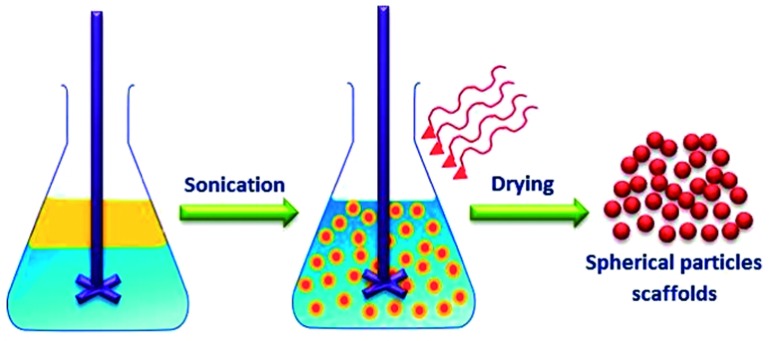
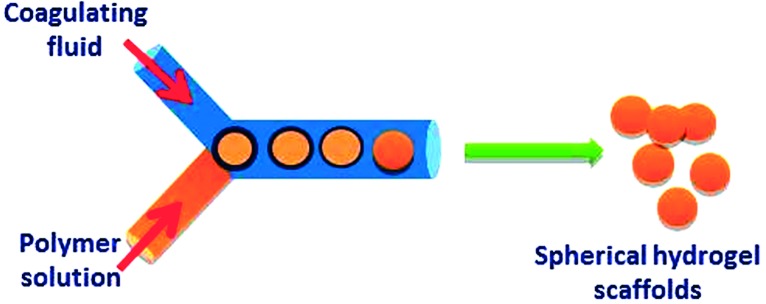
Emulsification is the most commonly used technique for fabricating hydrogel nano- and microparticles as illustrated in (Figure 10). Emulsification process involves agitation of a multi-phase mixture to generate small aqueous droplets of hydrogel precursors within a hydrophobic medium (such as oil or organic solvent). The droplets size can be controlled by the viscosity of the hydrogel precursor, the extent of mechanical agitation and through using of surfactants that can control the surface tension between the two phases as well as preventing aggregation of the resulting hydrogel particles. The hydrogel precursor droplets can be crosslinked using different crosslinking mechanisms to produce spherical nano- or microgels.
Figure 10. .
Schematic illustration of emulsification technique for fabrication of hydrogel particles scaffolds for tissue engineering applications.
Emulsification can be utilized to develop gel particles from a wide range of natural and synthetic polymers such as chitosan, polylactic acid, polylactic-co-glycolic acid, collagen, agarose and alginate. Cell-laden gel particles can be fabricated through the addition of cells to the aqueous phase containing the hydrogel precursor.138 Emulsification can be also used to encapsulate embryonic stem cells (ESCs) within hydrogel microparticles as an in vitro culture, to develop more controllable environments for differentiation.139 The main advantage of the emulsification process is the ease with which it can be used to develop gel particles. However, emulsification also has a number of potential limitations. For instance, the shape of the fabricated gels is limited to spheres and in spite of the ability to control the resulting sizes, there will always be a wide particle size distribution.
Lyophilization
Lyophilization (freeze-drying) depends on the rapid cooling of a sample to produce thermodynamic instability within it, leading to a kind of phase separation. This is followed by [under vacuum] sublimation of the solvent, leaving behind voids and pores. This approach has been used extensively for production of porous hydrogel matrices for tissue engineering. For instance, Wu et al.140 have reported the preparation and assessment of collagen-chitosan hydrogel scaffolds crosslinked with glutaraldehyde for adipose tissue engineering. Both in vitro and in vivo characterizations demonstrated that the developed collagen-chitosan hydrogel scaffolds seeded by preadipocytes cells were biocompatible, induced vascularization and were able to form adipose tissue. In another study, agarose hydrogel scaffolds with linear porous channels were fabricated using a modified lyophilization method.141 In this method, one end of a pillar of agarose was subjected to a block of dry ice immersed within a pool of liquid nitrogen. Hence, the resulting uni-axial temperature gradient led to the formation of ice crystals, oriented in the direction of the gradient. Then, upon sublimation of water during the second step of the lyophilization process, a highly linear network of porous channels was produced with dimensions suitable for cell infiltration. These agarose-based hydrogel scaffolds fabricated with this procedure showed promising axonal regeneration in a spinal cord injury model.142
Recently, an alternate lyophilization method was also reported by Ricciardi et al.143 for developing hydrogel scaffolds. This method involves repeated freeze–thaw cycles to avoid the incomplete phase separation occurring during the initial freezing step which may lead to formation of a polymer-lean phase inside the scaffold. Repeating the freezing step allows further phase separation of the polymer-lean phase within the matrix pores, forming a new more diluted polymer-lean phase and a more concentrated polymer-rich phase, and consequently allowing larger pores formation.
In spite of the suitability of lyophilization for fabrication of porous scaffolds, this technique demonstrates a difficulty in precisely tuning pores size, needs relatively long processing time, and results in relatively poor mechanical characteristics. In addition, lyophilization often leads to the formation of a surface skin, due to matrix collapse at the scaffold–air interface as a result of the interfacial tension change that occurs during solvent evaporation.144
Emulsification-lyophilization
In this fabrication technique, polymeric emulsions composed of dispersed aqueous phase and organic dispersion medium or vice versa, with a suitable biodegradable polymer dissolved in the dispersed phase, are lyophilized to produce porous biodegradable scaffolds with different pore sizes and inter-connectivities. This method was utilized to develop scaffolds with porosity extent of about 95% and pore size of up to 200 μm.145
Solvent casting-leaching
Solvent casting–leaching can be considered as the simplest technique for developing porous scaffolds with almost uniform pore size.146 The procedure includes the casting of an organic polymer solution containing a crosslinker and salt particulates, followed by solvent evaporation and dissolution of the entrapped salt particulates in water. This approach however, has some shortcomings, in that the resulting scaffold might contain residual salt particulates. This technique can also only be used to develop thin film scaffolds. Production of thin scaffold membranes with an open-cell morphology and relatively high porosity (up to 93%) has been reported using this technique.147 For developing a 3D scaffold, porous scaffold membranes were laminated as multi-layers with different anatomical structures.147
Gas foaming-leaching
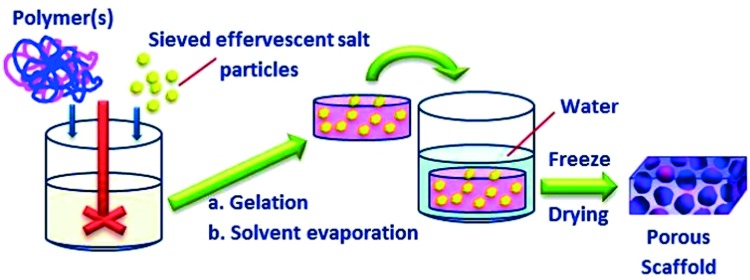
In this method, an effervescent salt is used as a gas foaming agent to develop the porous structure of the scaffolds. As illustrated in (Figure 11), a polymeric gel containing homogeneously dispersed salt particles such as ammonium bicarbonate is cast in a suitable mold, followed by immersion in hot water. The evolution of carbon dioxide and ammonia gases, followed by the leaching out of residual ammonium bicarbonate particles from the solidifying hydrogel lead to the formation of a porous matrix with high interconnectivity. The scaffolds resulting from this approach showed a macro-porous open cellular structure with uniform pore sizes in the range of 100 to 200 μm.148 The method was further modified by adding acidic salt, citric acid, into the hot water before immersing the gel mold.149 This citric acid salt reacts with the ammonium bicarbonate, facilitating evolution of the gases and resulting in the production of macro-porous scaffolds with more than 90% porosity and pore sizes of 200 μm. In this modified method, both porosity and mechanical characteristics could be controlled via adjusting the rate of evolution of the foaming gases through controlling the reaction rate between the two salts. Using the same fabrication approach, injectable highly open porous microspheres scaffolds with average particle size of 250 μm were also developed.150 These injectable scaffolds had average pore size of 30 μm, which allowed promising cell infiltration and seeding, as demonstrated by cultivation with fibroblasts.
Figure 11. .
Schematic illustration of gas foaming-leaching technique for fabrication of porous hydrogel scaffolds for tissue engineering applications.
Photolithography
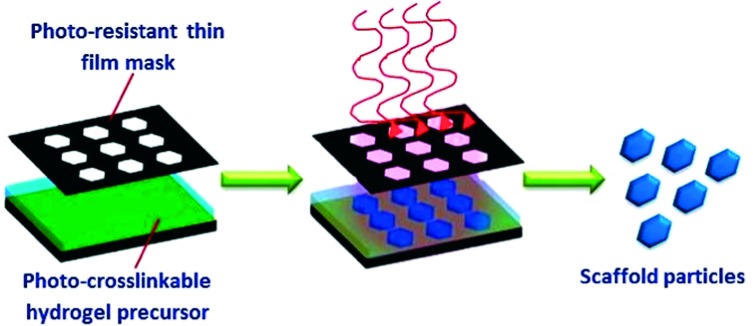
The photolithography technique was developed particularly for micro- and nano-electronics applications. More recently, photolithography has been utilized for a variety of biomedical applications to develop micro-engineered hydrogel scaffolds. This has been partly enabled by the preparation of different natural and synthetic photo-crosslinkable polymers that can undergo crosslinking to form hydrogels.151 Photolithography depends on the exposure of a thin film of photo-crosslinkable polymer to UV light through a mask as shown in (Figure 12). Then, as the light reaches the photo-sensitive polymer through the transparent areas of the mask it causes photoreactions that crosslink the polymer.
Figure 12. .
Schematic illustration of photolithography technique for fabrication of hydrogel scaffolds for tissue engineering applications.
Recently, other similar techniques have also been utilized to develop hydrogel matrices through focusing and scanning light. For instance, the development of photo-crosslinkable matrices that utilize blue light for crosslinking has further advanced and enhanced the safety of this technology for tissue engineering purposes.49 Also, in a similar photolithography technique known as laser scanning lithography, a laser light has been applied to crosslink photo-sensitive hydrophilic polymers in specific sites.152 With the aid of similar strategies, it was possible to construct 3D complex tissue scaffolds one layer at a time.153 Moreover, the use of focused light to conjugate bioactive moieties to and/ or within pre-fabricated hydrogel scaffolds was also reported.154 For instance, this method has been used to pattern photo-active RGD peptides within agarose-based hydrogel, to develop adhesive pathways that enabled directed cell migration into the hydrogel.
In spite of the significance of photolithography as a fabrication technique for hydrogel scaffolds, it has some potential disadvantages that include the necessity for photo-crosslinkable polymers and the harmful effects of UV light on cell function, in addition to the cytotoxicity associated with the use of photoinitators. Moreover, because photolithography is essentially a 2D method, it develops matrices that may require further assembly to generate 3D scaffolds.
Electrospinning
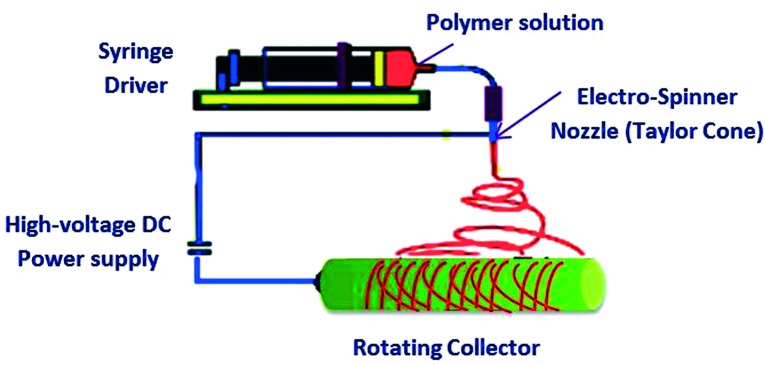
Electrospinning is one of the most significant techniques used for the fabrication of interconnected porous scaffolds for tissue engineering purposes. As schematically illustrated in (Figure 13), the electrospinning technique depends in particular on the use of an external electric field to draw microfibers from a charged polymer solution at the end of a capillary tube. Charging of the polymer is achieved through the application of a high voltage, and is then drawn as a thin jet filament toward an oppositely charged plate or rotating collector according to the desired orientation of the collected fibers.155 The characteristics of the resulting fibers such as diameter, morphology and porosity can be controlled through adjusting the processing parameters including the applied voltage, temperature, polymer solution viscosity and conductivity.156
Figure 13. .
Schematic illustration of the basic electrospinning setup.
Electrospinning has been utilized to develop various types of hydrogel scaffolds such as the ultrafine (submicron) porous fibrous hydrogels based on a combination of polyacrylic acid and polyvinyl alcohol (PVA).157 In these fabricated fibrous hydrogel scaffolds, the majority of the interfiber pores were inter-connected, which is advantageous for tissue engineering to allow cell–cell interaction and migration.157 In another study, a combination of electrospinning and salt-leaching methods was used to fabricate collagen-hyaluronic acid hydrogel nanofibers.158 In this study, NaCl salt particles were used to induce interfiber porosity. In vitro studies revealed that the fabricated collagen-hyaluronic acid hydrogel fibers could support adhesion, proliferation and retention of the in vivo morphology of bovine chondrocyte cells.158 In other reported investigations, various nano- and micro-nonwoven hydrogels based on natural polymers such as fibroin, fibrinogen, silk, and collagen have been fabricated for tissue engineering purposes with the aid of electrospinning.159 It was also reported that different types of cells can attach, proliferate and differentiate within these kinds of fibrous hydrogel scaffolds, demonstrating their potential in tissue engineering applications.155 However, using electrospinning to develop hydrogel scaffolds for tissue engineering is still limited due to many drawbacks, such as limited control over the porosity and pore size, relatively bad mechanical properties and, in particular, the inability of this technique to fabricate 3D hydrogel scaffolds.160
Microfluidic
The microfluidic technique was used to create a variety of hydrogel microstructures, through the creation of single or multi-phase flows within microfluidic channels. In most cases, cells, and the selected polymeric hydrogel precursors, are allowed to flow through microchannels that control the resulting shape of the developed hydrogel as shown in (Figure 14).161 By layering these cell-loaded microgels on each other, complex 3D structures can be developed in which multiple types of cells can be patterned relative to each other to recreate tissue-like complexity. Also, two-phase systems composed of hydrophilic droplets in a hydrophobic medium were used to develop hydrogel droplets with controllable physicochemical characteristics.
Figure 14. .
Schematic illustration of microfluidic technique.
A combination of microfluidics and photolithography has been used recently to engineer unique hydrogel scaffolds.162,163 In this study, microfluidic channels were used to produce micro-engineered hydrogel scaffolds, through the exposure of a stream of polymeric gel precursors to light that passed through a pre-designed mask and was focused with a microscope.162 As the fluid was exposed to the light, the polymeric precursors were crosslinked to produce microgels that were then collected at the outlets of the microchannels. With this combined fabrication approach, it was possible to encapsulate cells in hydrogel scaffolds of carefully controlled shapes.162,163
Micromolding
Micromolding is another fabrication technique that is capable of generating hydrogels with controlled size and porosity. This technique has become particularly attractive with the development of soft lithography, which has enabled the easy fabrication of molds based on poly(dimethyl siloxane) from prefabricated silicon wafers, and also the development of fluoro-based micro-mold nanoscale particles of controlled structures.164 In this technique, to develop a micro-molded hydrogel, polymeric hydrogel precursors are initially molded and subsequently gelled to produce structures of variety of shapes, morphologies and sizes.165–167 Micromolding has been used to fabricate micro-engineered hydrogels from different polymers such as chitosan,165 PEG168 and hyaluronic acid.166 However, this technique was unable to fabricate micro-engineered hydrogels of controlled features from polymers such as fibrin and alginates that require the addition of gelling agents such as divalent and polyvalent ions. To avoid this shortcoming, a new method has been applied which includes micro-molding of alginate hydrogels using other gels as templates.169 In this method, the polymeric gel precursor is initially formed using the hydrogel mold, followed by adding a crosslinking agent across the mold to crosslink the resulting matrices into a new gel. Using this procedure, alginate microstructures were fabricated and have revealed a promising ability to encapsulate cells within controlled structures. Micromolding has also been a successful technique in developing 3D interconnected macro-porous hydrogel matrices, where the hydrogel structures were formed around a packed bed of polymeric beads that were subsequently dissolved.170 Hydrogels with microfluidic channels within their structures were developed with the aid of a dissolvable gelatin-based template.171
Three-dimensional organ/tissue printing
Three-dimensional (3D) organ/tissue printing is a novel approach in tissue engineering and it is based on layered strand- or dropwise deposition of cell-laden hydrogels.172,173 This rapid prototyping-derived technique is beneficial in developing 3D scaffolds with a predesigned external shape and internal morphology, and also with definite cell placement.
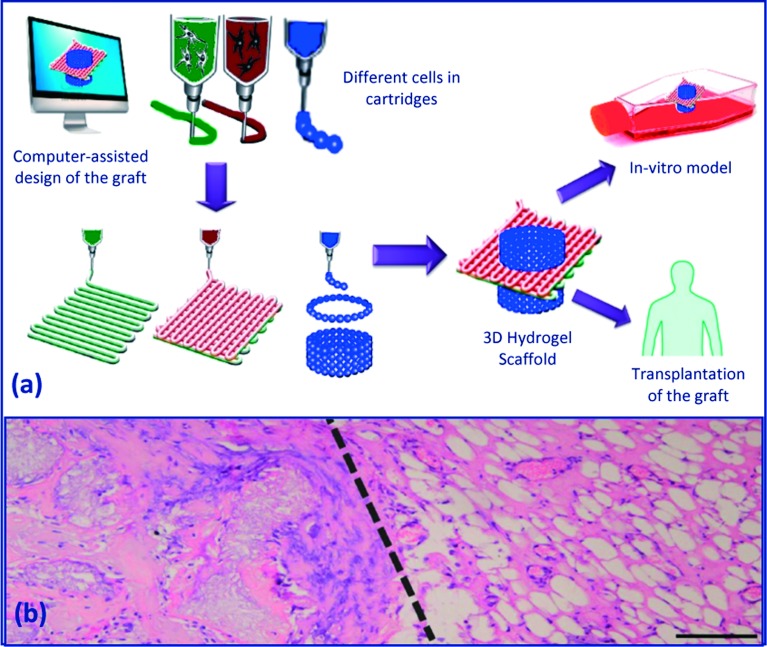
In this technique (Figure 15), a computer-aided design of the implant is translated by the rapid prototyping machine into a layered, cell-laden hydrogel construct with predefined external shape and internal morphology, for the use either in an in vitro model or in vivo grafting. This can be achieved via using multiple printing heads, each containing a specific cell type and/or hydrogel, which enables printing of heterogeneous constructs.
Figure 15. .
(a) Organ/tissue printing using fiber deposition. Illustration was adapted with modification from 174, (b) macroscopic view of the dual graft (heterogeneous tissue formation in a printed construct implanted subcutaneously in mice) at 6 weeks; dashed line represents the transition zone between (left) printed MSCs in Matrigel and (right) EPCs in Matrigel/hematoxylin and eosin staining, scale bar = 200 mm. Image was adapted from 175.
In 3D organ/tissue printing, the use of hydrogel scaffolds is important as they provide a support matrix for the embedded cells with a highly hydrated microenvironment that is adjustable to nutrient and oxygen diffusion. In general, the main hydrogel requirements for 3D organ printing involve: (a) non cytotoxicity, (b) preservation of the printed shape and internal morphology after the deposition, (c) conferring adequate stability and mechanical characteristics for in vitro culture and in vivo implantation, (d) preserving cell viability and function, and (e) easy handling of the printed scaffolds. Besides, the hydrogel should ideally provide the embedded cells with the suitable biochemical and physical stimuli to guide various cellular processes such as migration, proliferation, and differentiation. Furthermore, for optimal use in organ printing, fast gelation is essential during stacking of subsequent cell-laden hydrogel layers.
Some of the common hydrogels used for 3D organ/tissue printing include alginates,176,177 collagen, and Pluronics.178 Most of these hydrogels however, showed some shortcomings such as a lack of adhesive/biomimetic sequences, limited mechanical characteristics, and instability in culture. Hydrogels have been used through 3D organ/ tissue printing technique to develop tubular-like structures laden with endothelial cells176 and to design various co-culture systems.179–181
Hydrogel scaffolds for cardiac tissue engineering
Cardiac tissue engineering is an integrated process involving both cells (such as cardiomyocytes and stem cells) and supporting matrices. Because of their softness, viscoelastic nature, and tissue-like characteristics, hydrogels have been used as supporting matrices in cardiac tissue engineering and to deliver cells into infarcted cardiac muscle. These hydrogels not only maintain cells in the infarcted area, but also offer support for restoring myocardial wall stress, and cell survival and functioning.
Hydrogels based on both natural and synthetic polymers are suitable for cardiac tissue engineering. Chitosan, collagen, laminin, gelatin, matrigel, sodium aliginate, and hyaluronic acid (hyaluronan) are the most commonly used natural polymers in developing hydrogels for cardiac tissue engineering applications. These natural polymers have structures very similar to the molecules in biological organisms, thus reducing the possibility of immune response when implanted in vivo. Synthetic polymers used for developing hydrogel matrices for cardiac tissue engineering include polylactide (PLA), polylactide-co-glycolic acid copolymer (PLGA), poly(ethylene glycol) (PEG), polycaprolactone (PCL), polyurethane (PU), and polyacrylamide (PAAm). The use of synthetic polymers is advantageous over natural polymers due to ease of tailoring their physicochemical characteristics, such as water affinity, modulus, and degradation rate, to meet the requirements of cardiac muscle tissue engineering. However, potential cytotoxicity is a major concern upon using synthetic polymers. To date, only PLA, PEG, and PLGA have been approved by the FDA for clinical applications but some other polymers such as PU and PAAm have already been found to be non-toxic in vitro and in vivo.
Over the last decade, many hydrogel matrices have been developed for cardiac tissue engineering purposes. For instance, Singelyn et al.182 have generated injectable porcine myocardium hydrogel, which supported the survival of cardiomyocytes and the migration of ECs and SMCs in vivo. Further in vivo study revealed the infiltration of both the ECs and SMCs into the hydrogel, and showed that vascularization was improved within the hydrogel matrices. However, this in vivo study was performed on a normal rat heart, thus the findings need to be verified using a myocardial infarction (MI) heart model. In another study,183 hollow fibrin hydrogel tubes populated with neonatal cardiomyocytes were implanted into the femoral artery of adult rats. These fibrin hydrogel/cardiomyocytes constructs formed a mature cardiac tissue with a relatively dense capillary network after 3 weeks of implantation. The resulting cardiac tissue demonstrated all normal cardiac functions, including the contractility under electric stimulation and synchronous pacing with an external electric signal. Huang et al. in their recent studies184–186 have embedded rat cardiomyocytes within fibrin hydrogel and found that their contractility can be maintained up to two months with normal pacing ability.
Matrigel, an ECM-mimicking hydrogel produced by mouse Engelbreth-Holm-Swarm tumors, has been used as a delivery carrier to deliver genetically modified human MSCs into an infarcted heart. The study demonstrated that MSCs survival was significantly improved under the ischemic and apoptotic environment.187 Matrigel has also been combined with other natural polymers to improve cell proliferation and angiogenesis in vivo. For instance, Giraud et al. showed that Matrigel/collagen hydrogel significantly enhanced heart function after implantation into acute MI rat hearts.188 Besides, the implantation of Matrigel/collagen hydrogel and H9C2 cardiomyoblasts in an acute rat MI model was found to significantly increase the cell engraftment rate.189 In a relatively recent study, Matrigel was also incorporated into fibrin hydrogel to encapsulate cardiomyocytes. The encapsulated cardiomyocytes were able to retain their normal function for 10 days.190
Synthetic polymer-based hydrogels have also been investigated for cardiac tissue engineering. For instance, Wang et al.191 developed a hydrogel based on PEG derivative (PEG–PCL–PEG triblock copolymer) in a combination with α-cyclodextrin to encapsulate bone marrow MSCs and delivered it to a rabbit MI site. Walker et al. have developed and implanted poly(ethylene terephthalate) (PET) mesh reinforced PHEMA hydrogel into the canine epicardium.192,193 This study demonstrated absence of any significant fibrosis or thickening for 12 months after the implantation. However, a trace calcification was observed on the gel after 9 and 12 months of implantation, raising the concern of biocompatibility of the developed PET/PHEMA constructs over a long timeframe.
Challenges associated with the use of hydrogel scaffolds for tissue engineering
In spite of the relatively successful clinical translation of several engineered tissues, such as lung tracheal segments194 and tissue engineered bladders,195 various significant challenges are still associated with the use of hydrogel scaffolds and also with the applied scaffolding techniques in mimicking natural ECMs.196 Some of these challenges include, for instance; (1) poor cell penetration and irregular cell seeding due to the lack of appropriate spatial and temporal control (despite great advances in scaffold fabrication methods), (2) difficulties associated with engineering complex tissues with multiple cell types and unique ECM composition in spite of the promising success in engineering tissues composed of a single type of cells, (3) the poor mechanical properties of hydrogels at both macroscopic and microscopic levels, which tends to limit their applications to soft and non load-bearing tissues, and (4) the lack of complex microvasculature in most of the engineered tissues, which leads to a considerable loss of both viability and function of the seeded cells due to a resulting deficiency in transportation of nutrients and signaling molecules.
Conclusions
In summary, the scientific research on development, characterization and evaluation of various classes of hydrogels as potential scaffolds for tissue engineering applications has much progressed over the last decade. This recent focus on using hydrogels is mainly attributed to their superior biocompatibility and inherent similarity to ECM. Besides, hydrogels are highly customizable as 2D and 3D networks, with a wide range selection of available natural or synthetic constituents and fabrication techniques.
In this review, an attempt was made, from the materials science point of view, to provide an overview of the different classes of hydrogels, the available approaches for fabricating hydrogel scaffolds with specific features, and the recent discoveries and applications of hydrogels in tissue engineering. Special attention was also given to the various design criteria toward an efficient hydrogel scaffold. The challenges associated with the use of hydrogel scaffolds were also described.
References
- 1.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 2.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 3.Kyung JH, Yeon KS, Jeong KS, Moo LY. pH / temperature-responsive semi-IPN hydrogels composed of alginate and poly(N-isopropylacrylamide) J Appl Polym Sci. 2002;83:128–136. [Google Scholar]
- 4.Wichterle O, Lim D. Hydrophilic gels for biological use. Nature. 1960;185:117–129. [Google Scholar]
- 5.Hoffman AS. Hydrogels for biomedical applications. Ann N Y Acad Sci. 2001;944:62–73. doi: 10.1111/j.1749-6632.2001.tb03823.x. [DOI] [PubMed] [Google Scholar]
- 6.Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50:27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 7.Guenet JM. Thermoreversible gelation of polymers and biopolymers. New York: Academic Press; 1992. p. 89. [Google Scholar]
- 8.In: Markey ML, Bowman ML, Bergamini MY.Chitin and chitosan London: Elsevier Appl. Sci; 1989713 [Google Scholar]
- 9.Klech CM. Gels and jellies. In: Swarbrick J, Boylan JC, editors. Encyclopedia of pharmaceutical technology. Marcel Dekker, New York; 1990. p. 415. [Google Scholar]
- 10.Gehrke SH, Lee PI.Hydrogels for drug delivery systems Tyle P, ed. Specialized drug delivery systems Marcel Dekker, New York; 1990333 [Google Scholar]
- 11.Kunzier JF.Hydrogels Hoboken NJ, ed. Encyclopedia of polymer science and technology vol 2John Wiley & Sons, Hoboken, New Jersey 2003691 [Google Scholar]
- 12.Zhu W, Ding J. Synthesis and characterization of a redox-initiated, injectable, biodegradable hydrogel. J Appl Polym Sci. 2006;99:2375. [Google Scholar]
- 13.Gutowska A, Bae YH, Feijen J, Kim SW. Heparin release from thermosensitive hydrogels. J Control Release. 1992;22:95–104. [Google Scholar]
- 14.Ferreira L, Vidal MM, Gil MH. Evaluation of poly(2-hydroxyethyl methacrylate) gels as drug delivery systems at different pH values. Int J Pharm. 2000;194:169–180. doi: 10.1016/s0378-5173(99)00375-0. [DOI] [PubMed] [Google Scholar]
- 15.D'Emanuele A, Staniforth JN. An electrically modulated drug delivery device: I. Pharm Res. 1991;8:913–918. doi: 10.1023/a:1015815931739. [DOI] [PubMed] [Google Scholar]
- 16.Shantha KL, Harding DRK. Synthesis and evaluation of sucrose-containing polymeric hydrogels for oral drug delivery. J Appl Polym Sci. 2002;84:2597–2604. [Google Scholar]
- 17.Shantha KL, Harding DRK. Synthesis, characterisation and evaluation of poly[lactose acrylate-N-vinyl-2-pyrrolidinone] hydrogels for drug delivery. Eur Polym J. 2003;39:63–68. [Google Scholar]
- 18.El-Sherbiny IM, Lins RJ, Abdel-Bary EM, Harding DRK. Preparation, characterization, swelling and in vitro drug release behaviour of poly[N-acryloylglycine-chitosan] interpolymeric pH and thermally-responsive hydrogels. Eur Polym J. 2005;41:2584–2591. [Google Scholar]
- 19.El-Sherbiny IM, Abdel-Bary EM, Harding DRK. Preparation and swelling study of a pH-dependent interpolymeric hydrogel based on chitosan for controlled drug release. Int J Polym Mater. 2006;55:789–802. [Google Scholar]
- 20.Jabbari E, Nozari S. Swelling behavior of acrylic acid hydrogels prepared by γ-radiation crosslinking of polyacrylic acid in aqueous solution. Eur Polym J. 2000;36:2685–2692. [Google Scholar]
- 21.Rosiak JM, Ulanski P. Synthesis of hydrogels by irradiation of polymers in aqueous solution. Radiat Phys Chem. 1999;55:139–151. [Google Scholar]
- 22.El-Sherbiny IM, Smyth HDC. Poly(ethylene glycol)/carboxymethyl chitosan-based pH-responsive hydrogels: Photo-induced synthesis, characterization, swelling and in-vitro evaluation as potential drug carriers. Carbohydr Res. 2010;345(14):2004–2012. doi: 10.1016/j.carres.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 23.Yamada K, Tabata Y, Yamamoto K, Miyamoto S, Nagata I, Kikuchi H, Ikada Y. Potential efficacy of basic fibroblast growth factor incorporated in biodegradable hydrogels for skull bone regeneration. J Neurosurg. 1997;86:871–875. doi: 10.3171/jns.1997.86.5.0871. [DOI] [PubMed] [Google Scholar]
- 24.Akin H, Hasirci N. Thermal properties of crosslinked gelatin microspheres. Polym Preprint. 1995;36:384–385. [Google Scholar]
- 25.Anal AK, Stevens WF. Chitosan-alginate multilayer beads for controlled release of ampicillin. Int J Pharm. 2005;290:45–54. doi: 10.1016/j.ijpharm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Nakashima T, Takakura K, Komoto Y. Thromboresistance of graft-type copolymers with hydrophilic-hydrophobic microphase-separated structure. J Biomed Mater Res. 1977;11:787–798. doi: 10.1002/jbm.820110512. [DOI] [PubMed] [Google Scholar]
- 27.Cruise GM, Hegre OD, Lamberti FV, Hager SR, Hill R, Scharp DS, Hubbell JA. In vitro and in vivo performance of porcine islets encapsulated in interfacially photopolymerized poly(ethylene glycol) diacrylate membranes. Cell Transplant. 1999;8(3):293–306. doi: 10.1177/096368979900800310. [DOI] [PubMed] [Google Scholar]
- 28.Kraehenbuehl TP, Ferreira LS, Zammaretti P, Hubbell JA, Langer R. Cell-responsive hydrogel for encapsulation of vascular cells. Biomaterials. 2009;30(26):4318–4324. doi: 10.1016/j.biomaterials.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31:5536–5544. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin H, Mikos AG. Biomimetic materials for tissue engineering. Biomaterials. 2003;24:4353–4364. doi: 10.1016/s0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 31.Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24:4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 32.Hill-West JL, Chowdhury SM, Slepian MJ, Hubbell JA. Inhibition of thrombosis and intimal thickening by in situ photopolymerization of thin hydrogel barriers. Proc Natl Acad Sci USA. 1994;91(13):5967–5971. doi: 10.1073/pnas.91.13.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.West JL, Hubbell JA. Separation of the arterial wall from blood contact using hydrogel barriers reduces intimal thickening after balloon injury in the rat: The roles of medial and luminal factors in arterial healing. Proc Natl Acad Sci USA. 1996;93:13188–13193. doi: 10.1073/pnas.93.23.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill-West JL, Dunn RC, Hubbell JA. Local release of fibrinolytic agents for adhesion prevention. J Surg Res. 1995;59:759–763. doi: 10.1006/jsre.1995.1236. [DOI] [PubMed] [Google Scholar]
- 35.Sawhney AS, Pathak CP, van Rensburg JJ, Dunn RC, Hubbell JA. Optimization of photopolymerized bioerodible hydrogel properties for adhesion prevention. J Biomed Mater Res. 1994;28:831–838. doi: 10.1002/jbm.820280710. [DOI] [PubMed] [Google Scholar]
- 36.Bergmann NM, Peppas NA. Molecularly imprinted polymers with specific recognition for macromolecules and proteins. Prog Polym Sci. 2008;33:271–288. [Google Scholar]
- 37.Peppas NA, Kim B. Stimuli-sensitive protein delivery systems. J Drug Del Sci Technol. 2006;16:11–18. [Google Scholar]
- 38.Peppas NA. Intelligent biomaterials as pharmaceutical carriers in microfabricated and nanoscale devices. MRS Bull. 2006;31:888–893. [Google Scholar]
- 39.Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23:4307–4314. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 40.Lu SX, Ramirez WF, Anseth KS. Photopolymerized, multilaminated matrix devices with optimized nonuniform initial concentration profiles to control drug release. J Pharm Sci. 2000;89:45–51. doi: 10.1002/(SICI)1520-6017(200001)89:1<45::AID-JPS5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 41.Guyton A, Hall J. Textbook of medical physiology. 10th ed. Philadelphia, PA: Elsevier Saunders; 2000. p. 1064. [Google Scholar]
- 42.Elisseeff J, Anseth K, Sims D, McIntosh W, Randolph M, Langer R. Transdermal photopolymerization for minimally invasive implantation. Proc Natl Acad Sci USA. 1999;96:3104–3107. doi: 10.1073/pnas.96.6.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res. 2000;51:164–171. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 44.Suggs LJ, Mikos AG. Synthetic biodegradable polymers for medical. An injectable carrier for endothelial cells. Cell Transplant. 1999;8:345–350. doi: 10.1177/096368979900800402. [DOI] [PubMed] [Google Scholar]
- 45.Ratner BD. Biomaterials science: An introduction to materials in medicine. 2nd ed. Amsterdam: Elsevier Academic Press; 2004. p. 851. [Google Scholar]
- 46.Hubbell JA. Materials as morphogenetic guides in tissue engineering. Curr Opin Biotechnol. 2003;14(5):551–558. doi: 10.1016/j.copbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 48.Williams DF. The Williams', dictionary of biomaterials. Liverpool: Liverpool University Press; 1999. [Google Scholar]
- 49.Bryant SJ, Nuttelman CR, Anseth KS. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J Biomater Sci Polym Ed. 2000;11:439–457. doi: 10.1163/156856200743805. [DOI] [PubMed] [Google Scholar]
- 50.Léon CA, Léon Y. New perspectives in mercury porosimetry. Adv Colloid Interface Sci. 1998;76-77:341–372. [Google Scholar]
- 51.Yang S, Leong KF, Du Z, Chua CK. The design of scaffolds for use in tissue engineering—part I: traditional factors. Tissue Eng. 2001;7(6):679–689. doi: 10.1089/107632701753337645. [DOI] [PubMed] [Google Scholar]
- 52.Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004;4(8):743–765. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 53.Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC. Neovascularization of synthetic membranes directed by membrane micro architecture. J Biomed Mater Res. 1995;29:1517–1524. doi: 10.1002/jbm.820291208. [DOI] [PubMed] [Google Scholar]
- 54.Klawitter JJ, Hulbert SF. Application of porous ceramics for the attachment of load-bearing internal orthopedic applications. J Biomed Mater Res A Symposium. 1971;2:161–168. [Google Scholar]
- 55.Yannas IV, Lee E, Orgill DP, Skrabut EM, Murphy GF. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc Natl Acad Sci USA. 1989;86:933–937. doi: 10.1073/pnas.86.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whang K, Healy KE, Elenz DR. Engineering bone regeneration with bioabsorbable scaffolds with novel microarchitecture. Tissue Eng. 1999;5(1):35–51. doi: 10.1089/ten.1999.5.35. [DOI] [PubMed] [Google Scholar]
- 57.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 58.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 59.Bryant SJ, Anseth KS, Lee DA, Bader DL. Crosslinking density influences the morphology of chondrocytes photoencapsulated in PEG hydrogels during the application of compressive strain. J Orthop Res. 2004;22:1143–1149. doi: 10.1016/j.orthres.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 60.Bryant SJ, Chowdhury TT, Lee DA, Bader DL, Anseth KS. Crosslinking density influences chondrocyte metabolism in dynamically loaded photocrosslinked poly(ethylene glycol) hydrogels. Ann Biomed Eng. 2004;32:407–417. doi: 10.1023/b:abme.0000017535.00602.ca. [DOI] [PubMed] [Google Scholar]
- 61.Boyan BD, Hummert TW, Dean DD, Schwartz Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials. 1996;17(2):137–146. doi: 10.1016/0142-9612(96)85758-9. [DOI] [PubMed] [Google Scholar]
- 62.Elbert DL, Hubbell JA. Surface treatments of polymers for biocompatibility. Ann Rev Mater Sci. 1996;26(1):365–394. [Google Scholar]
- 63.Stevens MM, Marini RP, Schaefer D, Aronson J, Langer R, Shastri VP. In vivo engineering of organs. The bone bioreactor. Proc Natl Acad Sci USA. 2005;102:11450–11455. doi: 10.1073/pnas.0504705102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tabata Y, Hijikata S, Ikada Y. Enhanced vascularization and tissue granulation by basic fibroblast growth factor impregnated in gelatin hydrogels. J Control Release. 1994;31:189–199. [Google Scholar]
- 65.Lee KY, Peters MC, Mooney DJ. Comparison of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in SCID mice. J Control Release. 2003;87:49–56. doi: 10.1016/s0168-3659(02)00349-8. [DOI] [PubMed] [Google Scholar]
- 66.Lee KY, Peters MC, Anderson KW, Mooney DJ. Controlled growth factor release from synthetic extracellular matrices. Nature. 2000;408:998–1000. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- 67.Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, Davies N, Schmokel H, Bezuidenhout D, Djonov V, Zilla P, Hubbell JA. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J. 2003;17:2260–2262. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 68.Peattie RA, Nayate AP, Firpo MA, Shelby J, Fisher RJ, Prestwich JD. Stimulation of In-vivo angiogenesis by cytokine loaded hyaluronic acid hydrogel implants. Biomaterials. 2004;25:2789–2798. doi: 10.1016/j.biomaterials.2003.09.054. [DOI] [PubMed] [Google Scholar]
- 69.Peattie RA, Rieke E, Hewett E, Fisher RJ, Shu XZ, Prestwich GD. Dual growth factor-induced angiogenesis in vivo using hyaluronan hydrogel implants. Biomaterials. 2006;27:1868–1875. doi: 10.1016/j.biomaterials.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 70.Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:11298–11303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shu XZ, Ghosh K, Liu YC, Palumbo FS, Luo Y, Clark RA, Prestwich GD. Attachment and spreading of fibroblasts on an RGD peptide-modified injectable hyaluronan hydrogel. J Biomed Mater Res A. 2004;68:365–375. doi: 10.1002/jbm.a.20002. [DOI] [PubMed] [Google Scholar]
- 72.Pape LG, Balazs EA. The use of sodium hyaluronate (Healon®) in human anterior segment surgery. Ophthalmology. 1980;87:699–705. doi: 10.1016/s0161-6420(80)35185-3. [DOI] [PubMed] [Google Scholar]
- 73.Duranti F, Salti G, Bovani G, Calandra M, Rosati ML. Injectable hyaluronic acid gel for soft tissue augmentation. A clinical and histological study. Dermatol Surg. 1998;24:1317–1325. doi: 10.1111/j.1524-4725.1998.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 74.Burns JM, Skinner K, Colt J, Sheidlin A, Bronson R, Yaacobi Y, Goldberg EP. Prevention of tissue injury and postsurgical adhesions by precoating tissues with hyaluronic acid solutions. J Surg Res. 1995;59:644–652. doi: 10.1006/jsre.1995.1218. [DOI] [PubMed] [Google Scholar]
- 75.Ghosh KK, Ren XD, Shu XZ, Prestwich GD, Clark RAF. Fibronectin functional domains coupled to hyaluronan stimulate adult human dermal fibroblast responses critical for wound healing. Tissue Eng. 2006;12:601–613. doi: 10.1089/ten.2006.12.601. [DOI] [PubMed] [Google Scholar]
- 76.Sargeant TD, Guler MO, Oppenheimer SM, Mata A, Satcher RL, Dunand DC, Stupp SI. Hybrid bone implants: Self-assembly of peptide amphiphile nanofibers within porous titanium. Biomaterials. 2008;29:161–171. doi: 10.1016/j.biomaterials.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 77.Shu XZ, Ahmad S, Liu YC, Prestwich GD. Synthesis and evaluation of injectable, in situ crosslinkable synthetic extracellular matrices for tissue engineering. J Biomed Mater Res A. 2006;79:902. doi: 10.1002/jbm.a.30831. [DOI] [PubMed] [Google Scholar]
- 78.Dare EV, Vascotto SG, Carlsson DJ, Hincke MT, Griffith M. Differentiation of a fibrin gel encapsulated chondrogenic cell line. Int J Artif Organs. 2007;30:619–627. doi: 10.1177/039139880703000710. [DOI] [PubMed] [Google Scholar]
- 79.Ryu JH, Kim IK, Cho MC, Hwang KK, Piao H, Piao S, Lim SH, Hong YS, Choi CY, Yoo KJ, Kim BS. Implantation of bone marrow mononuclear cells using injectable fibrin matrix enhances neovascularization in infarcted myocardium. Biomaterials. 2005;26:319–326. doi: 10.1016/j.biomaterials.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 80.Currie LJ, Sharpe JR, Martin R. The use of fibrin glue in skin grafts and tissue-engineered skin replacements: a review. Plast Reconstr Surg. 2001;108:1713–1726. doi: 10.1097/00006534-200111000-00045. [DOI] [PubMed] [Google Scholar]
- 81.Park SH, Park SR, Chung SI, Pai KS, Min BH. Tissue-engineered cartilage using fibrin/hyaluronan composite gel and its in vivo implantation. Artif Organs. 2005;29:838–845. doi: 10.1111/j.1525-1594.2005.00137.x. [DOI] [PubMed] [Google Scholar]
- 82.Stevens MM, Qanadilo HF, Langer R, Shastri VP. A rapid-curing alginate gel system: utility in periosteum-derived cartilage tissue engineering. Biomaterials. 2004;25:887–894. doi: 10.1016/j.biomaterials.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 83.Chang SCN, Rowley JA, Tobias G, Genes NG, Roy AK, Mooney DJ, Vacanti CA, Bonassar LJ. Injection molding of chondrocyte/alginate constructs in the shape of facial implants. J Biomed Mater Res. 2001;55:503–511. doi: 10.1002/1097-4636(20010615)55:4<503::aid-jbm1043>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 84.Willers C, Chen J, Wood D, Zheng MH. Autologous chondrocyte implantation with collagen bioscaffold for the treatment of osteochondral defects in rabbits. Tissue Eng. 2005;11:1065–1076. doi: 10.1089/ten.2005.11.1065. [DOI] [PubMed] [Google Scholar]
- 85.Thacharodi D, Rao KP. Rate-controlling biopolymer membranes as transdermal delivery systems for nifedipine: development and in vitro evaluations. Biomaterials. 1996;17:1307–1311. [PubMed] [Google Scholar]
- 86.Itoh S, Takakuda K, Kawabata S, Aso Y, Kasai K, Itoh H, Shinomiya K. Evaluation of cross-linking procedures of collagen tubes used in peripheral nerve repair. Biomaterials. 2002;23:4475–4481. doi: 10.1016/s0142-9612(02)00166-7. [DOI] [PubMed] [Google Scholar]
- 87.Joosten EAJ, Veldhuis WB, Hamers FBT. Collagen containing neonatal astrocytes stimulates regrowth of injured fibers and promotes modest locomotor recovery after spinal cord injury. J Neurosci Res. 2004;77:127–142. doi: 10.1002/jnr.20088. [DOI] [PubMed] [Google Scholar]
- 88.Hahn MS, Teply BA, Stevens MM, Zeitels SM, Langer R. Collagen composite hydrogels for vocal fold lamina propria restoration. Biomaterials. 2006;27:1104–1109. doi: 10.1016/j.biomaterials.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 89.Liao E, Yaszemski M, Krebsbach P, Hollister S. Tissue-engineered cartilage constructs using composite hyaluronic acid/collagen I hydrogels and designed poly(propylene fumarate) scaffolds. Tissue Eng. 2007;13:537–550. doi: 10.1089/ten.2006.0117. [DOI] [PubMed] [Google Scholar]
- 90.Gong YH, He LJ, Li J, Zhou QL, Ma ZW, Gao CY, Shen JC. Hydrogel-filled polylactide porous scaffolds for cartilage tissue engineering. J Biomed Mater Res B. 2007;82:192–198. doi: 10.1002/jbm.b.30721. [DOI] [PubMed] [Google Scholar]
- 91.Tang SQ, Vickers SM, Hsu HP, Spector M. Fabrication and characterization of porous hyaluronic acid-collagen composite scaffolds. J Biomed Mater Res A. 2007;82:323–335. doi: 10.1002/jbm.a.30974. [DOI] [PubMed] [Google Scholar]
- 92.Magnani A, Rappuoli R, Lamponi S, Barbucci R. Novel polysaccharide hydrogels: characterization and properties. Polym Adv Technol. 2000;11:488–495. [Google Scholar]
- 93.Duflo S, Thibeault SL, Li WH, Shu XZ, Prestwich GD. Vocal fold tissue repair in vivo using a synthetic extracellular matrix. Tissue Eng. 2006;12:2171–2180. doi: 10.1089/ten.2006.12.2171. [DOI] [PubMed] [Google Scholar]
- 94.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 95.Kirker KR, Luo Y, Nielson JH, Shelby J, Prestwich GD. Glycosaminoglycan hydrogel films as biointeractive dressings for wound healing. Biomaterials. 2002;23:3661–3671. doi: 10.1016/s0142-9612(02)00100-x. [DOI] [PubMed] [Google Scholar]
- 96.Chang C, Peng N, He M, Teramoto Y, Nishio Y, Zhang L. Fabrication and properties of chitin/hydroxyapatite hybrid hydrogels as scaffold nano-materials. Carbohydr Polym. 2013;91(1):7–13. doi: 10.1016/j.carbpol.2012.07.070. [DOI] [PubMed] [Google Scholar]
- 97.Park KM, Yang JA, Jung H, Yeom J, Park JS, Park K-H, Hoffman AS, Hahn SK, Kim K. In situ supramolecular assembly and modular modification of hyaluronic acid hydrogels for 3d cellular engineering. ACS Nano. 2012;6(4):2960–2968. doi: 10.1021/nn204123p. [DOI] [PubMed] [Google Scholar]
- 98.Rossi F, Perale G, Storti G, Masi M. A library of tunable agarose carbomer-based hydrogels for tissue engineering applications: The role of cross-linkers. J Appl Polym Sci. 2012;123(4):2211–2221. [Google Scholar]
- 99.Buehler MJ. Molecular nanomechanics of nascent bone: fibrillar toughening by mineralization. Nanotechnology. 2007;18:295102. [Google Scholar]
- 100.Burdick JA, Anseth KS. Photoencapsulation of osteoblasts in injectable rgd-modified peg hydrogels for bone tissue engineering. Biomaterials. 2002;23:4315–4323. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 101.Burdick JA, Mason MN, Hinman AD, Thorne K, Anseth KS. Delivery of osteoinductive growth factors from degradable peg hydrogels influences osteoblast differentiation and mineralization. J Control Release. 2002;83:53–63. doi: 10.1016/s0168-3659(02)00181-5. [DOI] [PubMed] [Google Scholar]
- 102.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, Hubbell JA. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. Proc Natl Acad Sci USA. 2003;100:5413–5418. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kraehenbuehl TP, Zammaretti P, Van der Vlies AJ, Schoenmakers RG, Lutolf MP, Jaconi ME, Hubbell JA. Three-dimensional extracellular matrix-directed cardioprogenitor differentiation: systematic modulation of a synthetic cell-responsive PEG-hydrogel. Biomaterials. 2008;29:2757–2766. doi: 10.1016/j.biomaterials.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 104.Elisseeff J, McIntosh W, Fu K, Blunk T, Langer R. Controlled-release of IGF-I and TGF-beta1 in a photopolymerizing hydrogel for cartilage tissue engineering. J Orthop Res. 2001;19:1098–1104. doi: 10.1016/S0736-0266(01)00054-7. [DOI] [PubMed] [Google Scholar]
- 105.Hwang NS, Varghese S, Theprungsirikul P, Canver A, Elisseeff J. Enhanced chondrogenic differentiation of murine embryonic stem cells in hydrogels with glucosamine. Biomaterials. 2006;27:6015–6023. doi: 10.1016/j.biomaterials.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 106.Lee HJ, Lee JS, Chansakul T, Yu C, Elisseeff JH, Yu SM. Collagen mimetic peptide-conjugated photopolymerizable PEG hydrogel. Biomaterials. 2006;27:5268–5276. doi: 10.1016/j.biomaterials.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 107.Yang F, Williams CG, Wang DA, Lee H, Manson PN, Elisseeff J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials. 2005;26:5991–5998. doi: 10.1016/j.biomaterials.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 108.Varghese S, Hwang NS, Canver AC, Theprungsirikul P, Lin DW, Elisseeff J. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol. 2008;27:12–21. doi: 10.1016/j.matbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 109.Park Y, Lutolf MP, Hubbell JA, Hunziker EB, Wong M. Bovine primary chondrocyte culture in synthetic matrix metalloproteinase-sensitive poly(ethylene glycol)-based hydrogels as a scaffold for cartilage repair. Tissue Eng. 2004;10:515–522. doi: 10.1089/107632704323061870. [DOI] [PubMed] [Google Scholar]
- 110.Bryant SJ, Chowdhury TT, Lee DA, Bader DL, Anseth KS. Cross-linking density influences chondrocyte metabolism in dynamically loaded photocrosslinked PEG hydrogels. Ann Biomed Eng. 2004;32:407–417. doi: 10.1023/b:abme.0000017535.00602.ca. [DOI] [PubMed] [Google Scholar]
- 111.Williams CG, Kim TK, Taboas A, Malik A, Manson P, Elisseeff J. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng. 2003;9:679–688. doi: 10.1089/107632703768247377. [DOI] [PubMed] [Google Scholar]
- 112.Elisseeff J, Anseth K, Sims D, McIntosh W, Randolph M, Yaremchuk M, Langer R. Transdermal photopolymerization of poly(ethylene oxide)-based injectable hydrogels for tissue-engineered cartilage. Plast Reconstr Surg. 1999;104:1014–1022. doi: 10.1097/00006534-199909040-00017. [DOI] [PubMed] [Google Scholar]
- 113.Sharma B, Williams CG, Khan M, Manson P, Elisseeff JH. In vivo chondrogenesis of mesenchymal stem cells in a photopolymerized hydrogel. Plast Reconstr Surg. 2007;119:112–120. doi: 10.1097/01.prs.0000236896.22479.52. [DOI] [PubMed] [Google Scholar]
- 114.Chowdhury SM, Hubbell JA. Adhesion prevention with ancrod release via a tissue-adherent hydrogel. J Surg Res. 1996;61:58–64. doi: 10.1006/jsre.1996.0081. [DOI] [PubMed] [Google Scholar]
- 115.Cruise GM, Hegre OD, Lamberti FV, Hager SR, Hill R, Scharp DS, Hubbell JA. In vitro and in vivo performance of porcine islets encapsulated in interfacially photopolymerized poly(ethylene glycol) diacrylate membranes. Cell Transplant. 1999;8:293–306. doi: 10.1177/096368979900800310. [DOI] [PubMed] [Google Scholar]
- 116.Adelow C, Segura T, Hubbell JA, Frey P. The effect of enzymatically degradable poly(ethylene glycol) hydrogels on smooth muscle cell phenotype. Biomaterials. 2008;29:314–326. doi: 10.1016/j.biomaterials.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 117.Mann BK, Gobin AS, Tsai AT, Schmedlen RH. West Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: synthetic ECM analogs for tissue engineering. Biomaterials. 2001;22:3045–3051. doi: 10.1016/s0142-9612(01)00051-5. [DOI] [PubMed] [Google Scholar]
- 118.Seliktar D, Zisch AH, Lutolf MP, Wrana JL, Hubbell JA. MMP-2 sensitive, VEGF-bearing bioactive hydrogels for promotion of vascular healing. J Biomed Mater Res A. 2004;68:704–716. doi: 10.1002/jbm.a.20091. [DOI] [PubMed] [Google Scholar]
- 119.An YJ, Hubbell JA. Intraarterial protein delivery via intimally-adherent bilayer hydrogels. J Control Release. 2000;64:205–215. doi: 10.1016/s0168-3659(99)00143-1. [DOI] [PubMed] [Google Scholar]
- 120.Li H, Davison N, Moroni L, Feng F, Crist J, Salter E, Bingham CO, Elisseeff J. Evaluating osteoarthritic chondrocytes through a novel 3-dimensional in vitro system for cartilage tissue engineering and regeneration. Cartilage. 2012;3(2):128–140. doi: 10.1177/1947603511429698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bryant SJ, Durand KL, Anseth KS. Manipulations in hydrogel chemistry control photoencapsulated chondrocyte behavior and their extracellular matrix production. J Biomed Mater Res A. 2003;67:1430–1436. doi: 10.1002/jbm.a.20003. [DOI] [PubMed] [Google Scholar]
- 122.Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002;59:63–72. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 123.Cruise GM, Hegre OD, Scharp DS, Hubbell JA. A sensitivity study of the key parameters in the interfacial photopolymerization of poly(ethylene glycol) diacrylate upon porcine islets. Biotechnol Bioeng. 1998;57:655–665. doi: 10.1002/(sici)1097-0290(19980320)57:6<655::aid-bit3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 124.Martens PJ, Bryant SJ, Anseth KS. Tailoring the degradation of hydrogels formed from multivinyl poly(ethylene glycol) and poly(vinyl alcohol) macromers for cartilage tissue engineering. Biomacromolecules. 2003;4:283–292. doi: 10.1021/bm025666v. [DOI] [PubMed] [Google Scholar]
- 125.Belkas JS, Munro CA, Shoichet MS, Johnston M, Midha R. Long-term in vivo biomechanical properties and biocompatibility of poly(2-hydroxyethyl methacrylate-co-methyl methacrylate) nerve conduits. Biomaterials. 2005;26:1741–1749. doi: 10.1016/j.biomaterials.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 126.Vijayasekaran S, Chirila TV, Robertson TA, Lou X, Fitton JH, Hicks CR, Constable IJ. Calcification of poly(2-hydroxyethyl methacrylate) hydrogel sponges implanted in the rabbit cornea: a 3-month study. J Biomater Sci Polym Ed. 2000;11:599–615. doi: 10.1163/156856200743896. [DOI] [PubMed] [Google Scholar]
- 127.Bryant SJ, Cuy JL, Hauch KD, Ratner BD. Photo-patterning of porous hydrogels for tissue engineering. Biomaterials. 2007;28:2978–2986. doi: 10.1016/j.biomaterials.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bakshi A, Fisher O, Dagci T, Himes BT, Fischer I, Lowman A. Mechanically engineered hydrogel scaffolds for axonal growth and angiogenesis after transplantation in spinal cord injury. J Neurosurg Spine. 2004;1:322–329. doi: 10.3171/spi.2004.1.3.0322. [DOI] [PubMed] [Google Scholar]
- 129.Liu Y, Rayatpisheh S, Chew SY, Chan-Park MB. Impact of endothelial cells on 3D cultured smooth muscle cells in a biomimetic hydrogel. ACS Appl Mater Interfaces. 2012;4(3):1378–1387. doi: 10.1021/am201648f. [DOI] [PubMed] [Google Scholar]
- 130.Adams DJ, Holtzmann K, Schneider C, Butler MF. Self-assembly of peptide surfactants. Langmuir. 2007;23:12729–12736. doi: 10.1021/la7011183. [DOI] [PubMed] [Google Scholar]
- 131.Guler MO, Stupp SI. A self-assembled nanofiber catalyst for ester hydrolysis. J Am Chem Soc. 2007;129:12082–12083. doi: 10.1021/ja075044n. [DOI] [PubMed] [Google Scholar]
- 132.Williams BAR, Lund K, Liu Y, Yan H, Chaput JC. Self-assembled peptide nanoarrays: an approach to studying protein-protein interactions. Angew Chem Int Ed. 2007;46:3051–3054. doi: 10.1002/anie.200603919. [DOI] [PubMed] [Google Scholar]
- 133.Guler MO, Soukasene S, Hulvat JF, Stupp SI. Presentation and recognition of biotin on nanofibers formed by branched peptide amphiphiles. Nano Lett. 2005;5:249–252. doi: 10.1021/nl048238z. [DOI] [PubMed] [Google Scholar]
- 134.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294:1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 135.Hwang JJ, Lyer SN, Li LS, Claussen R, Harrington DA, Stupp SI. Self-assembling biomaterials: Liquid crystal phases of cholesteryl oligo(L-lactic acid) and their interactions with cells. Proc Natl Acad Sci USA. 2002;99:9662–9667. doi: 10.1073/pnas.152667399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Davis ME, Motion JPM, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, Zhang SG, Lee RT. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation. 2005;111:442–450. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 2003;21:1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 138.Dang SM, Kyba M, Perlingeiro R, Daley GQ, Zandstra PW. Efficiency of embryoid body formation and hematopoietic development from embryonic stem cells in different culture systems. Biotechnol Bioeng. 2002;78(4):442–453. doi: 10.1002/bit.10220. [DOI] [PubMed] [Google Scholar]
- 139.Magyar JP, Nemir M, Ehler E, Suter N, Perriard JC, Eppenberger HM. Mass production of embryoid bodies in microbeads. Ann NY Acad Sci. 2001;944:135–143. doi: 10.1111/j.1749-6632.2001.tb03828.x. [DOI] [PubMed] [Google Scholar]
- 140.Wu X, Black L, Santacana-Laffitte G, Patrick CW., Jr Preparation and assessment of glutaraldehyde-crosslinked collagen-chitosan hydrogels for adipose tissue engineering. J Biomed Mater Res A. 2007;81:59. doi: 10.1002/jbm.a.31003. [DOI] [PubMed] [Google Scholar]
- 141.Stokols S, Tuszynski MH. The fabrication and characterization of linearly oriented nerve guidance scaffolds for spinal cord injury. Biomaterials. 2004;25:5839. doi: 10.1016/j.biomaterials.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 142.Stokols S, Tuszynski MH. Freeze-dried agarose scaffolds with uniaxial channels stimulate and guide linear axonal growth following spinal cord injury. Biomaterials. 2006;27:443–451. doi: 10.1016/j.biomaterials.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 143.Ricciardi R, D'Errico G, Auriemma F, Ducouret G, Tedeschi AM, De Rosa C, Laupretre F, Lafuma F. Short time dynamics of solvent molecules and supramolecular organization of poly (vinyl alcohol) hydrogels obtained by freeze thaw techniques. Macromolecules. 2005;38:6629. [Google Scholar]
- 144.Ho MH, Kuo PY, Hsieh HJ, Hsien TY, Hou LT, Lai JY, Wang D. Preparation of porous scaffolds by using freeze-extraction and freeze-gelation methods. Biomaterials. 2004;25:129. doi: 10.1016/s0142-9612(03)00483-6. [DOI] [PubMed] [Google Scholar]
- 145.Whang K, Thomas CH, Healy KE. A novel method to fabricate bioabsorbable scaffolds. Polymer. 1995;36:837–842. [Google Scholar]
- 146.Mikos AG, Thorsen AJ, Czerwonka LA, Bao Y, Langer R, Winslow DN, Vacanti JP. Preparation and characterization of poly(L-lactic acid) foams. Polymer. 1994;35:1068–1077. [Google Scholar]
- 147.Mikos AG, Sarakinos G, Leite SM, Vacanti JP, Langer R. Laminated three-dimensional biodegradable foams for use in tissue engineering. Biomaterials. 1993;14:323–330. doi: 10.1016/0142-9612(93)90049-8. [DOI] [PubMed] [Google Scholar]
- 148.Nam YS, Yoon JJ, Park TG. A novel fabrication method for macroporous scaffolds using gas foaming salt as porogen additive. J Biomed Mater Res. 2000;53:1–7. doi: 10.1002/(sici)1097-4636(2000)53:1<1::aid-jbm1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 149.Yoon JJ, Park TG. Degradation behaviors of biodegradable macroporous scaffolds prepared by gas foaming of effervescent salts. J Biomed Mater Res. 2001;55:401–408. doi: 10.1002/1097-4636(20010605)55:3<401::aid-jbm1029>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 150.Kim TG, Yoon JJ, Lee DS, Park TG. Gas foamed open porous biodegradable polymeric microspheres. Biomaterials. 2006;27:152–159. doi: 10.1016/j.biomaterials.2005.05.081. [DOI] [PubMed] [Google Scholar]
- 151.Peppas N, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine. Adv Mater. 2006;18:1–17. [Google Scholar]
- 152.Hahn MS, Miller JS, West JL. Three-dimensional biochemical and biomechanical patterning of hydrogels for guiding cell behavior. Adv Mater. 2006;18:2679–2688. [Google Scholar]
- 153.Mapili G, Lu Y, Chen S, Roy K. Laser-layered microfabrication of spatially patterned functionalized tissue-engineering scaffolds. J Biomed Mater Res B. 2005;75B:414–424. doi: 10.1002/jbm.b.30325. [DOI] [PubMed] [Google Scholar]
- 154.Luo Y, Shoichet MS. A photolabile hydrogel for guided three-dimensional cell growth and migration. Nat Mater. 2004;3:249–253. doi: 10.1038/nmat1092. [DOI] [PubMed] [Google Scholar]
- 155.Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008;60:184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12:1197. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 157.Li L, Hsieh YL. Ultra-fine polyelectrolyte hydrogel fibers from poly (acrylic acid) poly (vinyl alcohol) Nanotechnology. 2005;16:2852. [Google Scholar]
- 158.Kim TG, Chung HJ, Park TG. Macroporous and nanofibrous hyaluronic acid-collagen hybrid scaffold fabricated by concurrent electrospinning and deposition leaching of salt particles. Acta Biomater. 2008;4:1611. doi: 10.1016/j.actbio.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 159.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 160.Heydarkhan-Hagvall S, Schenke-Layland K, Dhanasopon AP, Rofail F, Smith H, Wu BM, Shemin R, Beygui RE, Maclellan WR. Three-dimensional electronspun ECM-based hybrid scaffolds for cardiovascular tissue engineering. Biomaterials. 2008;29:2907. doi: 10.1016/j.biomaterials.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tan W, Desai TA. Layer-by-layer microfluidics for biomimetic three-dimensional structures. Biomaterials. 2004;25(7-8):1355–1364. doi: 10.1016/j.biomaterials.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 162.Dendukuri D, Pregibon DC, Collins J, Hatton TA, Doyle PS. Continuous-flow lithography for high-throughput microparticle synthesis. Nat Mater. 2006;5:365–369. doi: 10.1038/nmat1617. [DOI] [PubMed] [Google Scholar]
- 163.Panda P, Ali S, Lo E, Chung BG, Hatton TA, Khademhosseini A, Doyle PS. Stop-flow lithography to generate cell-laden microgel particles. Lab Chip. 2008;8(7):1056–1061. doi: 10.1039/b804234a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J Am Chem Soc. 2005;127(28):10096–10100. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- 165.Fukuda J, Khademhosseini A, Yeo Y, Yang X, Yeh J, Eng G. Micromolding of photocrosslinkable chitosan hydrogel for spheroid microarray and co-cultures. Biomaterials. 2006;27(30):5259–5267. doi: 10.1016/j.biomaterials.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 166.Yeh J, Ling Y, Karp JM, Gantz J, Chandawarkar A, Eng G. Micromolding of shape-controlled, harvestable cell-laden hydrogels. Biomaterials. 2006;27(31):5391–5398. doi: 10.1016/j.biomaterials.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 167.Ling Y, Rubin J, Deng Y, Huang C, Demirci U, Karp JM, Khademhosseini A. A cell-laden microfluidic hydrogel. Lab chip. 2007;7:756–762. doi: 10.1039/b615486g. [DOI] [PubMed] [Google Scholar]
- 168.Khademhosseini A, Yeh J, Jon S, Eng G, Suh KY, Burdick JA, Langer R. Molded polyethylene glycol microstructures for capturing cells within microfluidic channels. Lab Chip. 2004;4(5):425–430. doi: 10.1039/b404842c. [DOI] [PubMed] [Google Scholar]
- 169.Franzesi GT, Ni B, Ling Y, Khademhosseini A. A controlled-release strategy for the generation of cross-linked hydrogel microstructures. J Am Chem Soc. 2006;128:15064–15065. doi: 10.1021/ja065867x. [DOI] [PubMed] [Google Scholar]
- 170.Stachowiak AN, Bershteyn A, Tzatzalos E, Irvine DJ. Bioactive hydrogels with an ordered cellular structure combine interconnected macroporosity and robust mechanical properties. Adv Mater. 2005;17(4):399–403. [Google Scholar]
- 171.Golden AP, Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip. 2007;7(6):720–725. doi: 10.1039/b618409j. [DOI] [PubMed] [Google Scholar]
- 172.Fedorovich NE, Swennen I, Girones J, Moroni L, van Blitterswijk CA, Schacht E, Alblas J, Dhert WJA. Evaluation of photocrosslinked Lutrol hydrogel for tissue printing applications. Biomacromolecules. 2009;10:1689–1696. doi: 10.1021/bm801463q. [DOI] [PubMed] [Google Scholar]
- 173.Mironov V, Boland T, Trusk T. Organ printing: computer-aided jet-based 3D tissue engineering. Trend Biotechnol. 2003;21:157–161. doi: 10.1016/S0167-7799(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 174.Fedorovich NE, Alblas J, Hennink WE, Oner FC, Dhert WJ. Organ printing: the future of bone regeneration? Trends Biotechnol. 2011;29(12):601–606. doi: 10.1016/j.tibtech.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 175.Fedorovich NE, Wijnberg HM, Dhert WJ, Alblas J. Distinct tissue formation by heterogeneous printing of osteo- and endothelial progenitor cells. Tissue Eng A. 2011;17:2113–2121. doi: 10.1089/ten.TEA.2011.0019. [DOI] [PubMed] [Google Scholar]
- 176.Varghese D, Deshpande M, Xu T. Advances in tissue engineering: cell printing. J Thorac CardioVasc Surg. 2005;129:470–472. doi: 10.1016/j.jtcvs.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 177.Cohen DL, Malone E, Lipson H. Direct freeform fabrication of seeded hydrogels in arbitrary geometries. Tissue Eng. 2006;12:1325–1335. doi: 10.1089/ten.2006.12.1325. [DOI] [PubMed] [Google Scholar]
- 178.Smith CM, Stone AL, Parkhill RL. Three-dimensional bioassembly tool for generating viable tissue-engineered constructs. Tissue Eng. 2004;10:1566–1576. doi: 10.1089/ten.2004.10.1566. [DOI] [PubMed] [Google Scholar]
- 179.Bhatia SN, Balis UJ, Yarmush ML. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13:1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 180.Khademhosseini A, Langer R, Borenstein J. Microscale technologies for tissue engineering and biology. Pro Natl Acad Sci USA. 2006;103:2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fukuda J, Khademhosseini A, Yeo Y. Micromolding of photocrosslinkable chitosan hydrogel for spheroid microarray and cocultures. Biomaterials. 2006;27:5259–5267. doi: 10.1016/j.biomaterials.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 182.Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30:5409–5416. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Birla RK, Borschel GH, Dennis RG, Brown DL. Myocardial engineering in vivo: Formation and characterization of contractile, vascularized three-dimensional cardiac tissue. Tissue Eng. 2005;11:803–813. doi: 10.1089/ten.2005.11.803. [DOI] [PubMed] [Google Scholar]
- 184.Hecker L, Khait L, Radnoti D, Birla R. Development of a microperfusion system for the culture of bioengineered heart muscle. ASAIO J. 2008;54:284–294. doi: 10.1097/MAT.0b013e31817432dc. [DOI] [PubMed] [Google Scholar]
- 185.Huang Y, Khait L, Birla RK. Contractile three-dimensional bioengineered heart muscle for myocardial regeneration. J Biomed Mater Res A. 2007;80:719–731. doi: 10.1002/jbm.a.31090. [DOI] [PubMed] [Google Scholar]
- 186.Birla RK, Huang YC, Dennis RG. Development of a novel bioreactor for the mechanical loading of tissue-engineered heart muscle. Tissue Eng. 2007;13:2239–2248. doi: 10.1089/ten.2006.0359. [DOI] [PubMed] [Google Scholar]
- 187.Copland IB, Jolicoeur EM, Gillis M, Cuerquis J, Eliopoulos N, Annabi B, Calderone A, Tanguay J, Ducharme A, Galipeau J. Coupling erythropoietin secretion to mesenchymal stromal cells enhances their regenerative properties. Cardiovasc Res. 2008;79:405–415. doi: 10.1093/cvr/cvn090. [DOI] [PubMed] [Google Scholar]
- 188.Giraud M, Ayuni E, Cook S, Siepe M, Carrel TP, Tevaearai HT. Hydrogel-based engineered skeletal muscle grafts normalize heart function early after myocardial infarction. Artif Org. 2008;32:692–700. doi: 10.1111/j.1525-1594.2008.00595.x. [DOI] [PubMed] [Google Scholar]
- 189.Kutschka I, Chen IY, Kofidis T, Arai T, von Degenfeld G, Sheikh AY, Hendry SL, Pearl J, Hoyt G, Sista R, Yang PC, Blau HM, Gambhir SS, Robbins RC. Collagen matrices enhance survival of transplanted cardiomyoblasts and contribute to functional improvement of ischemic rat hearts. Circulation. 2006;114:I167–I173. doi: 10.1161/CIRCULATIONAHA.105.001297. [DOI] [PubMed] [Google Scholar]
- 190.Hansen A, Eder A, Bnstrup M, Flato M, Mewe M, Schaaf S, Aksehirlioglu B, Schwrer A, Uebeler J, Eschenhagen T. Development of a drug screening platform based on engineered heart tissue. Circ Res. 2010;107:35–44. doi: 10.1161/CIRCRESAHA.109.211458. [DOI] [PubMed] [Google Scholar]
- 191.Wang T, Jiang X, Tang Q, Li X, Lin T, Wu D, Zhang X, Okello E. Bone marrow stem cells implantation with alpha-cyclodextrin/MPEG-PCL-MPEG hydrogel improves cardiac function after myocardial infarction. Acta Biomater. 2009;5:2939–2944. doi: 10.1016/j.actbio.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 192.Allder MA, Guilbeau EJ, Brandon TA, Walker AS, Koeneman JB, Fisk RL. A hydrogel pericardial patch. ASAIO Trans. 1990;36:M572–M574. [PubMed] [Google Scholar]
- 193.Walker AS, Blue MA, Brandon TA, Emmanual J, Guilbeau EJ. Performance of a hydrogel composite pericardial substitute after long-term implant studies. ASAIO J. 1992;38:M550–M554. doi: 10.1097/00002480-199207000-00095. [DOI] [PubMed] [Google Scholar]
- 194.Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP, Dickinson SC, Hollander AP, Mantero S, Conconi MT, Birchall MA. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372(9655):2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 195.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 196.Geckil H, Xu F, Zhang X, Moon S, Demirci U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine. 2010;5(3):469–484. doi: 10.2217/nnm.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]