Abstract
Cannabinoid receptor (CBR) agonists produce antinociception in conventional preclinical assays of pain-stimulated behavior but are not effective in preclinical assays of pain-depressed behavior. Fatty acid amide hydrolase (FAAH) inhibitors increase physiological levels of the endocannabinoid anandamide (AEA), which may confer improved efficacy and/or safety relative to direct CBR agonists. To further evaluate FAAH inhibitors as candidate analgesics, this study assessed effects of the FAAH inhibitor URB597 in assays of acute pain-stimulated and pain-depressed behavior in male Sprague Dawley rats. Intraperitoneal injection of dilute lactic acid served as a noxious stimulus to stimulate a stretching response or depress positively reinforced operant behavior (intracranial self-stimulation; ICSS), and URB597 was tested 1 and 4 h after administration. Consistent with FAAH inhibitor effects in other assays of pain-stimulated behavior, URB597 (1–10 mg/kg IP) produced dose-related and CB1R-mediated decreases in acid-stimulated stretching. Conversely, in the assay of acid-depressed ICSS, URB597 produced a delayed, partial and non-CBR-mediated antinociceptive effect. The antinociceptive dose of URB597 (10 mg/kg) increased plasma and brain AEA levels. These results suggest that URB597 produces antinociception in these models of “pain stimulated” and “pain depressed” behavior, but with different rates of onset and differential involvement of CBRs.
Keywords: FAAH inhibitor, URB597, cannabinoid, antinociception, pain, intracranial self-stimulation, stretching, writhing, rat
INTRODUCTION
Fatty acid amide hydrolase (FAAH) is an enzyme that metabolizes endogenous fatty acid ethanolamines including the endocannabinoid anandamide (AEA). FAAH inhibitors can produce cannabinoid receptor (CBR)-mediated effects by blocking degradation and increasing tissue concentrations of AEA. In addition, FAAH inhibitor effects mediated by AEA and CBRs are “activity dependent” insofar as they depend on patterns of neural activity that trigger AEA synthesis and release (Cravatt et al., 1996; Cravatt et al., 2004). This activity dependence of FAAH inhibitor effects on AEA has been hypothesized to result in anatomical and temporal patterns of CBR activation that differ from those produced by direct agonists such as Δ9-tetrahydrocannabinol (THC), and this in turn may produce profiles of CBR-mediated effects that differ from those produced by direct agonists (Schlosburg et al., 2009; Alvarez-Jaimes and Palmer, 2011). For example, THC and other direct CBR agonists produce antinociception in many preclinical pain assays, but doses of direct CBR agonists that produce antinociception also often produce other effects such as motor impairment (De Vry et al., 2004; Kwilasz and Negus, 2012). Conversely, the FAAH inhibitor URB597 has been used extensively to investigate effects of FAAH inhibition, and it has been shown to produce antinociception in many preclinical assays of pain under conditions that produced little or no evidence of motor impairment (Jayamanne et al., 2006; Jhaveri et al., 2006; Hasanein et al., 2009; Naidu et al., 2009; Miller et al., 2012; Guindon et al., 2013). This type of behavioral selectivity to produce antinocicepton has been interpreted to suggest that FAAH inhibitors may display better efficacy and/or safety than CBR agonists as candidate analgesics for the treatment of pain (Cravatt et al., 1996; Cravatt et al., 2004; Schlosburg et al., 2009; Alvarez-Jaimes and Palmer, 2011).
Preclinical procedures used to evaluate candidate analgesics can be categorized as assays of “pain-stimulated” or “pain-depressed” behavior (Negus et al., 2006, 2010a, 2013b). In assays of pain-stimulated behavior, delivery of a noxious stimulus increases the rate, frequency, or intensity of a pain-related behavior (e.g. withdrawal responses), and antinociception is indicated by drug-induced decreases in this target behavior. However, decreases in pain-stimulated behaviors may be related to nonselective drug effects (e.g., sedation/motor impairment) that limit the subject’s ability to perform the behavior rather than to selective reduction in sensory sensitivity to the noxious stimulus. As a result, CBR agonists and other drugs that produce behavioral depression are especially prone to produce false-positive antinociception in preclinical assays of pain-stimulated behavior (De Vry et al., 2004; Finn et al., 2004; Kwilasz and Negus, 2012). Conversely, in assays of pain-depressed behavior, delivery of a noxious stimulus decreases an ongoing behavior (e.g. feeding, locomotion, or operant behavior), and antinociception is indicated by drug-induced increases in the target behavior. Drugs that produce nonselective behavioral depression do not produce false-positive antinociception in assays of pain-depressed behavior, although these assays may be vulnerable to drugs that produce nonselective stimulation of behavior (Negus et al., 2010a,b, 2013a; Kwilasz and Negus, 2012).
When used in concert, assays of pain-stimulated and pain-depressed behavior may facilitate distinction between analgesic drugs and drugs that nonselectively depress or stimulate behavior (Negus et al., 2006, 2010a, 2013b). For example, we have shown in rats that a visceral chemical noxious stimulus (i.p. injection of dilute lactic acid) both stimulates a stretching response and depresses operant responding in an assay of intracranial self-stimulation (ICSS). Clinically effective analgesics, including the mu opioid agonist morphine and the nonsteroidal anti-inflammatory drug (NSAID) ketoprofen, produced antinociception in assays of both acid-stimulated stretching and acid-depressed ICSS. Conversely, several drugs that lack reliable analgesic efficacy in humans (e.g. kappa opioid receptor agonists) failed to produced antinociception in both procedures (Pereira Do Carmo et al., 2009; Negus et al., 2010b, 2012, 2013a; Kwilasz and Negus, 2012; Rosenberg et al., 2013). With regard to cannabinoids, we reported previously that the CBR agonists THC and CP55940 produced antinociception in the assay of acid-stimulated stretching but not in the assay of acid-depressed ICSS (Kwilasz and Negus, 2012), and this failure of CBR agonists to produce antinociception in both procedures agrees with the poor clinical efficacy of CBR agonists as analgesics for acute pain in humans (Rice, 2006; Karst et al., 2010; Kraft, 2012). The goal of the present study was to examine effects of URB597 in the same assays of acid-stimulated stretching and acid-depressed ICSS. Significant effects were challenged with CB1 and CB2 receptor antagonists to assess the role of cannabinoid receptor mechanisms of action, and plasma and brain AEA levels were measured as a biomarker of FAAH inhibition. Given previous evidence that URB597 and other FAAH inhibitors can produce antinociception with little or no motor impairment, we predicted that URB597 would be more likely than CBR agonists to produce antinociception in assays of both acid-stimulated stretching and acid-depressed ICSS
METHODS
Subjects
Forty-six male Sprague-Dawley rats (Harlan, Frederick, MD, USA) weighing approximately 300 g at the time of delivery were individually housed and maintained on a 12 h light/dark cycle with lights on from 06:00 to 18:00 h. Rats had free access to food and water except during testing. Animal maintenance and research were in compliance with National Institutes of Health guidelines on care and use of animal subjects in research and adhered to guidelines of the National Research Council (National Research Council, 2011). All animal use protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Assay of acid-stimulated stretching
Behavioral procedure
To complement studies in the assay of pain-depressed ICSS (see below), 10 rats that failed to meet the criteria for ICSS within 4 weeks (see below) were used for studies of acid-stimulated stretching, as described previously (Pereira Do Carmo et al., 2009; Negus et al., 2010b; Kwilasz and Negus, 2012). During test sessions, rats were placed into an acrylic test chamber (31.0 × 20.1 × 20.0 cm) for a 30 min observation period that began immediately after I.p. injection of dilute lactic acid (1.8% in a volume of 1 ml/kg). A stretch was operationally defined as a contraction of the abdomen followed by a stretching of the hind limbs, and the number of stretches during the observation period was counted.
Studies on acid-stimulated stretching were conducted in three phases (see Figure 1). In two separate groups of rats, dose-effect curves were determined for URB597 (1–10 mg/kg or vehicle) administered 60 min (phase one) or 240 min (phase two) prior to acid. These pretreatment times were selected on the basis of previous studies that reported peak brain levels of AEA approximately 60 to 240 min after treatment with URB597 (Fegley et al., 2005). In phase three, to determine the involvement of CBRs in URB597-induced antinociception, URB597 (10 mg/kg) was administered 240 min before acid in combination with the CB1R-selective antagonist rimonabant (1 mg/kg) or the CB2R-selective antagonist SR144528 (1 mg/kg) administered 30 min before acid. These antagonist doses and pretreatment times were based on previous studies that have demonstrated antagonism of cannabinoid agonist-induced antinociception with rimonabant (Kwilasz and Negus, 2012) and SR144528 (Hohmann et al., 2004). Rimonabant and SR144528 were administered after URB597 because of their potentially shorter duration of action relative to URB597 and their ability to act as competitive antagonists of endocannabinoids at CB1Rs (Thomas et al., 1998; Jarbe et al., 2010) and CB2Rs (Griffin et al., 1999), respectively. Doses/treatments for all phases were delivered in a randomized dose order across rats and separated by at least one week.
Figure 1.

Diagram of experimental design used for drug treatments and behavioral testing in the assay of acid-depressed intracranial self-stimulation (ICSS).
Data Analysis
Drug effects on acid-stimulated stretching were evaluated by repeated measures one-way analysis of variance (ANOVA). A significant ANOVA was followed by Newman Keuls post hoc test, and the criterion for significance was set at p < 0.05.
Assay of intracranial self-stimulation (ICSS)
Surgery
All rats weighed approximately 300–320 g at the time of surgery and were anesthetized with isoflurane gas (2.5–3% in oxygen; Webster Veterinary, Phoenix, AZ, USA) for implantation of stainless steel electrodes (Plastics One, Roanoke, VA, USA). The cathode of each electrode was implanted in the left medial forebrain bundle at the level of the lateral hypothalamus (2.8 mm posterior to bregma, 1.7 mm lateral from the midsagittal suture, and 8.8 mm below the skull). The anode was wrapped around one of the three skull screws to serve as the ground, and the electrode assembly was secured to the skull with orthodontic resin. Rats received 5 mg/kg ketoprofen as a postoperative analgesic immediately and 24 h after surgery, and rats were allowed to recover for at least 7 days prior to commencing ICSS training.
Apparatus
Experiments were conducted in sound-attenuating boxes that contained modular acrylic test chambers (29.2 × 30.5 × 24.1 cm) equipped with a response lever, stimulus lights above the lever, a 2 W house light, and an ICSS stimulator (Med Associates, St. Albans, VT, USA). Electrodes were connected to the stimulator via a swivel connector (Model SL2C, Plastics One, Roanoke, VA, USA). The stimulator was controlled by computer software that also controlled programming parameters and data collection (Med Associates, St. Albans, VT, USA).
Behavioral procedure
After initial shaping of lever press responding, rats were trained and tested under a fixed-ratio (FR) 1 schedule of brain stimulation identical to that described previously (Carlezon and Chartoff, 2007; Do Carmo et al., 2009; Negus et al., 2010a; Kwilasz and Negus, 2012). During experimental sessions, each lever press resulted in the delivery of a 0.5 s train of square wave cathodal pulses (0.1 ms pulse duration), and stimulation was accompanied by illumination of the stimulus lights over the lever. Responses during the 0.5 s stimulation period did not earn additional stimulation. Initially, the frequency of stimulation was held constant at 158 Hz, and the stimulation intensity for each rat was adjusted gradually to the lowest value that would sustain a high rate of reinforcement (> 30 stimulations/min) during sessions lasting 30–60 min. This intensity (107–250 µA across rats) was then held constant for the remainder of the study, and frequency manipulations were introduced. Sessions involving frequency manipulations consisted of sequential 10 min components. During each component, a descending series of 10 current frequencies (158 to 56 Hz in 0.05 log increments) was presented, with each frequency available during sequential 1 min frequency trials. Each frequency trial began with a 10 s time out, during which responding had no scheduled consequences. During the last 5 s of this time out, five noncontingent “priming” stimulations were delivered at the frequency available during that trial, and the lever lights were illuminated during each stimulation. This noncontingent stimulation was then followed by a 50 s “response” phase, during which responding produced electrical stimulation under the FR 1 schedule. Training continued with presentation of three to six sequential components per day until rats reliably responded for only the first four to six frequency trials of all components for at least three consecutive days. In general, rats were implanted with electrodes and trained on ICSS procedures in groups of 10–12. The first six rats in each group to meet training criteria were then advanced to ICSS testing, while the remaining rats were assigned to assays of acid-stimulated stretching as described above. Overall, 13 rats completed ICSS studies, and 10 rats completed stretching studies.
Once training was completed, testing was initiated. The first component of each test session was considered to be an acclimation component, and data were discarded. Data from the second and third “baseline” components were used to calculate baseline parameters of the frequency-rate curves for that session (see “Data Analysis”). Drugs were administered immediately after removing the subjects from the operant chamber after the third baseline component. Studies of URB597 effects on ICSS were conducted in three phases, and the experimental design of each phase is shown in Figure 1. In the first phase, subjects were treated with URB597 (1–10 mg/kg or vehicle) at time “0” and with the i.p. acid noxious stimulus (1.8% lactic acid in a volume of 1 ml/kg) administered 60 min later. ICSS was evaluated for 20 min before and 20 min after acid injection to assess URB597 effects in the absence and presence of the noxious stimulus. Specifically, ICSS was evaluated from 40–60 min to assess effects of URB597 in the absence of the noxious stimulus and again from 60–80 min to assess effects of URB597 on acid-induced depression of ICSS. The second phase was identical except that the acid noxious stimulus was administered 260 min after URB597, and ICSS was assessed from 240–260 min (URB597 effects on ICSS in the absence of the noxious stimulus) and from 260–280 min (URB597 effects on acid-induced depression of ICSS). Lastly, in the third phase, to determine whether URB597-induced antinociception in the assay of acid-depressed ICSS was mediated by CB1Rs or CB2Rs, URB597 (10 mg/kg) was administered at time “0”, either rimonabant (1 mg/kg) or SR144528 (1 mg/kg) was administered after 210 min, and the acid noxious stimulus was administered after 260 min. All ICSS tests utilized consecutive control ICSS and acid-depressed ICSS tests in the same experimental session because of limited availability of URB597. URB597 doses and cannabinoid antagonist + URB597 combinations were administered in randomized order and were separated by at least one week. Training and test sessions were conducted Monday–Friday, with test sessions conducted on Wednesdays, Thursdays, or Fridays.
Data Analysis
The primary dependent variable was the total number of stimulations per component and was calculated as the sum of stimulations delivered across all 10 frequency-trials of each component. Test data were then normalized to individual baseline data using the equation Percent Baseline Total Stimulations per Component = (Mean Total Stimulations per Test Component ÷ Mean Total Stimulations per Baseline Component) × 100. Data were then averaged across rats in each experimental condition and compared by repeated measures one-way ANOVA. A significant ANOVA was followed by Newman Keuls post hoc test, and the criterion for significance was set at p < 0.05.
Measurement of brain and plasma endocannabinoids
Experimental procedure
Twenty-three rats were used for the measurement of brain and plasma endocannabinoids following URB597 administration. The endocannabinoids evaluated were anandamide (AEA) and 2-arachidonoylglycercol (2-AG). Rats were dosed in their home cages with URB597 (10 mg/kg or vehicle) and sacrificed via decapitation with guillotine at 60 or 240 min after drug administration. Naive rats that did not receive any injection prior to sacrifice were also included as controls. Immediately after decapitation, brains were harvested, quickly frozen in isopentane on dry ice, and stored at −80°C until assay. For plasma samples, trunk blood was collected immediately after decapitation and centrifuged at 1250 × g to separate plasma from blood cells. Plasma samples were then stored at −80°C until assay.
Procedure for tissue extraction
On the day of assay, the pre-weighed rat brains were homogenized with 5 ml chloroform:methanol (2:1 v/v containing 0.0348 g PMSF/ml). One quarter of the homogenate (1.25 ml) was taken and diluted to 1.4 ml with the chloroform:methanol used for homogenization. Internal standards (50 µl of each of 2 pmol AEA-d8 and 1 nmol 2-AG-d8) were added to each sample. Homogenates were vortexed and mixed with 0.3 ml of 0.73% w/v NaCl, vortexed again, and then centrifuged for 10 min at 3200 × g and 4°C. The aqueous phase plus debris were collected and extracted again twice with 0.8 ml chloroform. The organic phases from the three extractions were pooled, and organic solvents were evaporated under nitrogen gas. Dried samples were reconstituted with 0.1 ml chloroform and mixed with 1 ml cold acetone. The mixtures were then centrifuged for 5 min at 1800 × g and 4°C to precipitate proteins. The upper layer of each sample was collected and evaporated under nitrogen gas. Dried samples were reconstituted with 0.1 ml methanol and placed in auto-sample vials for analysis.
Rat plasma (200 µl) was mixed with 50 µl of each of the internal standards mentioned above and then mixed with 2.8 ml chloroform:methanol (2:1 containing 0.0348 g PMSF/ml). Samples were vortexed and 0.6 ml of 0.73% w/v NaCl was added to each sample, vortexed, and then centrifuged for 10 min at 3200 × g and 4°C. The aqueous phase plus debris were collected and extracted again twice with 1.6 ml chloroform. The organic phases from the three extractions were pooled and the organic solvents were evaporated under nitrogen gas. The dried samples were reconstituted with 0.1 ml chloroform and mixed with 1 ml cold acetone. The upper layer of each sample was then collected and processed as described above.
High performance liquid chromatography tandem mass spectrometry (HPLC/MS/MS) quantification
An HPLC/MS/MS system was used to identify and quantify AEA and 2-AG in brain and plasma samples. The system used was a 3200 Q trap with a turbo V source for TurbolonSpray (Applied Biosystems, Carlsbad, CA, USA) attached to a SCL HPLC system (Shimadzu, Kyoto, Japan) controlled by Analyst 1.4.2 software (AB Sciex, Framingham, MA, USA). The chromatographic separation was performed using a Discovery® HS C18, 4.6 × 15 cm, 3 micron (Supelco, Bellefonte, PA, USA). The mobile phase consisted of (10:90) water:methanol with 0.1% ammonium acetate and 0.1% formic acid and was delivered at a flow rate of 0.3 ml/min. The source temperature was set at 600°C and had a curtain gas at a flow rate of 30 ml/min. The ionspray voltage was 5000 V with ion source gases 1 and 2 flow rates of 60 and 50 ml/min, respectively. The mass spectrometer was run in positive ionization mode, and the acquisition mode used was multiple reaction monitoring. The following transitions were monitored: (348>62) and (348>91) for AEA; (356>62) for AEA-d8; (379>287) and (279>269) for 2-AG; and (387>96) for 2-AG-d8. Calibration curves were constructed with each analytical batch for each analyte. Curves were constructed using linear regression based on the peak area ratios of each analyte and its deuterated internal standard. The extracted calibration curves ranged from 0.039 pmol to 40 pmol for AEA and from 0.0625 nmol to 64 nmol for 2-AG. The total run time for the analytical method was 8 minutes.
Data Analysis
Prior to data analysis, AEA brain level data were transformed to pmol/g of brain tissue, 2-AG brain level data were transformed to nmol/g of brain tissue, and AEA plasma level data were transformed to pmol/ml of plasma. 2-AG plasma levels were below the threshold of detection. Statistical analysis of brain and plasma levels of AEA and brain levels of 2-AG did not reveal significant differences between naïve and vehicle-treated animals; thus naïve group data were excluded from final data analysis. Drug effects on brain and plasma endocannabinoids were evaluated by two-way ANOVA, with treatment and treatment time as the two factors. A significant ANOVA was followed by Newman-Keuls post hoc test, and the criterion for significance was set at p < 0.05.
Drugs
Lactic acid was purchased from Sigma Chemical Co. (St. Louis, MO, USA). AEA, AEA-d8, 2-AG, and 2-AG-d8 were purchased from Cayman Chemical (Ann Arbor, MI, USA). URB597 (cyclohexyl carbamic acid 3'-carbamoyl-biphenyl-3-yl ester) (Fegley et al., 2005), rimonabant, and SR144528 (N-{(1S)-endo-1,3,3-trimethyl bicyclo[2.2.1] heptan-2yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide) (Griffin et al., 1999) were provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD, USA). Lactic acid was prepared in sterile water. URB597 was prepared in a vehicle consisting of 1% carboxymethylcellulose (Sigma), 1% Tween 80 (Sigma), 2% dimethyl sulfoxide (Sigma), and 96% sterile saline. Rimonabant and SR144528 were prepared in a vehicle consisting of 5% ethanol, 5% cremophor (Sigma), and 90% sterile saline. All solutions were injected i.p. in a volume of 1 ml/kg except for URB597, which was injected i.p. in a volume of 2 ml/kg.
RESULTS
Effects of URB597 on acid-stimulated stretching
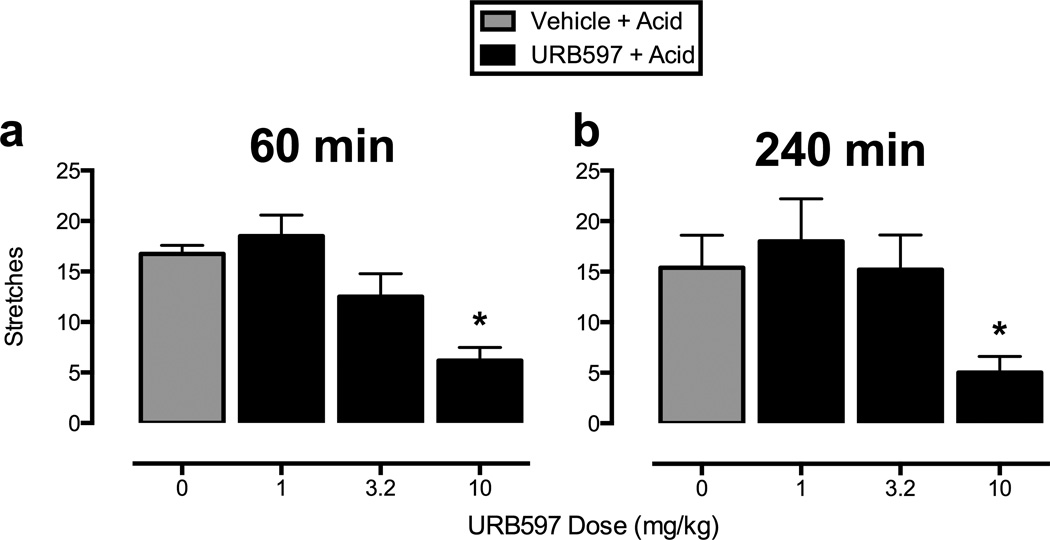
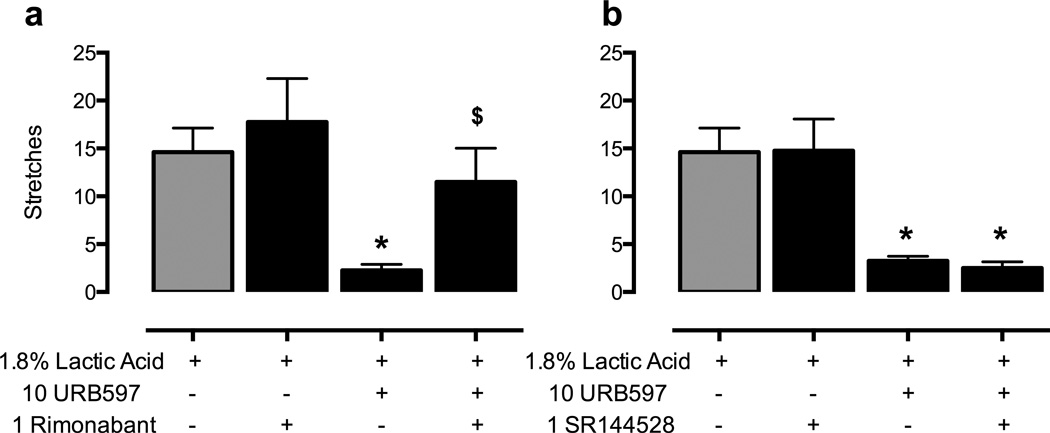
Figure 2 shows that pi.p. administration of lactic acid (1.8% in a volume of 1 ml/kg) stimulated approximately 15 stretches after administration of vehicle (gray bars in all panels). URB597 (1–10 mg/kg) produced a significant decrease in stretching after both 60 and 240 min at the highest dose of 10 mg/kg (panels 2a and 2b, respectively). Figure 3 shows that the antinociceptive effect of URB597 at 240 min was antagonized by administration of the CB1R antagonist rimonabant (1 mg/kg, panel 3a), but not by the CB2R antagonist SR144528 (1 mg/kg, panel 3b). Rimonabant and SR144528 did not alter acid-stimulated stretching when administered alone.
Figure 2.
Effects of URB597 on acid-stimulated stretching. Horizontal axes: dose of URB597 in milligrams per kilogram. Vertical axes: number of stretches observed during a 30 min observation period. Panels a and b show the effects of URB597 (1–10 mg/kg or vehicle) administered 60 min (panel a) or 240 min (panel b) before acid treatment. One-way repeated-measures ANOVA indicated significant main effects of URB597 after 60 min (panel a) [F(3,15)=9.59; p<0.001] and 240 min (panel b) [F(3,12)=14.63; p<0.001]. * significantly different from vehicle + acid in all panels as determined by Newman-Keuls post hoc test, p < 0.05. All bars show mean + SEM in six (60 min) or five (240 min) rats.
Figure 3.
URB597-induced antinociception in the assay of acid-stimulated stretching is antagonized by rimonabant but not by SR144528. Horizontal axes: Treatment conditions. Vertical axes : number of stretches observed during a 30 min observation period. The left panel (a) shows the effect of URB597 (10 mg/kg or vehicle) administered 240 min before acid treatment in combination with rimonabant (1 mg/kg or vehicle) administered 30 min before acid treatment. One-way repeated--easures ANOVA indicated a significant main effect of treatment in panel a [F(3,9)=9.47; p<0.005]. The right panel (b) shows the effect of URB597 (10 mg/kg or vehicle) administered 240 min before acid treatment in combination with SR144528 (1 mg/kg or vehicle) administered 30 min before acid treatment. One-way repeated measures ANOVA indicated a significant main effect of treatment in panel b [F(3,9)=17.03; p<0.001]. * in all panels: significantly different from vehicle + acid; $ in panel a: significantly different from URB597 (10 mg/kg) + acid as determined by Newman-Keuls post hoc test, p < 0.05. All bars show mean + SEM in four rats.
Effects of URB597 on ICSS in the absence of a noxious stimulus
Over the course of the entire ICSS study, the average baseline number of stimulations per component was 179.47 ± 37.65, and data in Figures 4–6 show URB597 effects expressed as a percent of the baseline number of stimulations per component in each group of rats. Figure 4 shows that, in the absence of the noxious stimulus, URB597 (1–10 mg/kg) dose-responsively decreased ICSS from 40–60 min after treatment (panel 4a), but not from 240–260 min after treatment (panel 4b). Supplemental Figure 1 shows the full ICSS frequency-rate curves for Figure 4.
Figure 4.
Effects of URB597 on control ICSS in the absence of a noxious stimulus. Horizontal axes: dose URB597 in milligrams per kilogram. Vertical axes: percent baseline total number of stimulations per component. Panels a and b show the effects of URB597 (1–10 mg/kg or vehicle) administered 40 min (panel a) or 240 min (panel b) before ICSS. One-way repeated-measures ANOVA indicated a significant main effect of URB597 treatment 40 min post-treatment (panel a) [F(3,12)=13.47; p<0.001].* significantly different from vehicle treatment as indicated by Newman-Keuls post hoc test, p < 0.05. All bars show mean + SEM in five rats. See Supplemental Figure 1 for full ICSS frequency-rate curves.
Figure 6.
URB597-induced antinociception in the assay of acid depressed ICSS is not antagonized by rimonabant or SR144528. Horizontal axes: Treatment conditions. Vertical axes : percent baseline total number of stimulations per component. The left panel (a) shows the effects of URB597 (10 mg/kg or vehicle) administered 260 min before acid treatment in combination with rimonabant (1 mg/kg or vehicle) administered 50 min before acid treatment. One-way repeated-measures ANOVA indicated a significant main effect of treatment in panel a [F(4,16)=13.68; p<0.001]. The right panel (b) shows the effects of URB597 (10 mg/kg or vehicle) administered 260 min before acid treatment in combination with SR144528 (1 mg/kg or vehicle) administered 50 min before acid treatment. One-way repeated-measures ANOVA indicated a significant main effect of treatment in panel b [F(4,16)=14.53; p<0.001]. * significantly different from vehicle + acid vehicle treatment; $ significantly different from vehicle + acid treatment as indicated by Newman-Keuls post hoc test, p < 0.05. All bars show mean + SEM in five rats.
Effects of URB597 on acid-induced depression of ICSS
Figure 5 shows that the lactic acid noxious stimulus significantly decreased ICSS when it was administered 60 min or 260 min after vehicle. This acid-induced depression of ICSS served as a manifestation of pain-related behavioral depression, and URB597 was evaluated for its ability to block acid-induced depression of ICSS. Figure 5 also shows that URB597 produced dose- and time-dependent antinociception in the assay of acid-depressed ICSS. Specifically, URB597 (1–10 mg/kg) did not significantly alter acid-induced depression of ICSS after 60 min (panel 5a), but it significantly attenuated acid-induced depression of ICSS at 260 min (panel 5b). These results are in contrast to results in the assay of acid-stimulated stretching, in which URB597 produced significant antinociception at both early and late time points (Figure 2). Higher URB597 doses were not tested due to limited solubility of the compound. Supplemental Figure 2 shows the full ICSS frequency-rate curves for Figure 5.
Figure 5.
Effects of URB597 on acid-induced depression of ICSS. Horizontal axes: dose FAAH inhibitor in milligrams per kilogram. Vertical axes : percent baseline total number of stimulations per component. Panels a and b show the effects of URB597 (1–10 mg/kg or vehicle) administered 60 min (panel a) or 260 min (panel b) before acid treatment. One-way repeated-measures ANOVA indicated a significant main effect of treatment in panels a [F(4,16)=4.59; p<0.02] and b [F(4,16)=9.83; p<0.001]. * in all panels: significantly different from vehicle + acid vehicle treatment;$ in panel b: significantly different from vehicle + acid treatment as indicated by Newman-Keuls post hoc test, p < 0.05. All bars show mean + SEM in five rats. See Supplemental Figure 2 for full ICSS frequency-rate curves.
Figure 6 shows that neither rimonabant nor SR144528 reversed URB597-induced antinociception in the assay of acid-depressed ICSS. Administration of 1 mg/kg rimonabant (panel 6a) or 1 mg/kg SR144528 (panel 6b) 50 min before the ICSS session had no effect on the antinociceptive effects of 10 mg/kg URB597 administered 260 min before the ICSS session. Furthermore, these doses of rimonabant (panel 6a) and SR144528 (panel 6b) produced no effects on acid-induced depression of ICSS alone.
Effects of URB597 on plasma and brain endocannabinoids
Figure 7 shows that URB597 (10 mg/kg) increased plasma and brain AEA levels. In plasma, URB597 significantly increased plasma AEA levels after both 60 and 240 min, although in brain, AEA levels were significantly increased only after 240 min. Brain AEA levels were also significantly higher 240 min versus 60 min after URB597. No significant differences were found in brain levels of 2-AG after URB597 administration or its vehicle at any time point (data not shown). AEA levels in naïve rats were 0.90 ± 0.18 pmol/ml in plasma and 5.26 ± 1.41 pmol/g in brain. Levels of 2-AG were below the limits of detection in plasma and 9.49 ± 1.04 nmol/g in brain.
Figure 7.
URB597 produced time-dependent increases in plasma and brain anandamide. Horizontal axes: time post-drug administration in minutes. Vertical axes: percent of vehicle-treatment. Panels a and b show the effects of URB597 (10 mg/kg or vehicle) on plasma (panel a) and brain (panel b) levels of anandamide (AEA). Two-way ANOVA indicated a significant main effect of URB597 treatment in plasma (panel a) [F(1,11)=23.66; p<0.001] and in brain (panel b) [F(2,17)=26.15; p<0.001]). Filled symbols indicate significantly different from vehicle treatment. All points show mean ± SEM in four rats except for URB597 60 min post-treatment which shows mean ± SEM in three rats.
DISCUSSION
This study assessed effects of the FAAH inhibitor URB597 in assays of pain-stimulated and pain-depressed behavior in rats. There were two main findings. First, URB597 produced significant antinociception in both assays, and these results provide some support for further consideration of URB597 as a candidate analgesic. In particular, antinociceptive effects of URB597 in the assay of acid-depressed ICSS were greater than those produced by CBR agonists, although weaker than those produced by clinically approved opioid and NSAID analgesics (Pereira Do Carmo et al., 2009; Kwilasz and Negus, 2012; Negus, 2013a). Second, URB597 increased plasma and brain AEA levels at a dose that also produced rimonabant-sensitive antinociception in the assay of acid-stimulated stretching; however, antinociception in the assay of acid-depressed ICSS was not antagonized by either rimonabant or SR144528. These results suggest that CB1Rs can play differential roles in the antinociceptive effects of URB597 in assays of pain-stimulated versus pain-depressed behavior.
URB597 effects on pain-stimulated behavior
In assays of pain-stimulated behavior, delivery of a noxious stimulus increases the rate or intensity of the target behavior, and antinociception is inferred from drug-induced decreases in the target behavior (Negus et al., 2010a). In the present study, acid stimulated a stretching response in rats, and URB597 produced CB1R-mediated antinociception insofar as it produced a rimonabant-reversible decrease in this stretching behavior. The antinociceptive effects of URB597 are in agreement with previous studies showing that URB597 produces CB1R-mediated antinociception in preclinical assays of pain-stimulated behavior, such as stretching elicited by intraperitoneal acetic acid administration (Naidu et al., 2009; Clapper et al., 2010; Miller et al., 2012), first and second phases of nociceptive behavior elicited by intraplantar formalin injection (Hasanein et al., 2009), tail-flick responses elicited by noxious heat (Hasanein et al., 2009), and hypersensitive withdrawal responses elicited by thermal/mechanical stimuli in inflammatory or neuropathic pain models (Jayamanne et al., 2006; Jhaveri et al., 2006; Guindon et al., 2013). These results thus add to a growing body of literature that supports the antinociceptive effects of URB597 in various preclinical models of pain-stimulated behavior. Nonetheless, many drugs that produce antinociception in assays of pain-stimulated behavior fail to produce significant clinical analgesia when tested in humans. One source of this poor translation may be the vulnerability of assays of pain-stimulated behavior to drug effects that impair motor performance rather than alter sensory sensitivity to noxious stimuli. One approach to addressing this vulnerability is to complement assays of pain-stimulated behavior with assays of pain-depressed behavior.
URB597 effects on pain-depressed behavior
In assays of pain-depressed behavior, delivery of a noxious stimulus decreases the rate or intensity of the target behavior, and antinociception is inferred from drug-induced increases in the target behavior. URB597 failed to produce antinociception in the assay of acid-depressed ICSS after 1 h, a time when URB597 was effective in the assay of acid-stimulated stretching, but at which it also produced potential motor impairment as indicated by significant depression of control ICSS. However, after 4 h, the depressant effects of URB597 on control ICSS dissipated, and URB597 produced significant though partial antinociception in the assay of acid-depressed ICSS. Thus, at 4 h, URB597 significantly attenuated acid-induced depression of ICSS without facilitating control ICSS. This partial efficacy of URB597 agrees with a previous study that reported partial efficacy of URB597 in assays of intraperitoneal acid-induced depression of wheel-running and feeding in mice (Miller et al., 2012). Moreover, the present finding that URB597 produced antinociception in assays of both acid-stimulated stretching and acid-depressed ICSS without altering control ICSS at the 4 h test time further suggests that URB597 effects included analgesic attenuation of sensitivity to the noxious stimulus rather than (or in addition to) non-selective motor effects.
The profile of effects produced by URB597 is distinct from effects of the CBR agonists THC and CP55940, which failed to produce antinociception at any dose or time in the assay of acid-depressed ICSS (Kwilasz and Negus, 2012). However, URB597 was less effective than clinically approved opioids (e.g. morphine) or NSAIDs (e.g. ketoprofen), which completely block acid-induced depression of ICSS at doses that do not alter control ICSS (Pereira Do Carmo et al., 2009; Kwilasz and Negus, 2012; Negus, 2013a). Although higher doses of URB597 may have produced greater effects, they could not be tested due to limited solubility of the compound, and the dose range tested was sufficient to produce significant behavioral effects and significant increases in AEA levels. Taken together, these results support further consideration of URB597 as an alternative to CBR agonists as candidate cannabinoid-based treatments for prevention of acute pain. However, these results do not provide evidence to suggest that URB597 or other FAAH inhibitors would be equally efficacious to or safer than existing opioid or NSAID analgesics. Clinical correlates of these findings remain to be determined. However, in the only clinical trial conducted to date with a FAAH inhibitor, the compound PF04457845 was compared to the NSAID naproxen in osteoarthritis patients. Naproxen significantly decreased pain scores, whereas PF04457845 was ineffective despite producing a significant increase in AEA and other FAAH substrate biomarkers (Huggins et al., 2012). Thus, this clinical study also suggests lower analgesic efficacy of a FAAH inhibitor relative to an NSAID.
Mechanisms of URB597 antinociception
Consistent with its function as a FAAH inhibitor (Fegley et al., 2005), URB597 significantly increased plasma and brain AEA levels at a dose that also produced significant behavioral effects. These findings agree with other reports that behaviorally active URB597 doses increase plasma and/or brain AEA levels in rats and mice (Kathuria et al., 2003; Gobbi et al., 2005; Jhaveri et al., 2006; Bortolato et al., 2007). These results are also consistent with the proposition that URB597 effects could be mediated in part by agonist effects of AEA at CBRs, and as noted above, rimonabant antagonism of URB597 antinociception in the assay of acid-stimulated stretching is consistent with this proposition. Rimonabant also blocked URB597 antinociception in assays of acid-depressed-wheel running and -feeding in mice (Miller et al., 2012). However, URB597 antinociception in the assay of acid-depressed ICSS was not blocked by either rimonabant or by a CB2R antagonist, which implies a role for non-CBR effects of URB597.
The nature of this non-CBR effect remains to be determined, but three possibilities will be suggested here. First, in addition to functioning as an agonist at CBRs, AEA is also an agonist at some non-CBRs such as transient receptor potential vanilloid 1 (TRPV1) ion channels (Ross, 2003). AEA treatment can desensitize TRPV1 (Lizanecz et al., 2006), and this desensitization could block pro-nociceptive effects of acid that are also mediated at least in part through TRPV1 (Tang et al., 2007). Second, antinociceptive effects of URB597 may be mediated by FAAH substrates other than AEA. For example, palmitoylethanolamide and oleoylethanolamide are also substrates of FAAH, and they have been shown to decrease nociceptor receptive field expansion through their effects on peroxisome-proliferator activated receptor-α (PPAR-α) (Sagar et al., 2008). Furthermore, inhibition of FAAH can increase physiological levels of the FAAH substrates N-acyl taurines, which have been shown to activate a variety of other TRP ion channels, including TRPV1 and TRPV4 (Saghatelian et al., 2006; Long et al., 2011). Finally, there are several off-target mechanisms by which URB597 could have produced non-CBR-mediated antinociception. For example, URB597 has been shown to directly inhibit liver carboxylesterases (Zhang et al., 2007; Xie et al., 2010) as well as to decrease tyrosine-hydroxylase mRNA expression, an effect mediated by abnormal-cannabidiol-sensitive receptors and PPAR-α (Bosier et al., 2012). Moreover, OL135, an earlier generation FAAH inhibitor, was shown to produce antinociception mediated by mu-opioid receptors (Chang et al., 2006), and thus URB597 may have also produced antinociception through these receptors. Lastly, URB597 may alter arachidonate metabolism to reduce synthesis of pro-nociceptive cyclooxygenase-mediated products such as prostaglandins (Fowler, 2007).
In conclusion, URB597 significantly increased plasma and brain AEA levels and produced CB1R-mediated antinociception in the assay of acid-stimulated stretching. In contrast, URB597 produced partial and delayed antinociception in the assay of acid-depressed ICSS that was not mediated by either CB1Rs or CB2Rs. These results suggest that FAAH inhibitors can produce antinociception through different mechanisms on different behavioral manifestations of pain.
Supplementary Material
Acknowledgments
Sources of Funding: R01 NS0070715 (SN), P01 DA009789 (AL), and F31 DA032267 (AK)
Footnotes
Conflicts of Interest: none declared
REFERENCES
- Alvarez-Jaimes LJ, Palmer JA. The role of endocannabinoids in pain modulation and the therapeutic potential of inhibiting their enzymatic degradation. Curr Pharm Biotechnol. 2011;12:1644–1659. doi: 10.2174/138920111798357357. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol Psychiatry. 2007;62:1103–1110. doi: 10.1016/j.biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Bosier B, Muccioli GG, Lambert DM. The FAAH inhibitor URB597 efficiently reduces tyrosine hydroxylase expression through CB(1) and FAAH-independent mechanisms. Br J Pharmacol. 2012;169:794–807. doi: 10.1111/j.1476-5381.2012.02208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Chang L, Luo L, Palmer JA, Sutton S, Wilson SJ, Barbier AJ, Breitenbucher JG, Chaplan SR, Webb M. Inhibition of fatty acid amide hydrolase produces analgesia by multiple mechanisms. Br J Pharmacol. 2006;148:102–113. doi: 10.1038/sj.bjp.0706699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapper JR, Moreno-Sanz G, Russo R, Guijarro A, Vacondio F, Duranti A, Tontini A, Sanchini S, Sciolino NR, Spradley JM, Hohmann AG, Calignano A, Mor M, Tarzia G, Piomelli D. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat Neurosci. 2010;13:1265–1270. doi: 10.1038/nn.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Saghatelian A, Hawkins EG, Clement AB, Bracey MH, Lichtman AH. Functional disassociation of the central and peripheral fatty acid amide signaling systems. Proc Natl Acad Sci U S A. 2004;101:10821–10826. doi: 10.1073/pnas.0401292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vry J, Jentzsch KR, Kuhl E, Eckel G. Behavioral effects of cannabinoids show differential sensitivity to cannabinoid receptor blockade and tolerance development. Behav Pharmacol. 2004;15:1–12. doi: 10.1097/00008877-200402000-00001. [DOI] [PubMed] [Google Scholar]
- Do Carmo GP, Folk JE, Rice KC, Chartoff E, Carlezon WA, Jr, Negus SS. The selective non-peptidic delta opioid agonist SNC80 does not facilitate intracranial self-stimulation in rats. Eur J Pharmacol. 2009;604:58–65. doi: 10.1016/j.ejphar.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3'-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- Finn DP, Beckett SR, Roe CH, Madjd A, Fone KC, Kendall DA, Marsden CA, Chapman V. Effects of coadministration of cannabinoids and morphine on nociceptive behaviour, brain monoamines and HPA axis activity in a rat model of persistent pain. Eur J Neurosci. 2004;19:678–686. doi: 10.1111/j.0953-816x.2004.03177.x. [DOI] [PubMed] [Google Scholar]
- Fowler CJ. The contribution of cyclooxygenase-2 to endocannabinoid metabolism and action. Br J Pharmacol. 2007;152:594–601. doi: 10.1038/sj.bjp.0707379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin G, Wray EJ, Tao Q, McAllister SD, Rorrer WK, Aung MM, Martin BR, Abood ME. Evaluation of the cannabinoid CB2 receptor-selective antagonist, SR144528: further evidence for cannabinoid CB2 receptor absence in the rat central nervous system. Eur J Pharmacol. 1999;377:117–125. doi: 10.1016/s0014-2999(99)00402-1. [DOI] [PubMed] [Google Scholar]
- Guindon J, Lai Y, Takacs SM, Bradshaw HB, Hohmann AG. Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: Effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment. Pharmacol Res. 2013;67:94–109. doi: 10.1016/j.phrs.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanein P, Parviz M, Keshavarz M, Roohbakhsh A. URB597, an inhibitor of fatty acid amide hydrolase, reduces hyperalgesia in diabetic rats. Canadian journal of physiology and pharmacology. 2009;87:432–439. doi: 10.1139/y09-026. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Farthing JN, Zvonok AM, Makriyannis A. Selective activation of cannabinoid CB2 receptors suppresses hyperalgesia evoked by intradermal capsaicin. J Pharmacol Exp Ther. 2004;308:446–453. doi: 10.1124/jpet.103.060079. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, Gifford RS, Makriyannis A. Antagonism of (9)-THC induced behavioral effects by rimonabant: time course studies in rats. Eur J Pharmacol. 2010;648:133–138. doi: 10.1016/j.ejphar.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayamanne A, Greenwood R, Mitchell VA, Aslan S, Piomelli D, Vaughan CW. Actions of the FAAH inhibitor URB597 in neuropathic and inflammatory chronic pain models. Br J Pharmacol. 2006;147:281–288. doi: 10.1038/sj.bjp.0706510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri MD, Richardson D, Kendall DA, Barrett DA, Chapman V. Analgesic effects of fatty acid amide hydrolase inhibition in a rat model of neuropathic pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:13318–13327. doi: 10.1523/JNEUROSCI.3326-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst M, Wippermann S, Ahrens J. Role of cannabinoids in the treatment of pain and (painful) spasticity. Drugs. 2010;70:2409–2438. doi: 10.2165/11585260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nature medicine. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Kraft B. Is There Any Clinically Relevant Cannabinoid-Induced Analgesia? Pharmacology. 2012;89:237–246. doi: 10.1159/000337376. [DOI] [PubMed] [Google Scholar]
- Kwilasz AJ, Negus SS. Dissociable effects of the cannabinoid receptor agonists {Delta}9-tetrahydrocannabinol and CP55940 on pain-stimulated versus pain-depressed behavior in rats. J Pharmacol Exp Ther. 2012;343:389–400. doi: 10.1124/jpet.112.197780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizanecz E, Bagi Z, Pasztor ET, Papp Z, Edes I, Kedei N, Blumberg PM, Toth A. Phosphorylation-dependent desensitization by anandamide of vanilloid receptor-1 (TRPV1) function in rat skeletal muscle arterioles and in Chinese hamster ovary cells expressing TRPV1. Molecular pharmacology. 2006;69:1015–1023. doi: 10.1124/mol.105.015644. [DOI] [PubMed] [Google Scholar]
- Long JZ, LaCava M, Jin X, Cravatt BF. An anatomical and temporal portrait of physiological substrates for fatty acid amide hydrolase. J Lipid Res. 2011;52:337–344. doi: 10.1194/jlr.M012153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LL, Picker MJ, Umberger MD, Schmidt KT, Dykstra LA. Effects of alterations in cannabinoid signaling, alone and in combination with morphine, on pain-elicited and pain-suppressed behavior in mice. J Pharmacol Exp Ther. 2012;342:177–187. doi: 10.1124/jpet.112.191478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu PS, Booker L, Cravatt BF, Lichtman AH. Synergy between enzyme inhibitors of fatty acid amide hydrolase and cyclooxygenase in visceral nociception. J Pharmacol Exp Ther. 2009;329:48–56. doi: 10.1124/jpet.108.143487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- Negus SS. Expression and treatment of pain-related behavioral depression. Lab Animal. 2013a;42:292–300. doi: 10.1038/laban.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS. Intracranial self-stimulation as an operant procedure to investigate expression and treatment of pain-related behavioral depression in rats. Lab Animal. 2013b doi: 10.1038/laban.255. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. Preclinical assessment of candidate analgesic drugs: recent advances and future challenges. J Pharmacol Exp Ther. 2006;319:507–514. doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- Negus SS, Bilsky EJ, Do Carmo GP, Stevenson GW. Rationale and methods for assessment of pain-depressed behavior in preclinical assays of pain and analgesia. Methods Mol Biol. 2010a;617:79–91. doi: 10.1007/978-1-60327-323-7_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Morrissey EM, Rosenberg M, Cheng K, Rice KC. Effects of kappa opioids in an assay of pain-depressed intracranial self-stimulation in rats. Psychopharmacology (Berl) 2010b;210:149–159. doi: 10.1007/s00213-009-1770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Rosenberg MB, Altarifi AA, O'Connell RH, Folk JE, Rice KC. Effects of the delta opioid receptor agonist SNC80 on pain-related depression of intracranial self-stimulation (ICSS) in rats. The journal of pain : official journal of the American Pain Society. 2012;13:317–327. doi: 10.1016/j.jpain.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS. Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: further studies on pain-depressed behavior. Pain. 2009;144:170–177. doi: 10.1016/j.pain.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice A, editor. Wall and Melzack's Textbook of Pain. Philadelphia: Churchill Livingstone; 2006. [Google Scholar]
- Rosenberg MB, Carroll FI, Negus SS. Effects of Monoamine Reuptake Inhibitors in Assays of Acute Pain-Stimulated and Pain-Depressed Behavior in Rats. The journal of pain : official journal of the American Pain Society. 2013 doi: 10.1016/j.jpain.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA. Anandamide and vanilloid TRPV1 receptors. Br J Pharmacol. 2003;140:790–801. doi: 10.1038/sj.bjp.0705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar DR, Kendall DA, Chapman V. Inhibition of fatty acid amide hydrolase produces PPAR-alpha-mediated analgesia in a rat model of inflammatory pain. Br J Pharmacol. 2008;155:1297–1306. doi: 10.1038/bjp.2008.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghatelian A, McKinney MK, Bandell M, Patapoutian A, Cravatt BF. A FAAH-regulated class of N-acyl taurines that activates TRP ion channels. Biochemistry. 2006;45:9007–9015. doi: 10.1021/bi0608008. [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Kinsey SG, Lichtman AH. Targeting fatty acid amide hydrolase (FAAH) to treat pain and inflammation. Aaps J. 2009;11:39–44. doi: 10.1208/s12248-008-9075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Chen Y, Chen Z, Blumberg PM, Kozikowski AP, Wang ZJ. Antinociceptive pharmacology of N-(4-chlorobenzyl)-N'-(4-hydroxy-3-iodo-5-methoxybenzyl) thiourea, a high-affinity competitive antagonist of the transient receptor potential vanilloid 1 receptor. J Pharmacol Exp Ther. 2007;321:791–798. doi: 10.1124/jpet.106.117572. [DOI] [PubMed] [Google Scholar]
- Thomas BF, Gilliam AF, Burch DF, Roche MJ, Seltzman HH. Comparative receptor binding analyses of cannabinoid agonists and antagonists. J Pharmacol Exp Ther. 1998;285:285–292. [PubMed] [Google Scholar]
- Xie S, Borazjani A, Hatfield MJ, Edwards CC, Potter PM, Ross MK. Inactivation of lipid glyceryl ester metabolism in human THP1 monocytes/macrophages by activated organophosphorus insecticides: role of carboxylesterases 1 and 2. Chemical research in toxicology. 2010;23:1890–1904. doi: 10.1021/tx1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Saraf A, Kolasa T, Bhatia P, Zheng GZ, Patel M, Lannoye GS, Richardson P, Stewart A, Rogers JC, Brioni JD, Surowy CS. Fatty acid amide hydrolase inhibitors display broad selectivity and inhibit multiple carboxylesterases as off-targets. Neuropharmacology. 2007;52:1095–1105. doi: 10.1016/j.neuropharm.2006.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.