Abstract
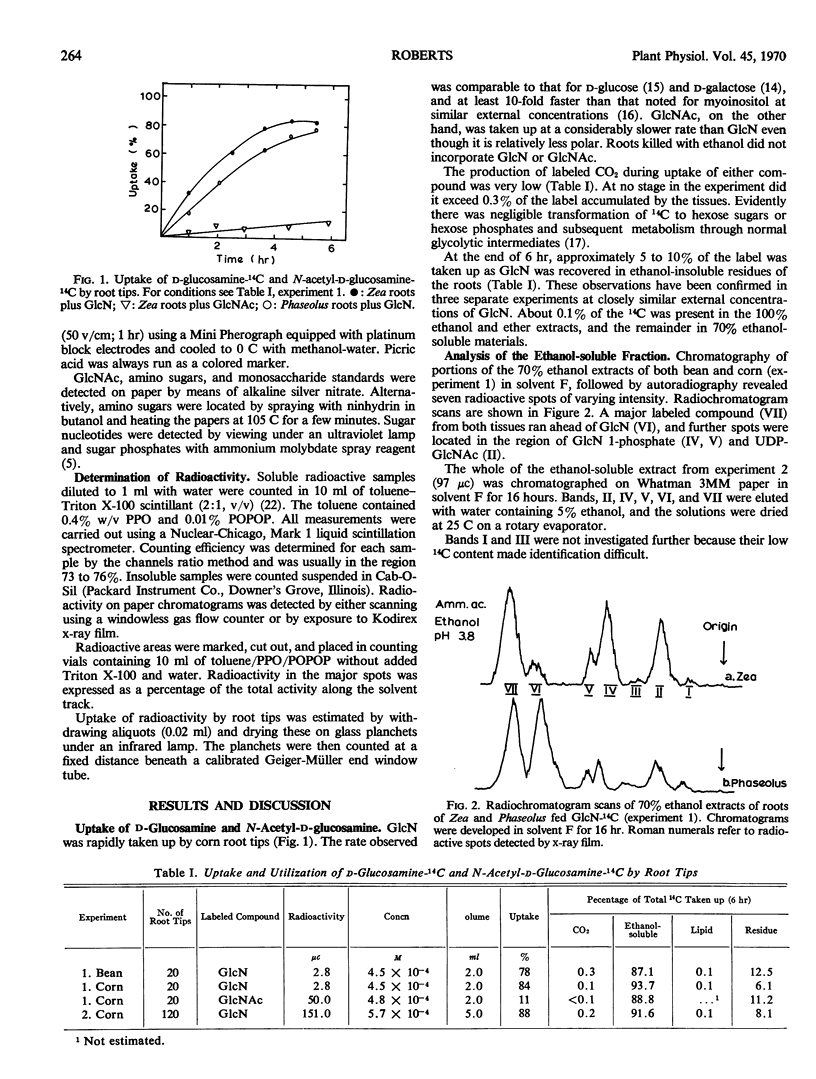
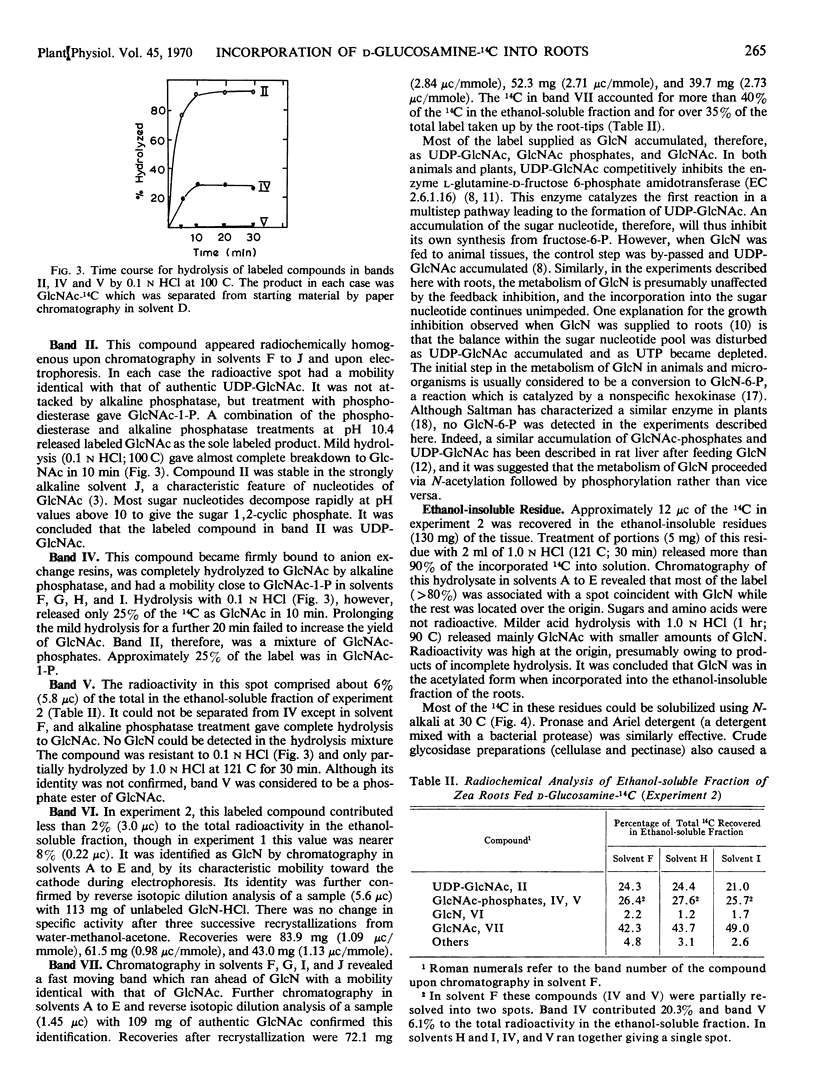
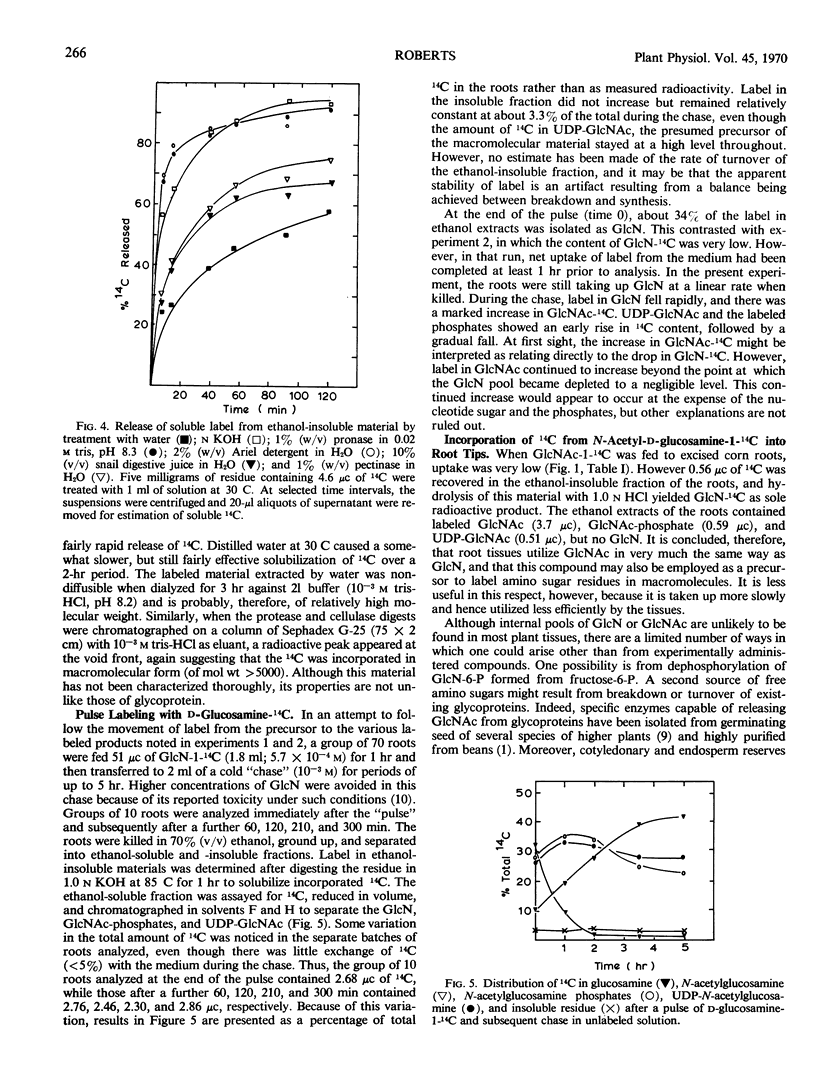
d-Glucosamine-1-14C was rapidly taken up from aqueous solution by both excised bean (Phaseolus vulgaris) and corn (Zea mays) root tips. The labeled glucosamine did not accumulate in the tissues, however, but was metabolized to N-acetyl-d-glucosamine, N-acetyl-d-glucosamine phosphates, and uridine diphosphate N-acetyl-d-glucosamine. Little or no label was detected in respiratory CO2, glycolytic intermediates, or d-glucosamine 6-phosphate. Between 5 and 10% of the 14C was recovered in high molecular weight ethanol-insoluble materials which could be solubilized readily with alkali or by treatment with proteases, and which yielded labeled glucosamine upon complete hydrolysis with HCl. Milder hydrolytic conditions released quantities of N-acetylglucosamine-14C plus labeled fragments of higher molecular weight. It is concluded that d-glucosamine-14C may be used to label specifically the amino sugar residues of plant as well as animal macromolecules. N-Acetyl-d-glucosamine acts similarly as a precursor, except that it is taken up at only about 1/10 the rate of glucosamine and hence is utilized less efficiently.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahl O. P., Agrawal K. M. Glycosidases of Phaseolus vulgaris. I. Isolation and characterization of beta-N-acetylglucosaminidase. J Biol Chem. 1968 Jan 10;243(1):98–102. [PubMed] [Google Scholar]
- Boundy J. A., Wall J. S., Turner J. E., Woychik J. H., Dimler R. J. A mucopolysaccharide containing hydroxyproline from corn pericarp. Isolation and composition. J Biol Chem. 1967 May 25;242(10):2410–2415. [PubMed] [Google Scholar]
- GONZALEZ N. S., PONTIS H. G. Uridine diphosphate fructose and uridine diphosphate acetylgalactosamine from dahlia tubers. Biochim Biophys Acta. 1963 Jan 1;69:179–181. doi: 10.1016/0006-3002(63)91243-5. [DOI] [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- KOHN P., WINZLER J., HOFFMAN R. C. Metabolism of D-glucosamine and N-acetyl-D-glucosamine in the intact rat. J Biol Chem. 1962 Feb;237:304–308. [PubMed] [Google Scholar]
- KORNFELD S., KORNFELD R., NEUFELD E. F., O'BRIEN P. J. THE FEEDBACK CONTROL OF SUGAR NUCLEOTIDE BIOSYNTHESIS IN LIVER. Proc Natl Acad Sci U S A. 1964 Aug;52:371–379. doi: 10.1073/pnas.52.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb A. J. The separation of three L-fucose-binding proteins of Lotus tetragonolobus. Biochim Biophys Acta. 1968 Dec 3;168(3):532–536. doi: 10.1016/0005-2795(68)90186-4. [DOI] [PubMed] [Google Scholar]
- Mayer F. C., Bikel I., Hassid W. Z. Pathway of Uridine Diphosphate N-Acetyl-d-Glucosamine Biosynthesis in Phaseolus aureus. Plant Physiol. 1968 Jul;43(7):1097–1107. doi: 10.1104/pp.43.7.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEMAN S. Metabolism of connective tissue. Annu Rev Biochem. 1959;28:545–578. doi: 10.1146/annurev.bi.28.070159.002553. [DOI] [PubMed] [Google Scholar]
- Roberts R. M., Deshusses J., Loewus F. Inositol Metabolism in Plants. V. Conversion of Myo-inositol to Uronic Acid and Pentose Units of Acidic Polysaccharides in Root-tips of Zea mays. Plant Physiol. 1968 Jun;43(6):979–989. doi: 10.1104/pp.43.6.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALTMAN P. Hexokinase in higher plants. J Biol Chem. 1953 Jan;200(1):145–154. [PubMed] [Google Scholar]
- SHETLAR M. R., CAPPS J. C., HERN D. L. INCORPORATION OF RADIOACTIVE GLUCOSAMINE INTO THE SERUM PROTEINS OF INTACT RATS AND RABBITS. Biochim Biophys Acta. 1964 Mar 2;83:93–101. doi: 10.1016/0926-6526(64)90055-2. [DOI] [PubMed] [Google Scholar]
- SOLMS J., HASSID W. Z. Isolation of uridine diphosphate N-acetylglucosamine and uridine diphosphate glucuronic acid from mung bean seedlings. J Biol Chem. 1957 Sep;228(1):357–364. [PubMed] [Google Scholar]
- Shannon L. M., Kay E., Lew J. Y. Peroxidase isozymes from horseradish roots. I. Isolation and physical properties. J Biol Chem. 1966 May 10;241(9):2166–2172. [PubMed] [Google Scholar]
- Turner J. C. Triton X-100 scintillant for carbon-14 labelled materials. Int J Appl Radiat Isot. 1968 Jul;19(7):557–563. doi: 10.1016/0020-708x(68)90065-3. [DOI] [PubMed] [Google Scholar]


