Abstract
Cotton plants, variety Acala 4-42 family 77 (Gossypium hirsutum L.,), were stem puncture-inoculated with either a defoliating isolate (T9) or a nondefoliating isolate (SS4) of Verticillium albo-atrum (Reinke and Berth.). As symptoms developed, growth regulators were assayed in diseased plants to discern their importance in the disease syndrome.
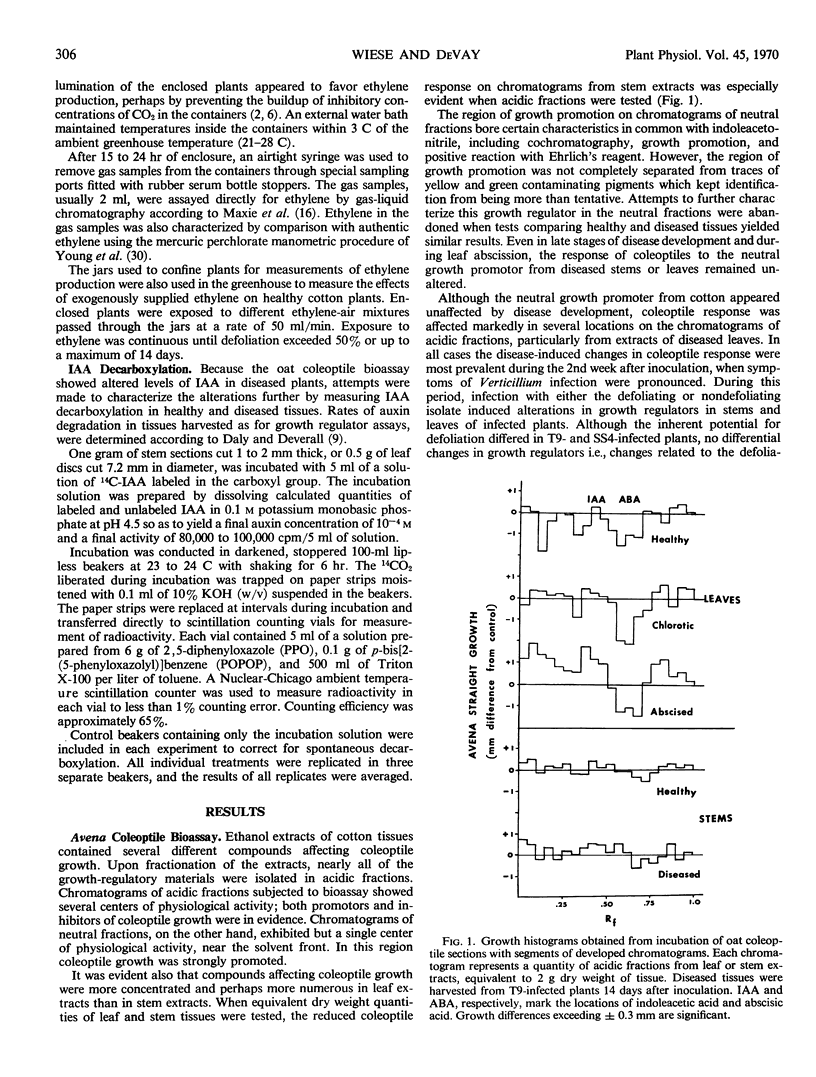
An Avena coleoptile straight growth bioassay demonstrated the presence of several growth-regulatory compounds in cotton tissue extracts. Indoleacetic acid was among the compounds whose effects on coleoptile growth were influenced by disease development. Coleoptile growth due to indoleacetic acid was greater in extracts of diseased stems and leaves than in extracts of comparable healthy tissues. During the defoliation period the T9 and SS4 isolates appeared equally effective in increasing indoleacetic acid and reducing indoleacetic acid decarboxylation. Preceding defoliation, however, in plants showing equivalent symptoms the degradation of auxin was reduced more by infection with T9, the defoliating isolate. The reduced auxin degradation appeared to be releated to concomitant increases in caffeic acid and other indoleacetic acid-oxidase inhibitors in the affected tissues.
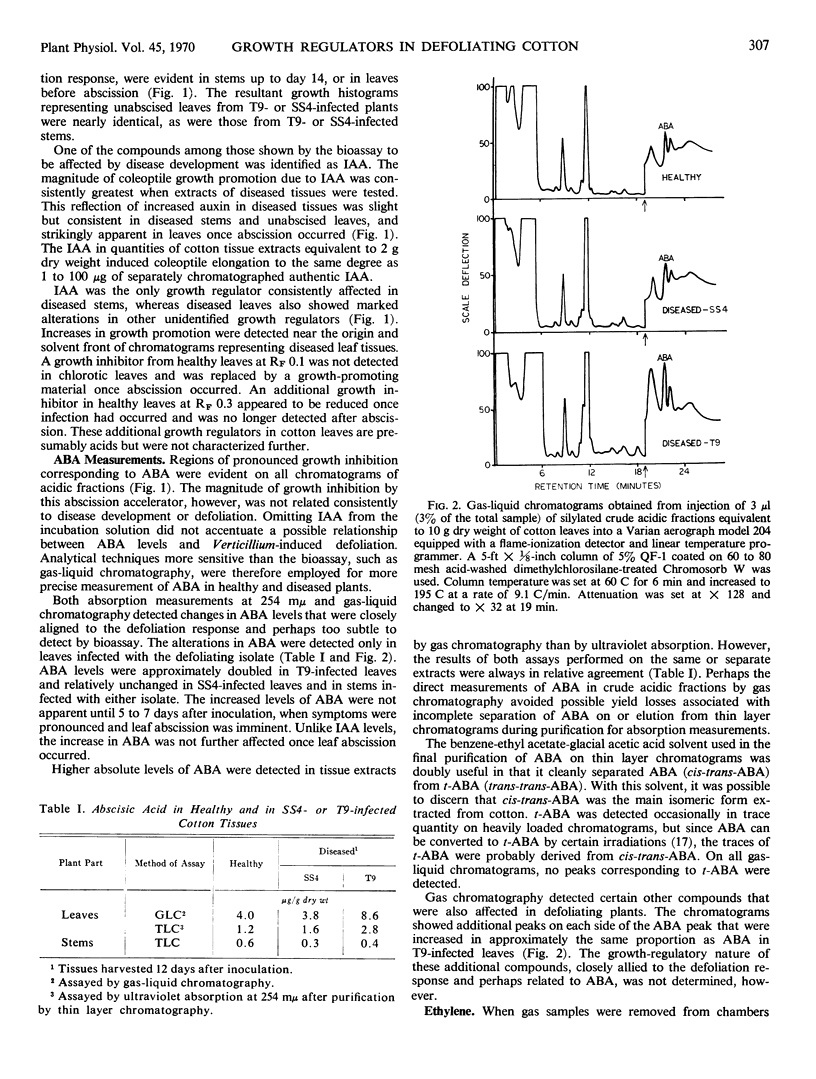
Abscisic acid in tissue extracts strongly inhibited coleoptile growth. During the defoliation period gas-liquid chromatographic and ultraviolet absorption measurements revealed that abscisic acid levels were approximately doubled in T9-infected leaves but were relatively unaffected in leaves infected with the nondefoliating isolate and in stems infected with either isolate.
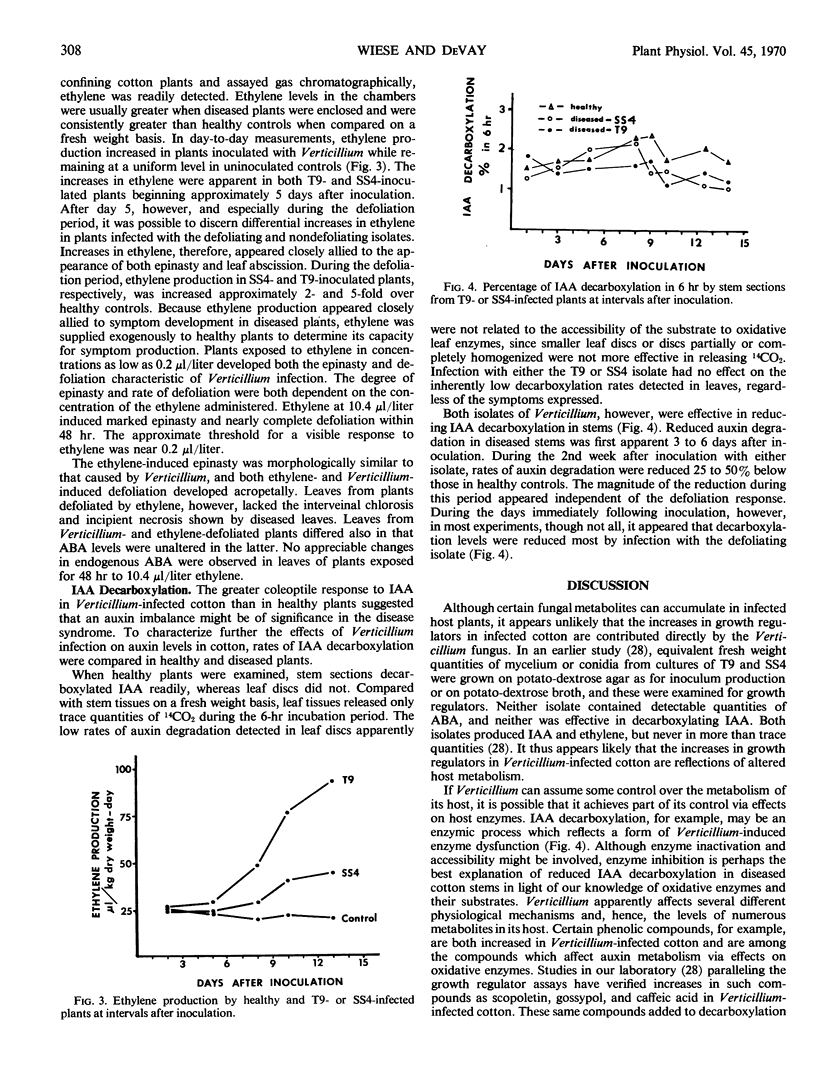
The onset of epinasty and especially defoliation was also accompanied by increased ethylene production in diseased plants. Ethylene in gas samples taken from jars confining plants infected with SS4 or T9, respectively, was increased 2- and 5-fold over uninoculated controls. Ethylene supplied exogenously to healthy plants in concentrations as low as 0.2 microliter per liter induced both the epinasty and defoliation symptoms characteristic of Verticillium infection. Ethylene treatment did not, however, induce other symptoms of Verticillium infection and did not affect endogenous levels of abscisic acid.
Defoliation of T9-but not SS4-infected plants apparently is related to the differential alterations in abscisic acid and ethylene levels induced by each isolate, and perhaps to differential alterations in initial rates of indoleacetic acid decarboxylation. These growth regulator alterations apparently are reflections of altered host metabolism rather than direct contributions of the invading fungus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles F. B., Gahagan H. E. Abscission: the role of ethylene, ethylene analogues, carbon dioxide, and oxygen. Plant Physiol. 1968 Aug;43(8):1255–1258. doi: 10.1104/pp.43.8.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeles F. B., Rubinstein B. Regulation of Ethylene Evolution and Leaf Abscission by Auxin. Plant Physiol. 1964 Nov;39(6):963–969. doi: 10.1104/pp.39.6.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Molecular requirements for the biological activity of ethylene. Plant Physiol. 1967 Jan;42(1):144–152. doi: 10.1104/pp.42.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. The interaction between auxin and ethylene and its role in plant growth. Proc Natl Acad Sci U S A. 1966 Feb;55(2):262–269. doi: 10.1073/pnas.55.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick A. V., Burg S. P. An explanation of the inhibition of root growth caused by indole-3-acetic Acid. Plant Physiol. 1967 Mar;42(3):415–420. doi: 10.1104/pp.42.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. M., Deverall B. J. Metabolism of Indoleacetic Acid in Rust Diseases. I. Factors Influencing Rates of Decarboxylation. Plant Physiol. 1963 Nov;38(6):741–750. doi: 10.1104/pp.38.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L. A., Heinz D. E., Addicott F. T. Gas-liquid chromatography of trimethylsilyl derivatives of abscisic Acid and other plant hormones. Plant Physiol. 1968 Sep;43(9):1389–1394. doi: 10.1104/pp.43.9.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. A., Weber R. P. COLORIMETRIC ESTIMATION OF INDOLEACETIC ACID. Plant Physiol. 1951 Jan;26(1):192–195. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxie E. C., Eaks I. L., Sommer N. F., Rae H. L., El-Batal S. Effect of Gamma Radiation on Rate of Ethylene and Carbon Dioxide Evolution by Lemon Fruit. Plant Physiol. 1965 May;40(3):407–409. doi: 10.1104/pp.40.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan P. W., Gausman H. W. Effects of ethylene on auxin transport. Plant Physiol. 1966 Jan;41(1):45–52. doi: 10.1104/pp.41.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan P. W., Hall W. C. Indoleacetic Acid Oxidizing Enzyme & Inhibitors from Light-Grown Cotton. Plant Physiol. 1963 Jul;38(4):365–370. doi: 10.1104/pp.38.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdovinos J. G., Ernest L. C., Henry E. W. Effect of ethylene and gibberellic Acid on auxin synthesis in plant tissues. Plant Physiol. 1967 Dec;42(12):1803–1806. doi: 10.1104/pp.42.12.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton D. C., Sondheimer E. Effects of Abscisin II on Phenylalanine Ammonia-Lyase Activity in Excised Bean Axes. Plant Physiol. 1968 Mar;43(3):467–469. doi: 10.1104/pp.43.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]


