Abstract
Prior genetic correlation analysis of 22 heritable behavioral measures of nociception and hypersensitivity in the mouse identified five genetically distinct pain types. In the present study, we reanalyzed that dataset and included the results of an additional nine assays of nociception and hypersensitivity to: 1) replicate the previously identified five pain types; 2) test whether any of the newly added pain assays represent novel genetically distinct pain types; 3) test the level of genetic relatedness among nine commonly employed neuropathic pain assays. Multivariate analysis of pairwise correlations between assays shows that the newly added zymosan-induced heat hypersensitivity assay does not conform to the two previously identified groups of heat hypersensitivity assays and cyclophosphamide-induced cystitis, the first organ-specific visceral pain model examined, is genetically distinct from other inflammatory assays. The four included mechanical hypersensitivity assays are genetically distinct, and do not comprise a single pain type as previously reported. Among the nine neuropathic pain assays including autotomy, chemotherapy, nerve ligation and spared nerve injury assays, at least four genetically distinct types of neuropathic sensory abnormalities were identified, corresponding to differences in nerve injury method. In addition, two itch assays and Comt genotype were compared to the expanded set of nociception and hypersensitivity assays. Comt genotype was strongly related only to spontaneous inflammatory nociception assays. These results indicate the priority for continued investigation of genetic mechanisms in several assays newly identified to represent genetically distinct pain types.
1. Introduction
Significant progress has been made in determining genetic loci and candidates genes underlying heritable differences in pain and analgesia traits in animals and humans [20]. More studies are needed to determine relative levels of genetic control of these modalities and how the genetic mechanisms differ between pain types. Thus, further analysis of the relationships among highly heritable assays of nociception and hypersensitivity is warranted to determine how genetically distinct or similar an assay is to other assays and traits [8,14,17,25] in order to identify types and specific examples of assays for which further investigation is a priority.
Previously, by investigating genetic correlations among 12 inbred strains on 22 common assays of nociception and hypersensitivity we provided evidence for at least five genetically distinct types of nociception and hypersensitivity: (1) thermal nociception; (2) spontaneous responses to noxious chemical/inflammatory stimuli; (3) thermal hypersensitivity; (4) mechanical hypersensitivity; (5) afferent input-dependent hypersensitivity [17,25], where hypersensitivity is defined as increased sensitivity to a response-evoking stimulus after treatment compared to prior to treatment. Inbred strains most sensitive to one assay within an assay type, or set of positively correlated assays, are more likely to show high sensitivity to other assays within that same type of assay but not to other assay types. For example, the strains most sensitive in the hot plate test are also most sensitive in Hargreaves’ thermal plantar hind paw test using radiant heat but not to mechanical stimulation after nerve injury [25].
It has been more than a decade since the previous installment of this analysis [17] and several highly relevant data sets from nociception and hypersensitivity assays have been produced since that time [5, 6, 12, 19, 22, 33]. The present study re-examines the genetic relationships among murine assays with the addition of nine new assays of nociception and hypersensitivity to: 1) test the robustness of previously identified pain types; 2) test whether any of the newly added pain assays represent novel genetically distinct pain types; 3) test the genetic relationships among commonly employed neuropathic pain assays. The new assays include: one thermal hypersensitivity assay induced by zymosan (ZYMHT); a cystitis model of cyclophosphamide-induced bladder inflammation (CPLOCO); two mechanical hypersensitivity assays induced by paclitaxel and spared nerve injury of the sciatic nerve branches (PACVF, SNIVF); an assay of spinal and saphenous nerve transection-induced autotomy (AUTSNN); four cold hypersensitivity assays induced by zymosan, paclitaxel and in the spared sciatic nerve injury assay assessed with acetone or tail withdrawal from cold liquid (PACACET, PACCOLD, SNIACET, ZYMACET). Two assays of itch sensitivity (CHLITCH, HISTITCH) have been included to test the genetic relatedness of pain and itch given the mounting evidence for interactions of these two systems [28,29]. Catechol-O-methyltransferase (Comt) is one of the first genes associated with pain in humans and is well tested across multiple experimental and patient cohorts [16]. Genotype status for Comt is also included to test specific hypotheses regarding its role in inflammatory and neuropathic pain and to test the ability to transfer defined human genetic mechanisms to their study in the mouse.
2. Methods
2.1. Subjects
Mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and bred in-house. Only data from male mice are included in the analysis. The following strains were used (all ‘J’ substrains): 129P3, A, AKR, BALB/c, C3H/He, C57BL/6, C57BL/10, C58, CBA, DBA/2, RIIIS, and SM. Mice were housed with same-sex littermates in groups of four or less, and maintained on a 12-h light/dark cycle (lights on at 07:00 h), with food (Harlan Teklad Laboratory Diet) and tap water available ad libitum. Mice in the spinal nerve neuroma autotomy assay (AUTSNN) had available ad libitum, solid pelleted food from Koffolk, Petah Tikva, Israel (product #19520), a standard rodent chow used in Israel that contains soy protein. All experiments were performed during the light phase. A single investigator performed all of the measurements for any given assay. Each mouse was used only once on a single assay. Experiments adhered to the guidelines of the Committee for Research and Ethical Issues of IASP [37] and were reviewed by the institutional animal care and use committee of each institution.
2.2. Nociception and hypersensitivity assays
2.2.1. Previously examined assays
Refer to Mogil et al., 1999 [24] for detailed protocols of acetic acid and magnesium sulfate abdominal constriction (writhing) tests (ACAA, ACMS), autotomy following sciatic and saphenous nerve transection (AUT), carrageenan-induced thermal hypersensitivity assessed with Hargreaves’ test (CARHT), the early/acute and late/tonic phases of the formalin test (FEarly, FLate), the hot-plate test (HP), Hargreaves’ thermal paw-withdrawal test (HT), peripheral spinal nerve ligation-induced thermal and mechanical hypersensitivity assessed with Hargreaves’ test and the von test (PNIHT, PNIVF; also known as spinal nerve ligation assays, often abbreviated SNL), the 49°C water tail-withdrawal test (TW49), and the von Frey monofilament test of punctate mechanical sensitivity (VF). Similarly, the reader is referred to Lariviere et al., 2002 [17] for detailed protocols of bee venom-induced spontaneous nociceptive hindpaw licking behavior (BV), bee venom-induced thermal hypersensitivity assessed with Hargreaves’ test in the ipsilateral and contralateral hind paws (BVHT, BVCON), capsaicin-induced spontaneous hindpaw licking (CAP), capsaicin-induced thermal hypersensitivity assessed with the 47.5°C water tail-withdrawal test (CAPTW), intrathecal dynorphin-induced mechanical hypersensitivity assessed with the von Frey test (DYNVF), the tail-clip test of mechanical nociception (TC), and tail withdrawal from −15°C ethanol (TW−15) or 47.5°C water (TW47.5). Abbreviations and assays are listed in Table 1 for quick reference.
Table 1.
Abbreviations of assays.*
| Abbreviation | Assay |
|---|---|
| ACAA | Abdominal constrictions following intraperitoneal injection of acetic acid |
| ACMS | Abdominal constrictions following intraperitoneal injection of magnesium sulfate |
| AUT | Autotomy following transection of sciatic and saphenous nerves |
| AUTSNN | Autotomy following spinal nerve neuroma induced by transection of lumbar nerves L4-L6 and saphenous nerve |
| BV | Bee venom, intraplantar injection-induced hindpaw licking |
| BVCON | Bee venom-induced thermal hypersensitivity assessed with Hargreaves’ test, contralateral hindpaw |
| BVHT | Bee venom-induced thermal hypersensitivity assessed with Hargreaves’ test, ipsilateral hindpaw |
| CAP | Capsaicin, intraplantar injection-induced hindpaw licking |
| CAPTW | Capsaicin, tail injection-induced thermal hypersensitivity assessed with tail withdrawal from 47°C water |
| CARHT | Carrageenan, intraplantar injection-induced thermal hypersensitivity assessed with Hargreaves’ test |
| CHLITCH | Chloroquine, subcutaneous injection-induced maximum itch duration |
| ComtB2i | Catechol-O-methyltransferase genotype as absence of B2 SINE in 3′ UTR |
| CPLOCO | Cyclophosphamide cystitis, intraperitoneal injection-induced hypolocomotion |
| DYNVF | Dynorphin, intrathecal injection-induced mechanical hypersensitivity assessed with von Frey test |
| FEarly | Formalin, intraplantar injection-induced hindpaw licking, early/acute phase |
| FLate | Formalin, intraplantar injection-induced hindpaw licking, late/tonic phase |
| HISTITCH | Histamine, subcutaneous injection-induced itch duration |
| HP | Hot-plate test, latency to paw withdrawal or jump response |
| HT | Hargreaves’ plantar thermal nociception test, latency to paw withdrawal from light focused on hindpaw |
| PACACET | Paclitaxel, intraperitoneal injection-induced cold hypersensitivity assessed with acetone test |
| PACCOLD | Paclitaxel, intraperitoneal injection-induced cold hypersensitivity assessed with tail withdrawal from −15°C ethanol |
| PACVF | Paclitaxel, intraperitoneal injection-induced mechanical hypersensitivity assessed with von Frey test |
| PNIHT | Peripheral nerve injury#-induced thermal hypersensitivity assessed with Hargreaves’ test |
| PNIVF | Peripheral nerve injury#-induced mechanical hypersensitivity assessed with von Frey test |
| SNIACET | Spared nerve injury-induced cold hypersensitivity assessed with acetone test |
| SNIVF | Spared nerve injury-induced mechanical hypersensitivity assessed with von Frey test |
| TC | Tail-clip test, latency to bring nose to alligator clip applied to base of tail |
| TF | Tail flick from radiant heat source, latency to flick tail |
| TW−15 | Tail withdrawal from −15°C ethanol, latency to withdraw tail |
| TW47.5 | Tail withdrawal from 47.5°C water, latency to withdraw tail |
| TW49 | Tail withdrawal from 49°C water, latency to withdraw tail |
| VF | von Frey monofilament test, 50% response threshold using up-down method |
| ZYMACET | Zymosan, intraplantar injection-induced cold hypersensitivity assessed with acetone test |
| ZYMHT | Zymosan, intraplantar injection-induced thermal hypersensitivity assessed with Hargreaves’ test |
Font color of abbreviations refers to the genetically distinct type of nociception or hypersensitivity identified previously [17]: (red) thermal nociception in otherwise naïve mice; (green) spontaneous responses to noxious chemical/inflammatory stimuli; (orange) thermal hypersensitivity; (blue) mechanical hypersensitivity; (purple) afferent input-dependent hypersensitivity. Brown font refers to mechanical sensitivity in naïve mice and mechanical nociception. Black font is used for assays newly included in the current analysis.
also referred to as the spinal nerve ligation (SNL) model.
2.2.2. Cyclophosphamide cystitis-induced hypolocomotion (CPLOCO)
Cyclophosphamide is an antitumor agent that has been shown to cause urinary bladder cystitis in humans and mice. It is converted by the kidney to acrolein which subsequently accumulates in the bladder causing painful cystitis when urine is retained within the bladder. As described previously [5], mice (n = 4–6/strain) were given a single intraperitoneal (i.p.) injection of cyclophosphamide (200 mg/kg) at the onset of the dark cycle and immediately placed in individual cages. Briefly, horizontal (walking) and vertical (rearing) locomotor activity were recorded as counts of infrared beam crosses using Opto-Varimex Animal Activity System (Columbus Instruments, Columbus, OH) for 4 hours following cyclophosphamide administration and mean percent hypolocomotion (CPLOCO) was calculated compared to locomotor activity for 4 hours prior to cyclophosphamide treatment.
2.2.3. Paclitaxel-induced neuropathic hypersensitivity (PACACET, PACCOLD, PACVF)
Paclitaxel is an antineoplastic agent that has been shown to cause dose-dependent peripheral sensory neuropathy. As described previously [33], paclitaxel (in 10% saline and Cremophor EL) was diluted in saline to a concentration of 0.1 mg/ml. Mice received a total of 4 i.p. injections of paclitaxel on days 1, 3, 5 and 7 in a volume of 10 ml/kg for a cumulative dose of 4 mg/kg. For mechanical hypersensitivity (PACVF), mice (n = 4–6/strain) were habituated to the plastic chamber testing apparatus on a wire mesh floor prior to each testing session. Prior to paclitaxel treatment and each day thereafter for 15 days, von Frey type fibers producing forces of 0.10 g, 0.45 g, and 1.40 g, respectively, were applied to the plantar surface of the hindpaw until bent and then held for 5 s or until a withdrawal response was made. Both right and left paws were tested. Each fiber was applied to the paw 5 times for a total of 30 fiber applications per mouse. Mechanical hypersensitivity was calculated as the percentage of the maximum possible effect for each testing day as ([(number of responses on the test day-number of responses prior to paclitaxel treatment)/30-number of responses prior to paclitaxel treatment] × 100) [33]. Two assays of cold sensitivity were used, the acetone test and cold liquid tail withdrawal. For the acetone test (PACACET), cold hypersensitivity was assessed for 15 days after the last paclitaxel injection by placing a small bubble of acetone against the plantar surface of the hind paw from below (n = 4 mice/strain). The time spent licking and/or shaking the paw was measured for 60 s after acetone exposure. For cold liquid tail withdrawal (PACCOLD), mice (n = 4–8/strain) were lightly restrained and their tails submerged in an ethanol solution maintained at −15°C as described previously [21]. The observed latency to withdraw the distal half of the tail from the solution was evaluated twice and averaged. This value was compared to the average latency prior to treatment to determine the percent hypersensitivity. For all variables (PACACET, PACCOLD, PACVF), the data presented are the maximum values recorded during the 15 days following the final paclitaxel administration compared to sensitivity assessed prior to paclitaxel treatment.
2.2.4. Spared-nerve injury-induced neuropathic hypersensitivity (SNIACET, SNIVF)
Spared nerve injury (SNI) was performed as described previously [9,23] and entailed transection of the tibial and common peroneal branches of the sciatic nerve and sparing of the sural branch. For mechanical threshold testing (SNIVF), mice (n = 9–16/strain) were placed in acrylic cubicles (5 cm wide × 8.5 cm long × 6 cm high), separated from neighboring mice with an opaque divider and placed on a mesh floor. All subjects were habituated for 2 h, after which nylon monofilaments were applied for 3 s to the lateral aspect of the hindpaw plantar surface until they bowed. Paw withdrawal performed obviously in response to the applied stimulus were considered positive responses. The lowest force von Frey fiber that evoked a positive response at least eight out of ten stimulations was recorded and the average of two trials 5 min apart was used as that animal’s mechanical threshold. Percent maximal mechanical hypersensitivity was calculated as the greatest percent reduction in mechanical threshold relative to prior to surgery observed over the 28 day post-operative period. For determination of cold hypersensitivity (SNIACET), mice (n = 9–16/strain) were tested by placing a small drop of acetone against the plantar surface of the hind paw from below. The time spent licking and/or shaking the paw was measured for 60 s after acetone exposure. There were no significant differences in strain means of time spent licking and shaking the paw before nerve injury, which ranged from 0.50–0.59 s across all strains (data not shown).
2.2.5. Spinal nerve neuroma-induced autotomy (AUTSNN)
All mice underwent a modified spinal nerve ligation procedure [19]. Briefly, while mice (n = 15–23/strain) were under chloral hydrate anesthesia, the lower lumbar spinal nerves were exposed unilaterally after removing the L6 transverse spinous process, and the L4, L5 and L6 lumbar spinal nerves were cut across about 4 mm from the corresponding dorsal root ganglion. Due to strain variability in the afferent contribution from L3 and to ensure uniform paw denervation across strains, the saphenous nerve was exposed on the same side, tightly ligated at mid-calf level and cut immediately distal to the ligature. A 3–4 mm segment of the resultant distal nerve stump was removed. Autotomy behavior (AUTSNN) was assessed weekly for 5 weeks post-surgery following the protocol of Wall et al. [36]. Briefly, one point was given for loss of one or more toe nails with an additional point for injury to the proximal or distal half of each digit for a maximum total score of 11. The maximum autotomy score assigned during the 5 week post-surgical observation period was used for the correlation analyses.
2.2.6. Zymosan-induced hypersensitivity (ZYMACET, ZYMHT)
Zymosan administration to the hindpaw results in dose-dependent thermal hypersensitivity that corresponds with the progression of edema in the area of administration [18]. As described previously [6], thermal hypersensitivity (ZYMHT) was determined with Hargreaves’ thermal plantar test. All mice (n = 4–8/strain) were habituated to the testing apparatus for 1 h prior to behavioral manipulations. Each mouse was placed in a small acrylic cubicle (4 cm wide × 8 cm deep × 4 cm high) on a glass surface. Prior to and following zymosan injection, a high-intensity light source (IITC Life Science Model 390 Plantar Analgesia meter, IITC, Inc., USA) at 20% of maximum intensity was focused on the plantar hindpaw and the latency to withdraw the paw was recorded twice on each hindpaw alternating side between trials. All mice received a single subcutaneous injection of zymosan (3 mg/mL; 20 uL volume) in the right plantar hindpaw and were immediately placed back in the testing apparatus. Thermal sensitivity was determined 3 h after zymosan administration, a time previously shown to be at the peak of the thermal hypersensitivity effects of zymosan [6]. Thermal hypersensitivity was determined by calculating the percent decrease in paw withdrawal latency following zymosan injection relative to the paw withdrawal latency of the same paw prior to zymosan injection. For evaluation of cold hypersensitivity following zymosan administration (ZYMACET), a drop of acetone was placed against the plantar surface of the hind paw from below a wire mesh floor (n = 4–5 mice/strain). The time spent licking and/or shaking the paw was measured for 60 s after acetone application prior to zymosan treatment and 60, 120, 180, 240 and 300 min after zymosan injection and the area under the curve used for analysis.
2.3. Itch assays
2.3.1. Chloroquine-induced itch (CHLITCH)
Mice (n = 4–5/strain) were individually habituated to an acrylic cylinder (15 cm diameter; 22 cm height) on a glass floor for 30 minutes. Each mouse was then removed from the cylinder, injected with chloroquine phosphate (Sigma, St. Louis, MO) in 50 μl of saline subcutaneously at the interscapular level of the mouse’s back and returned to the cylinder for observation as described previously [15]. Subjects were monitored for 60 min by video camera from below. Video analysis by a trained observer using specialized software (The Observer®, Noldus Information Technology, The Netherlands) involved the determination of total time (min) spent scratching the injected area. Each strain was given a range of doses of chloroquine guided by preliminary data suggesting the optimal single dose for inducing itch. Beginning with a dose of 400 μg, all strains were subject to chloroquine dose increases or decreases depending on data collected up to that point. Dosing continued until each strain was represented by a minimum of four doses and until the lowest and highest doses tested yielded similar total scratching times. Data used for analysis are maximal scratching times (s) elicited across doses (CHLITCH) for a given strain.
2.3.2. Histamine-induced itch (HISTITCH)
As described previously [12,15], histamine (375 μg/mouse in 50 μl saline) was injected subcutaneously at the interscapular level. Mice (n = 4–7/strain) were individually habituated to the acrylic observation cylinder on a glass floor for 30 minutes prior to injection. Each mouse was removed from the cylinder following habituation, injected with histamine, and returned to the cylinder. Subjects were monitored for 60 min by video camera from below and total time (min) spent scratching the injected area was determined by a trained observer using specialized video analysis software (The Observer®, Noldus Information Technology, The Netherlands).
2.4. Comt genotype (ComtB2i)
The Comt gene encodes for catecholamine-O-methyltransferase (COMT), an enzyme responsible for the elimination/inactivation of catecholamines [3]. Altered COMT enzymatic activity has been linked, albeit controversially, to differences in pain perception in humans [10,11,20,38]. In standard inbred strains of mice, the presence or absence of a B2 short interspersed nuclear element (SINE) in the 3′ UTR of the mRNA has been identified as part of a common haplotype that correlates highly with Comt gene expression [31]. Strains without the B2 SINE in the 3′ UTR (abbreviated in this report as ComtB2i for the absence of the B2 SINE) have lower COMT enzymatic activity and increased inflammatory pain sensitivity [31], whereas attenuating COMT activity has been hypothesized to protect against neuropathic pain [4,30,35]. In the present analysis, strains were assigned a 0 (A, AKR, BALB/c, C57BL/6, C57BL/10 and SM) or 1 (129P3, C3H/He, C58, CBA, DBA/2 and RIIIS) based on the presence or absence of the ComtB2i genotype, respectively, and this Comt genotype was compared to all nociception and hypersensitivity assays. Note that since these strains are inbred, they carry homozygous alleles throughout the genome, and thus, the genotyped markers comprise a diplotype that doubles the effect of carrying a ComtB2i genotype.
2.5. Data analysis
2.5.1. Strain differences
The significance of the effect of mouse strain on the assay means was determined for each behavioral assay with a one-way analysis of variance.
2.5.2. Correlations between assays
Correlation coefficients were calculated using Spearman’s statistic (rs) for strain mean rank data as previously described [17,25]. All data were corrected for sign, such that ranks closer to 1 represent greater sensitivity in each behavioral assay. Strain mean ranks are listed in the Supplementary Material. As described previously [17,25], the primary goal of the present study is exploratory, for hypothesis generation and testing regarding the interrelatedness of the assays. As such, no significance testing of correlations was performed.
2.5.3. Multivariate analysis
Principal components analysis (PCA) and multidimensional-scaling (MDS) were used to produce two-dimensional graphical representations of the pairwise correlations between assays and the interrelatedness of the assays as previously [17,25]. In a two-factor PCA, two linear combinations of the assays are constructed based on the correlation matrix and the combined weight is used to plot a two-dimensional vector for each assay. High positive correlations are represented as angles between vectors that approach 0° and high negative correlations are represented by angles close to 180°. Uncorrelated assays have angles between vectors around 90°. Additionally, the vector length from the origin to any given assay is indicative of how much assay variance is explained by the two factors. PCA plots shown are of the first two unrotated principal components. In a MDS plot, two-dimensional coordinates are computed to fit as closely as possible the matrix of pairwise correlations where high positive correlations are displayed with small inter-point distances, high negative correlations are displayed with large inter-object distances, and uncorrelated assays have intermediate inter-point distances.
While the correlation coefficient between any pair of assays is constant, the position of the assays in the PCA or MDS plot is determined as a function of every interrelationship present. The introduction of additional assays can exert substantial influence on the final position of assays and the appearance of relationships of assays with other assays, disrupting clusters of highly positively correlated assays that can be clearly seen when the additional assays are removed from the analysis or bringing uncorrelated or negatively correlated assays closer together in the plots. This influence can result in a graphical representation that is not true to all of the pairwise correlations between assays, not due to the properties of the assays themselves, but rather to the limitations and vagaries of the multivariate analyses as new assays are forced into the limited two-dimensional space. The two incidences of graphical misrepresentations of the pairwise correlations of the current study are fully described and highlighted with parentheses in the figures.
As a result of the increased number and complexity of interrelationships in this new analysis, and to test specific hypotheses, we have selected specific subsets of assays for PCA plots. This approach allows for testing of specific hypotheses about the relatedness of assays without the undue influence of assays unrelated to the hypothesis being tested. It is this approach that is recommended for subsequent, long term use of this data (publicly available at http://phenome.jax.org) for direct comparisons with new traits.
3. Results
3.1. Strain differences
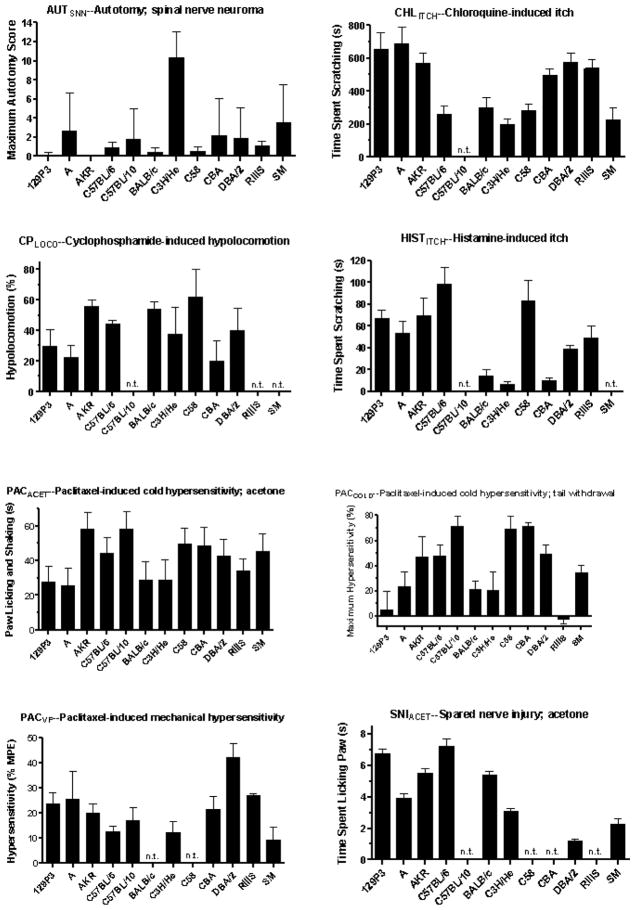
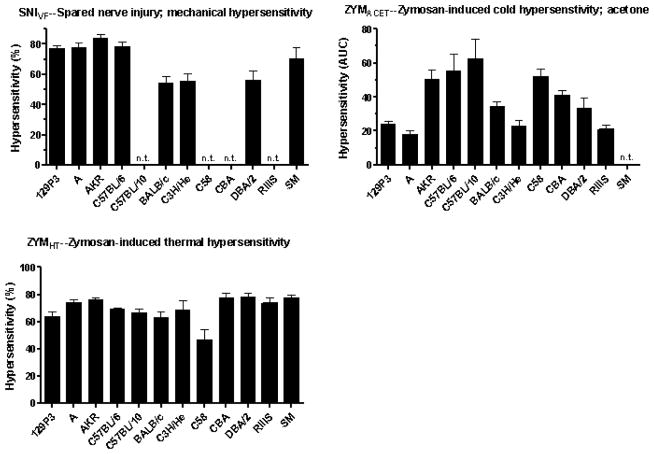
Strain means for newly included assays and traits are illustrated in Fig. 1. Strain means of the 22 previously analyzed assays have been published elsewhere [17,24]. As expected and observed numerous times previously, sensitivity varies greatly across mouse strains and a significant effect of mouse strain was obtained in all assays (P < 0.01).
Fig. 1.
Mean responses of up to 12 inbred mouse strains on 11 behavioral measures of nociception, hypersensitivity and itch. The strain means for AUTSNN and a portion of those for SNIVF have been published previously [19,34]. A significant effect of mouse strain is seen in all assays (P < 0.01). Abbreviations: AUC, area under the curve; MPE, maximum possible effect; n.t., strain not tested.
3.2. Correlations between assays
Spearman correlations (rs) between the new traits and all traits examined to date are shown in Table 2. Correlations between the previously examined assays have been published elsewhere [17,25].
Table 2.
Spearman coefficients of rank correlation between the newly added assays (top row) and all assays studied thus far (left column).*
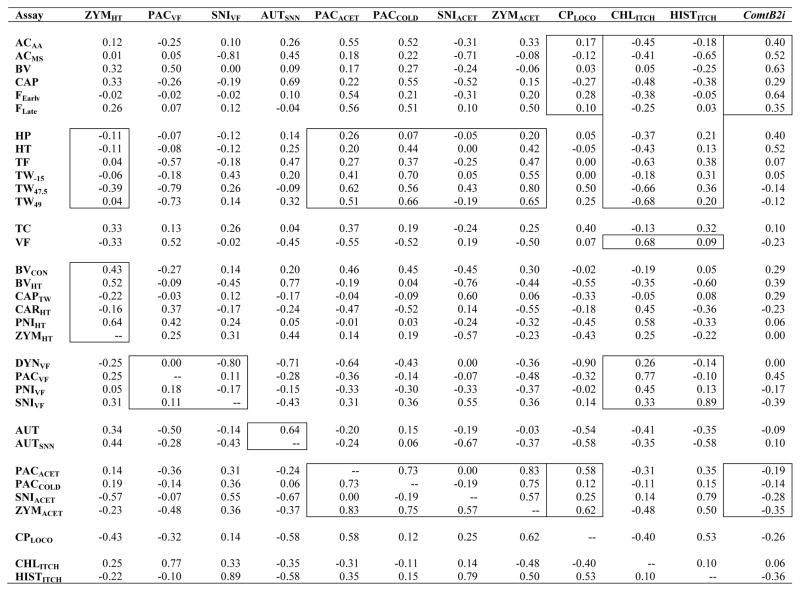
|
Correlations indicated within boxes, from left to right columns: zymosan-induced heat hypersensitivity (ZYMHT) is correlated close to zero with thermal nociception assays in otherwise naïve mice, and variably correlated with other heat hypersensitivity assays (Fig. 3); paclitaxel-induced and spared nerve injury-induced mechanical hypersensitivity (PACVF, SNIVF) are not strongly positively correlated with other assays of mechanical hypersensitivity (Fig. 5); autotomy induced by transection of spinal and saphenous nerves (AUTSNN) is moderately positively correlated with autotomy induced by transection of sciatic and saphenous nerves (Fig. 6); except for SNIACET, cold hypersensitivity assays (PACACET, PACCOLD, ZYMACET) are consistently positively correlated with thermal nociception assays, and highly positively correlated with one another (Fig. 2); cyclophosphamide cystitis-induced hypolocomotion (CPLOCO) is correlated around zero with spontaneous inflammatory nociception assays (Fig. 4A) and moderately positively correlated with two of the four cold hypersensitivity assays (Fig. 4B); both chloroquine-induced and histamine-induced itch (CHLITCH, HISTITCH) are negatively correlated with spontaneous inflammatory nociception assays, but only CHLITCH is negatively correlated with thermal nociception and consistently positively correlated with mechanical sensitivity and hypersensitivity (Fig. 7); Comt genotype associated with the absence of the B2 SINE in the 3′UTR (ComtB2i) is consistently moderately positively correlated only with spontaneous inflammatory nociception assays (Fig. 8A), and mildly negatively correlated with cold hypersensitivity assays (Fig. 8B). Font color reference of assay abbreviations is as in Table 1 legend. Correlations between previously examined assays have been published elsewhere [17,25].
3.3. Multivariate analyses
3.3.1. Overall principal component analysis (PCA) and multidimensional-scaling (MDS)
Multivariate analyses of the 31 assays of nociception and hypersensitivity resulted in two-factor PCA and MDS plots (Figs. 2A and 2B) that display less obvious isolated clustering and more overlap of previously identified assay types than observed with fewer assays [17,25]. This is because the final position of each assay in a PCA or MDS plot is determined by the interrelationships of every assay in the analysis, and several of the newly included assays show complex relationships (positive, negative and near zero correlations) with intercorrelated assays shown previously to cluster. Thermal nociception assays in otherwise naïve mice (HP, HT, TF, TW−15, TW47.5, TW49) and assays of spontaneous nociceptive responses to chemical/inflammatory stimuli in otherwise naïve mice (ACAA, AAMS, BV, CAP, FEarly, FLate) continue to group together (Fig. 2B), respectively, indicating strong genetic relatedness as previously [17,25]. These data continue to support earlier findings of clusters of thermal nociception and spontaneous inflammatory nociception assays, respectively, as the correlations between pairs of assays within these clusters display consistently moderately-to-highly positive correlations among the six assays in each cluster (average rS = 0.61 and 0.55 for the 15 correlations within clusters of inflammatory nociception assays and thermal nociception assays, respectively; 0.41 < rS < 0.79 between inflammatory nociception assays and 0.42 < rS < 0.82 for 13 of the 15 correlations between thermal nociception assays; [17,25]).
Fig. 2.
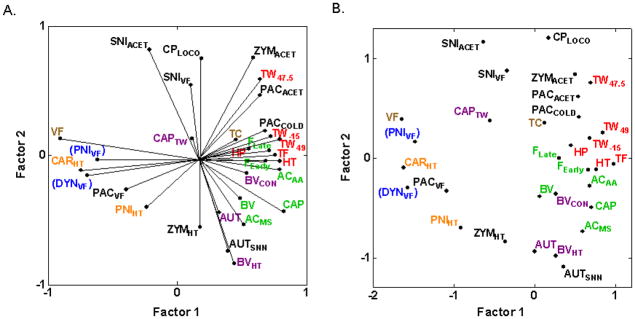
Multivariate analyses plots illustrating cross-correlations among inbred strain means for 31 assays of nociception and hypersensitivity. (A) PCA plot illustrating the first two unrotated principle components. The angle between rays projecting to the assays’ points is representative of their correlation: close to 0° indicates high positive correlations; close to 180° indicates high negative correlations; close to 90° indicates uncorrelated assays. The proportion of total variance accounted for by the two factors is 0.52. (B) MDS plot illustrating the cross-correlations in which the Euclidean distances between assays are representative of their correlation such that higher positive correlations are closer. The proportion of total variance accounted for is 0.74. A Kruskal loss function with monotonic regression was employed, resulting in a final stress of 0.23. Both plots show that thermal nociception assays in otherwise naïve mice (HP, HT, TF, TW−15, TW47.5, TW49) and assays of spontaneous nociceptive responses to chemical/inflammatory stimuli in otherwise naïve mice (ACAA, AAMS, BV, CAP, FEarly, FLate) continue to cluster together (Fig. 2B), respectively, indicating strong genetic relatedness as previously [17,25]. Cold hypersensitivity assays (PACACET, PACCOLD, SNIACET, ZYMACET) are shown to be positively correlated with thermal nociception assays. Note that graphical misrepresentation of the pairwise correlations with assays DYNVF and PNIVF exists in both PCA and MDS plots, as discussed in the text and indicated with parentheses around the assay labels. Font color refers to the genetically distinct type of nociception or hypersensitivity identified previously [17]: (red) thermal nociception in otherwise naïve mice; (green) spontaneous responses to noxious chemical/inflammatory stimuli; (orange) thermal hypersensitivity; (blue) mechanical hypersensitivity; (purple) afferent input-dependent hypersensitivity. Brown font refers to mechanical sensitivity in otherwise naïve mice and mechanical nociception. Black font is used for assays newly included in the current analysis.
Some graphical misrepresentation of the raw correlations exists in the all-inclusive PCA and MDS plots of Fig. 2, especially in the left-most portions where dynorphin-induced and spinal nerve ligation-induced mechanical hypersensitivity (DYNVF, PNIVF) have been forced toward the lower half of the graphs by the inclusion of several of the newly added assays (CPLOCO, PACACET, PACCOLD, SNIACET, SNIVF, ZYMACET) that are uncorrelated (close to zero) or negatively correlated with DYNVF and PNIVF. Most notably, although carrageenan-induced thermal hypersensitivity (CARHT) and spinal nerve ligation-induced mechanical hypersensitivity (PNIVF) are uncorrelated (rS = 0.01; [25]), they are juxtaposed in Figs. 2A and 2B. This graphical distortion does not occur when any new assay is added individually, is minimal when all new assays except AUTSNN, SNIACET and SNIVF are included, but is apparent when SNIACET and SNIVF are added in combination with any other new assays (graphs not shown). This indicates that the distortion effect is cumulative, that all graphical findings must be compared to the pairwise correlations, and that more accurate graphical representations are achieved with selective inclusion of assays to test specific hypotheses. Thus, we have selected specific subsets of assays for the subsequent multivariate analyses and PCA plots.
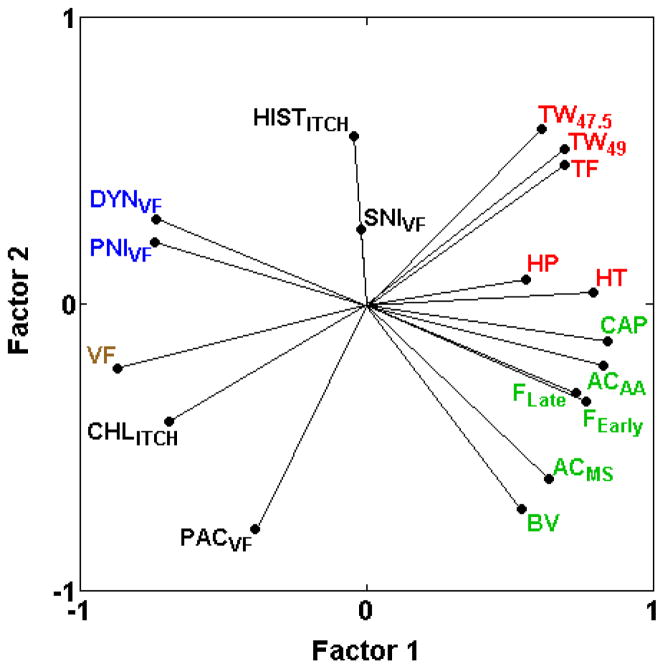
3.3.2. Relationships between heat hypersensitivity assays and thermal nociception assays in otherwise naïve mice
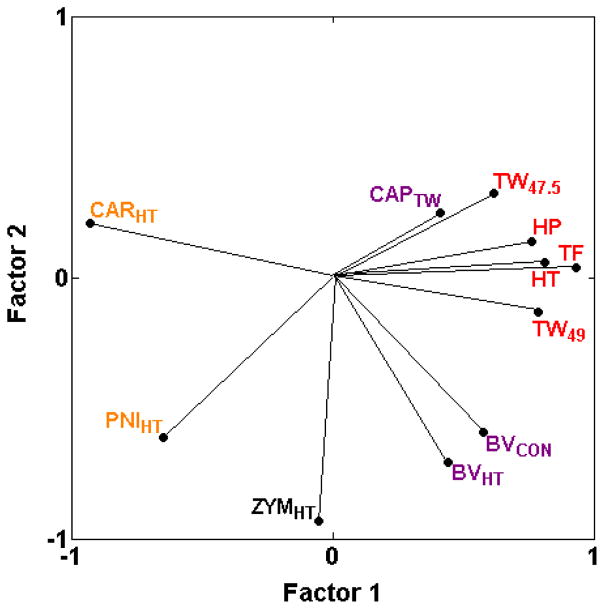
We previously reported two types of heat hypersensitivity assays, one type (BVCON, BVHT, CAPTW) showing mild to moderate positive genetic correlations with thermal nociception assays and another type (CARHT, PNIHT) displaying negative correlations with thermal nociception assays (HP, HT, TF, TW−15, TW47.5, TW49) [17]. The newly added assay of zymosan-induced heat hypersensitivity (ZYMHT) is unlike either type, displaying correlations near zero (−0.11 < rS < 0.04) with five of the six thermal nociception assays (Table 2; Fig. 3) and highly variable correlations with the heat hypersensitivity assays (Table 2).
Fig. 3.
PCA plot of cross-correlations among heat nociception assays in naïve mice and heat hypersensitivity assays. Heat hypersensitivity assays show a continuous range of relationships with heat nociception, from high positive (CAPTW) to moderate positive correlations (BVCON and BVHT), through correlations close to zero (ZYMHT) to negative correlations (PNIHT and CARHT) (Table 2; [19,28]). Thus, heat hypersensitivity assays are more or less genetically related to thermal nociception assays along a continuum. The proportion of total variance accounted for is 0.67. Font color reference is as in Fig. 2 legend.
3.3.3. Relationship of a cystitis inflammatory pain assay to other assays
The present analysis marks the first inclusion of a purely visceral pain assay, CPLOCO, induced by i.p. injection of cyclophosphamide resulting in an inflammatory cystitis confined to the bladder. As can be seen in Fig. 4A, the angles between vectors approaching 90° indicate that cyclophosphamide cystitis-induced hypolocomotion (CPLOCO) is genetically distinct from the assays of spontaneous pain behaviors induced by chemical/inflammatory irritants (ACAA, AAMS, BV, CAP, FEarly, FLate) (−0.27 ≤ rs ≤ 0.28) (Table 2). Also, CPLOCO is not positively correlated with, or is genetically distinct from, five of the six inflammatory hypersensitivity assays (−0.55 ≤ rs ≤ −0.02) (Fig. 4B) and all other types of assay considered except cold hypersensitivity assessed with acetone application (rs = 0.58 and 0.62 with PACACET and ZYMACET, respectively) (Table 2; Fig. 4B).
Fig. 4.
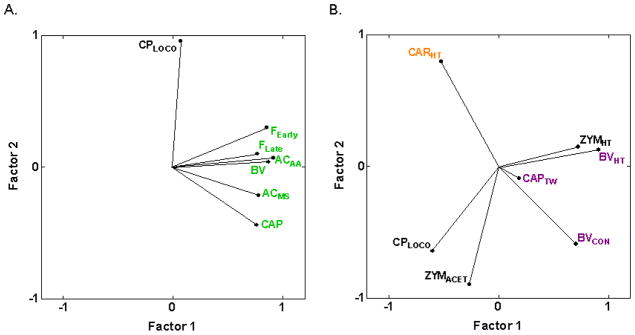
PCA plot of cross-correlations between cyclophosphamide cystitis-induced hypolocomotion (CPLOCO) and assays of spontaneous inflammatory nociception and inflammatory hypersensitivity. (A) CPLOCO is genetically distinct from all spontaneous inflammatory nociception assays as evidenced by the vector angles close to 90° between CPLOCO and the assays. The proportion of total variance accounted for is 0.76. (B) Similarly, CPLOCO is genetically distinct from all inflammation-induced hypersensitivity assays except zymosan-induced cold hypersensitivity assessed with acetone application (rs = 0.62 with ZYMACET). The proportion of total variance accounted for is 0.69. Font color reference is as in Fig. 2 legend.
3.3.4. Mechanical hypersensitivity is genetically distinct
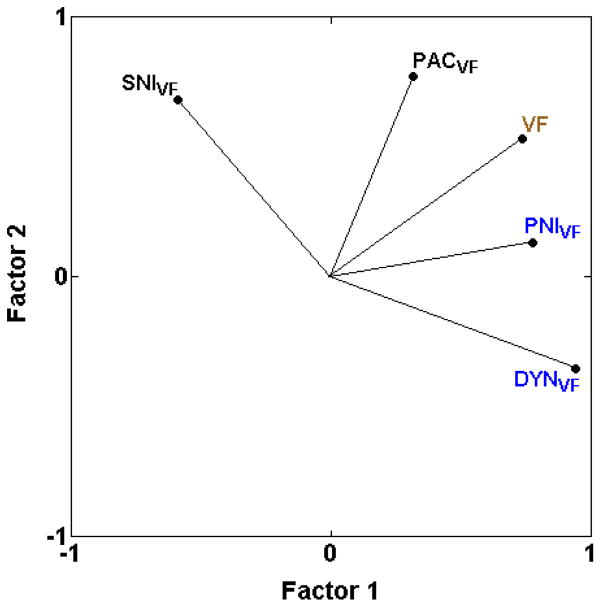
Based on the almost complete lack of strong positive correlations with other assays (with few correlations over 0.50), mechanical hypersensitivity assays (DYNVF, PACVF, PNIVF, SNIVF) do not appear to be systematically related to any other type of assay with the one exception that three of the four mechanical hypersensitivity assays are moderately positively correlated with mechanical sensitivity in otherwise naïve mice in the von Frey test (VF) (with VF = 0.54, 0.52 rS and 0.50 for DYNVF, PACVF, and PNIVF, respectively) (Table 2 and [17,25]). None of the heat hypersensitivity assays (BVHT, BVCON, CAPTW, CARHT, PNIHT, ZYMHT) or cold hypersensitivity assays (PACACET, PACCOLD, ZYMACET) are strongly positively correlated with these mechanical hypersensitivity assays (DYNVF, PACVF, PNIVF, SNIVF), even when induced by the same method of nerve injury (PACVF, PNIVF) (Table 2 and [17,25]) with only one exception. Mechanical hypersensitivity after spared nerve injury (SNIVF) is moderately positively correlated with cold hypersensitivity after the same nerve injury (SNIACET) (rS = 0.55). In addition, mechanical hypersensitivity in the two newly added assays (PACVF, SNIVF) is only weakly positively correlated with the other assays of mechanical hypersensitivity (rS ≤ 0.18; Table 2 and [17,25]; Fig. 5).
Fig. 5.
PCA plot illustrating cross-correlations among inbred strain means for mechanical sensitivity and mechanical hypersensitivity assays, illustrating the first two unrotated principle components. Mechanical hypersensitivity in the two newly added assays (PACVF and SNIVF) is not highly positively related with any of the other assays of mechanical hypersensitivity, indicating that mechanical hypersensitivity assays do not comprise a single pain type. The proportion of total variance accounted for by the two factors is 0.79. Font color reference is as in Fig. 2 legend.
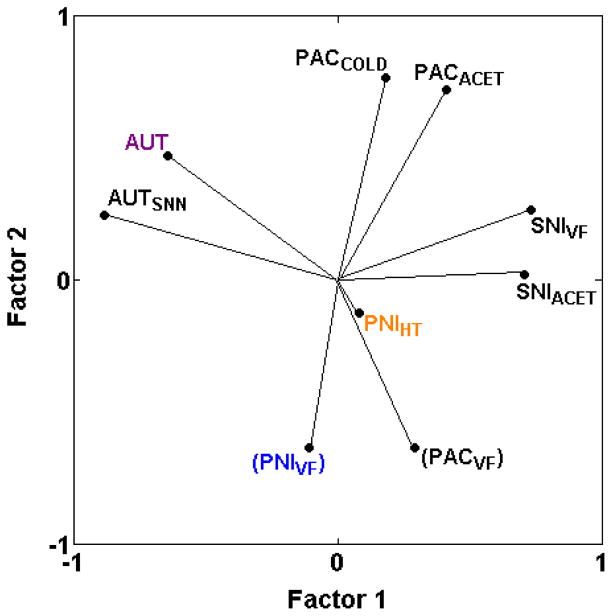
3.3.5. Relationships among assays of neuropathic pain
The addition of six new assays of neuropathic pain permits testing of several hypotheses: 1) that neuropathic mechanical and thermal hypersensitivities have different underlying genetic mechanisms; 2) that spontaneous behaviors in neuropathic pain assays have distinct genetic mechanisms; 3) that chemotherapy-induced neuropathic pain responses are distinct from physical nerve-injury induced pain responses.
The first hypothesis has been discussed above, with the conclusion that neuropathic mechanical hypersensitivity in two of three assays (PACVF, PNIVF) is genetically distinct from heat or cold hypersensitivity induced with the same methods (PACACET, PACCOLD, PNIHT). Paclitaxel-induced mechanical hypersensitivity (PACVF) is negatively correlated with cold hypersensitivity induced the same way (rs = −0.36 and −0.14 with PACACET and PACCOLD, respectively) (Table 2; Fig. 6) and mechanical hypersensitivity induced by spinal nerve ligation (PNIVF) is negatively correlated with thermal hypersensitivity induced the same way (PNIHT) (rs = −0.07) [25]. Mechanical hypersensitivity induced by spared nerve injury of branches of the sciatic nerve (SNIVF) remains the only exception (rS = 0.55 with SNIACET) (Table 2; Fig. 6).
Fig. 6.
PCA plot of cross-correlations among neuropathic pain assays. Neuropathic pain assays segregate based on whether nerve injury is caused by complete transection of spinal and/or sciatic and/or saphenous nerves (AUT, AUTSNN), transection of two of the three branches of the sciatic nerve (SNIACET, SNIVF), spinal nerve ligation (PNIHT, PNIVF), or chemotherapy drug-induced nerve injury (PACACET, PACCOLD, PACVF). Further segregation also occurs based on the method of assessment (PACVF versus PACACET, PACCOLD; PNIVF versus PNIHT). Thus, there are at least four genetically distinct types of neuropathic pain assay with little overlap across methods of nerve injury. PNIHT might represent a fifth type; the short vector for PNIHT indicates that these two principal components do not explain variance in this assay as well as in the other assays with long vectors. Note that despite the graphically close positioning of PACVF and PNIVF, these two assays are only mildly correlated (rs = 0.18; Table 2), as discussed in the text and indicated with parentheses around the assay label, indicating that the assays do not represent a single genetically distinct assay type. The proportion of total variance accounted for is 0.53. Font color reference is as in Fig. 2 legend.
Regarding the second hypothesis, the newly added assay of autotomy following transection of lumbar nerves and the saphenous nerve (AUTSNN) is positively correlated with autotomy following transection of the sciatic and saphenous nerves (AUT) (rs = 0.64). Both assays cluster together and are distinct from all neuropathic assays of mechanical, heat and cold hypersensitivities included here (PACACET, PACCOLD, PACVF, PNIHT, PNIVF, SNIACET, SNIVF) (Table 2; Fig. 6). Regarding the third hypothesis, paclitaxel-induced neuropathic cold hypersensitivity (PACACET, PACCOLD) appears to be distinct from all other neuropathic pain assays including cold hypersensitivity after spared nerve injury (SNIACET) (Table 2; Fig. 6). Similarly, paclitaxel-induced mechanical hypersensitivity (PACVF) is only weakly related to spinal nerve ligation-induced mechanical hypersensitivity (PNIVF) (rs = 0.18) and spared nerve injury-induced mechanical hypersensitivity (SNIVF) (rs = 0.11) (Table 2). However, the two spared nerve injury assays (SNIACET, SNIVF) are also distinct from all other neuropathic pain assays (Table 2, Fig. 6), as are the spinal nerve ligation assays (PNIHT, PNIVF) (Fig. 6; [25]).
Thus, the results indicate that neuropathic pain assays are genetically distinct for each method of nerve injury. That is, the neuropathic pain assays appear to segregate based on whether nerve injury is caused by complete transection of sciatic and saphenous nerves or spinal and saphenous nerves (AUT, AUTSNN), transection of two of the three branches of the sciatic nerve (SNIACET, SNIVF), spinal nerve ligation (PNIHT, PNIVF), or chemotherapy drug-induced nerve injury (PACACET, PACCOLD, PACVF). Further segregation also occurs based on the method of assessment (PACVF versus PACACET, PACCOLD; PNIVF versus PNIHT).
3.3.6. Relationship of two itch assays to assays of nociception and hypersensitivity
Despite similar outcomes, sensitivity to itch induced by chloroquine (CHLITCH) and histamine (HISTITCH) are only very weakly positively correlated (rs = 0.10; Table 2) and show different relationships with nociception and hypersensitivity assays (Fig. 7). Although both chloroquine- and histamine-induced itch are negatively correlated with most of the spontaneous inflammatory nociception assays (ACAA, AAMS, BV, CAP, FEarly, FLate), only chloroquine-induced itch consistently shows negative correlations with assays of thermal nociception (HP, HT, TF, TW−15, TW47.5, TW49) (−0.68 ≤ rs ≤ −0.18) (Table 2; Fig. 7). Furthermore, while chloroquine-induced itch shows mild to strong positive correlations with assays of mechanical sensitivity (rs = 0.68 with VF) and mechanical hypersensitivity (DYNVF, PACVF, PNIVF, SNIVF) (0.26 ≤ rs ≤ 0.77), histamine-induced itch is mostly unrelated to the same assays (−0.14 ≤ rs ≤ 0.13, except rs = 0.89 with SNIVF) (Table 2; Fig. 7). This is in agreement with previous research suggesting that itch and pain may share common signaling pathways and that itch is a heterogeneous phenomenon with mechanistic differences among methods of itch induction [29].
Fig. 7.
PCA plot of cross-correlations between chloroquine-induced and histamine-induced itch assays (CHLITCH, HISTITCH) and assays of thermal nociception (HP, HT, TF, TW47.5, TW49) spontaneous inflammatory nociception (ACAA, AAMS, BV, CAP, FEarly, FLate) in otherwise naïve mice, mechanical sensitivity (VF) and mechanical hypersensitivity (DYNVF, PACVF, PNIVF, SNIVF). Both CHLITCH and HISTITCH are negatively correlated with assays of spontaneous inflammatory nociception. CHLITCH, but not HISTITCH, exhibits strong positive correlations with mechanical sensitivity and mechanical hypersensitivity assays (except SNIVF), indicating that common genetic mechanisms contribute to these assays and itch induced by chloroquine. CHLITCH, but not HISTITCH, is also negatively correlated with assays of thermal nociception, indicating shared genetic mechanisms with opposite effects. The proportion of total variance accounted for is 0.63. Font color reference is as in Fig. 2 legend.
3.3.7. Relationship of Comt genotype to assays of nociception and hypersensitivity
The present analysis confirms our previous demonstration [31] of strong, consistently positive genetic correlations between Comt genotype (ComtB2i, the absence of the B2 SINE) and increased spontaneous pain behaviors in response to chemical/inflammatory irritants (ACAA, AAMS, BV, CAP, FEarly, FLate) (0.29 ≤ rs ≤ 0.64; Table 2; Fig. 8A), but not with inflammation-induced thermal hypersensitivity (BVCON, BVHT, CAPTW, CARHT) including two additional assays (ZYMACET, ZYMHT) in the current analysis (−0.35 ≤ rs ≤ 0.39; Table 2). Comt genotype is also unrelated to most of the assays of thermal nociception, and unrelated to mechanical sensitivity (VF) and mechanical nociception (TC) (rS = −0.23 and 0.10; Table 2; Fig. 8A; [31]). With six newly added assays of neuropathic pain, the results indicate that Comt genotype is not strongly related to the assays of neuropathic pain examined (AUT, AUTSNN, PACACET, PACCOLD, PNIHT, PNIVF, SNIACET, SNIVF) (−0.39 ≤ rs ≤ 0.10 with absence of B2 SINE, ComtB2i) with the possible exception of paclitaxel-induced mechanical hypersensitivity (PACVF, rs = 0.45). However, the presence of the B2 SINE, abbreviated as -(ComtB2i) in Fig. 8, is consistently but only mildly positively related to the four cold hypersensitivity assays (PACACET, PACCOLD, SNIACET, ZYMACET) (−0.35 ≤ rs ≤ −0.14 with absence of B2 SINE in Table 2; Fig. 8).
Fig. 8.
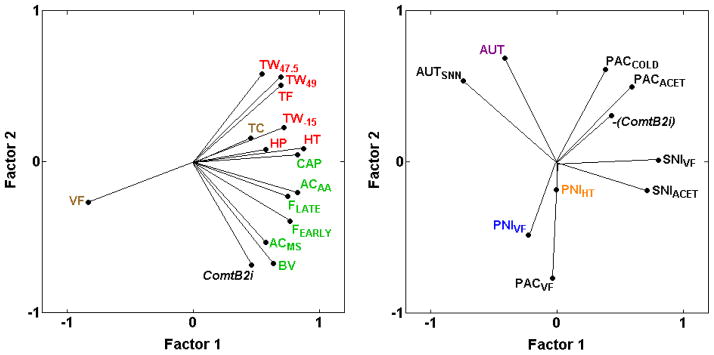
PCA plot of cross-correlations between Comt genotype (ComtB2i) and inflammatory and neuropathic pain assays. (A) Absence of the ComtB2i haplotype (and absence of the B2 SINE in the 3′ UTR) is shown to be closely related to spontaneous inflammatory nociception (ACAA, AAMS, BV, CAP, FEarly, FLate), but not to mechanical sensitivity (VF) and not to mechanical (TC) or thermal nociception (HP, HT, TF, TW−15, TW47.5, TW49). The proportion of total variance accounted for is 0.65. (B) ComtB2i haplotype is not strongly related to sensitivity in any of the neuropathic pain assays examined, represented graphically with a shorter vector for Comt genotype; however, presence of the ComtB2i haplotype, -(ComtB2i), is consistently mildly related to the neuropathic cold hypersensitivity assays (PACACET, PACCOLD, SNIACET) (0.14 ≤ rs ≤ 0.28 with absence of ComtB2i) (Table 2). The proportion of total variance accounted for is 0.50. Font color reference is as in Fig. 2 legend.
4. Discussion
4.1. Additions to previously identified, genetically distinct pain types
Whereas the current study confirms that thermal nociception in naïve mice and spontaneous inflammatory nociception in naïve mice remain genetically distinct types of pain, two exceptions to previously identified pain types have been detected. The newly added assay of zymosan-induced heat hypersensitivity (ZYMHT) highlights that heat hypersensitivities are genetically related to thermal heat nociception in naïve mice along a continuum from highly positively correlated, through correlated close to zero, to highly negatively correlated. This pattern may reflect the previously suggested dependence of the induction of heat hypersensitivity in positively correlated assays (BVCON, BVHT, CAPTW) on the initial afferent fiber activity at the time of induction [17]. In contrast, the negatively correlated PNIHT and CARHT heat hypersensitivity assays may not evoke sufficient initial afferent fiber activity to induce the same centrally-maintained mechanisms as discussed at length previously [1,17]. Variability in the newly added zymosan-induced heat hypersensitivity assay (ZYMHT) may, therefore, involve a combination of genetic mechanisms induced in the two types of assays or distinct mechanisms.
Cyclophosphamide cystitis-induced hypolocomotion (CPLOCO) was found to be genetically distinct from all assays examined, including assays of spontaneous inflammatory nociception and the majority of assays of inflammation-induced hypersensitivity. Although the ACMS and ACAA assays of i.p. inflammation-induced abdominal constriction were included in the previous analysis [17], the nociceptive response in these assays could be due to inflammation of the internal abdominal wall and thereby somatic rather than purely visceral in origin. In contrast, with cyclophosphamide cystitis, the inflammation is isolated to the bladder [5]. Note that cyclophosphamide-induced bladder mucosal edema and mucosal hemorrhage does not correlate with the reduced locomotor activity used to assess the nociception [5,23], further suggesting that non-inflammatory mechanisms underlie the observed strain differences. Additional assays of visceral pain and direct assessment of visceral nociception (e.g. bladder distension, colorectal distension) are needed to test whether CPLOCO represents a genetically distinct type of pain or if the unique behavior of reduced locomotion used to assess the effect of cyclophosphamide is responsible for the distinctiveness of the assay.
4.2. Mechanical hypersensitivity is genetically distinct
The inclusion of two additional mechanical hypersensitivity assays (PACVF, SNIVF) and two of the cold hypersensitivity assays (PACACET, PACCOLD) reinforces the previous genetic distinction between mechanical hypersensitivity and thermal (heat or cold) hypersensitivity [17], also shown in the rat [32]. The previously demonstrated lack of correlation between mechanical and thermal hypersensitivity in the same mice induced by tight ligation of a lumbar spinal nerve innervating the hindpaw (rs = −0.07 between PNIVF and PNIHT) [17,25] is now shown to also occur with paclitaxel-induced nerve injury (rs = −0.36 and −0.14 between PACVF and PACACET and PACCOLD, respectively) (Table 2; Fig. 2A). Thus, future efforts to determine heritable mechanisms of individual differences in a variety of prolonged pain outcomes must focus on the specific outcome in addition to outcome-independent etiological factors.
The genetic distinctiveness of mechanical hypersensitivity from all other pain types is supported by the inclusion of two new assays of mechanical hypersensitivity induced by physical nerve injury (SNIVF) and a chemotherapy drug (PACVF) in addition to the two assays (DYNVF and PNIVF) that previously [17] identified mechanical hypersensitivity as a genetically distinct pain type (Table 2). However, the current study indicates that mechanical hypersensitivity assays do not comprise a single pain type as previously suggested by the correlation (rS = 0.66) between the two assays previously analyzed (DYNVF, PNIVF) [17]. Instead, while it is predicted that these two correlated assays share common genetic mechanisms, the results indicate that mutually distinct genetic mechanisms are involved in the two newly added neuropathic mechanical hypersensitivity assays (PACVF, SNIVF). Interestingly, parallels for this genetic separation exist in the literature. Using expression arrays, global gene regulation both in the dorsal root ganglion [7] and the dorsal horn of the spinal cord [13] were determined in Sprague-Dawley outbred rats across four time points up to six weeks after physical nerve injury induced by chronic sciatic nerve constriction with a polyethylene cuff and the spinal nerve ligation and spared nerve injury methods used here. The results for both tissues show with multivariate analysis of gene expression that the data cluster according to method of nerve injury and not other variables such as time after nerve injury indicating that the methods of nerve injury have mutually distinct genome-wide transcriptional responses. Although common genetic mechanisms have been determined for mechanical hypersensitivity induced by multiple methods of nerve injury in the genetic background of Sprague-Dawley rats [13], the current results indicate that there are multiple genetic routes underlying the same mechanical hypersensitivity phenotype with different neuropathic induction methods.
Therefore, recently identified heritable mechanisms of sensitivity to mechanical hypersensitivity after spared nerve injury of branches of the sciatic nerve [34] or to autotomy after nerve transection [26] may not be common to mechanical hypersensitivity induced by other methods. If this pattern translates to human neuropathic pain patients, it could indicate that distinct therapeutic targets may be necessary for disparate types or sources of neuropathic mechanical allodynia in patients.
4.3. Neuropathic pain assays are of several genetically distinct types determined by method of nerve injury
The current study indicates that each method of nerve injury produces pain-related responses that are genetically distinct from responses produced in each other method (Fig. 5). In addition, multiple pain-related outcomes of the same method of nerve injury may be genetically distinct as mechanical hypersensitivity is following paclitaxel exposure (PACVF) and spinal nerve ligation (PNIVF) (Fig. 5). This is not always the case, however, as mechanical hypersensitivity and cold hypersensitivity following spared nerve injury of branches of the sciatic nerve (SNIACET, SNIVF) are genetically related (Fig. 5). Thus, among the nine assays of neuropathic pain included in this analysis, there are at least four genetically distinct types with little overlap across methods of nerve injury. PNIHT might represent a fifth pain type, but additional, similarly responding neuropathic pain assays are required to determine this.
Hence, each method of induction of neuropathic pain is expected to have distinct underlying genetic mechanisms responsible for the heritable sensitivity, which is in agreement with the distinct loci and candidate genes that have been discovered for autotomy after nerve transection (AUT) [9,26] and mechanical hypersensitivity after spared nerve injury (SNIVF) [34]. Because chemotherapy-induced cold hypersensitivity appears to represent a novel, genetically distinct neuropathic pain type, understanding the genetic factors that contribute to chemotherapy-induced cold hypersensitivity merits continued efforts. Note that paclitaxel-induced neuropathic cold hypersensitivity (PACACET, PACCOLD) and the highly correlated (rs = 0.83 and 0.75) zymosan-induced cold hypersensitivity (ZYMACET) are positively correlated with all six thermal nociception assays of otherwise naïve mice (Table 2; Fig. 2) indicating that paclitaxel-induced cold hypersensitivity shares common genetic mechanisms with thermal nociception. Candidate genes for thermal nociception are, therefore, also candidate genes for chemotherapy-induced cold hypersensitivity.
These data caution against basing conclusions regarding efficacy and effectiveness of specific drugs or mechanisms on testing with a single neuropathic pain assay as effects may be observed in other, genetically distinct neuropathic pain assays. Conversely, expectations of a broad effect across multiple neuropathic pain assays are not justified without positive evidence. If this pattern translates to humans, it would indicate that multiple sources of nerve injury may require different therapeutic strategies even though the clinical presentation may be similar.
4.4. Assays of itch show distinct genetic relationships with nociception and hypersensitivity assays
Comparison of itch assays with the nociception and hypersensitivity assays detected highly specific relationships between the assays. Chloroquine-induced itch, but not histamine-induced itch, was found to be strongly inversely related to assays of thermal nociception, as reported previously [12]. This is consistent with nociceptive mechanisms having opposite roles in pain and itch [28,29].
Pharmacogenetic explanations for variable sensitivity to itch induced by the anti-malarial chloroquine have already been proposed based on family clustering [2]. The present analysis advances our understanding and suggests that candidate genes for thermal nociception, and for select assays of mechanical sensitivity and hypersensitivity, may also underlie variability in chloroquine-induced itch and related puritis conditions.
4.5. Comt genotype is strongly related only to spontaneous chemical/inflammatory nociception
The current study found Comt genotype (for ComtB2i) to be related only to spontaneous inflammatory nociception (Fig 8A) as shown previously [31]; strains without the B2 SINE in the 3′ UTR show more pain behaviors in assays of spontaneous inflammatory nociception. It has also been hypothesized that the absence of the B2 SINE is related to protection from neuropathic pain [4,27,30,31]. The present analysis indicates for the first time that this Comt genotype is not strongly related to the nine neuropathic pain assays included here, but that the absence of the B2 SINE element is consistently, albeit mildly, related to decreased cold hypersensitivity following chemotherapy drug exposure (PACACET, PACCOLD) and spared nerve injury (SNIACET). Human genetic associations between COMT genotype and chronic and/or neuropathic pain have been mixed [35]. The present findings suggest that COMT genotype may be strongly related only to inflammatory, but not neuropathic, pain processes. There may be species differences between human and mice.
Supplementary Material
Summary.
Genetic correlation analysis of murine pain models indicates that novel genetic mechanisms remain to be determined for several mechanical hypersensitivity assays and neuropathic pain assays.
Acknowledgments
This study was supported by the National Institutes of Health (NIH) grant R01 DA021198 to W.R.L. and R01 NS074430 to M.C. The authors have no conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abdi S, Lee DH, Park SK, Chung JM. Lack of pre-emptive analgesic effects of local anaesthetics on neuropathic pain. Br J Anaesth. 2000;85:620–623. doi: 10.1093/bja/85.4.620. [DOI] [PubMed] [Google Scholar]
- 2.Ajayi AA, Oluokun A, Sofowora O, Akinleye A, Ajayi AT. Epidemiology of antimalarial-induced pruritus in Africans. Eur J Clin Pharmacol. 1989;37:539–540. doi: 10.1007/BF00558141. [DOI] [PubMed] [Google Scholar]
- 3.Axelrod J, Tomchick R. Enzymatic O-methylation of epinephrine and other catechols. J Biol Chem. 1958;233:702–705. [PubMed] [Google Scholar]
- 4.Belfer I, Segall S. COMT genetic variants and pain. Drugs Today. 2011;47:457–467. doi: 10.1358/dot.2011.47.6.1611895. [DOI] [PubMed] [Google Scholar]
- 5.Bon K, Lichtensteiger CA, Wilson SG, Mogil JS. Characterization of cyclophosphamide cystitis, a model of visceral and referred pain, in the mouse: species and strain differences. J Urol. 2003;170:1008–1012. doi: 10.1097/01.ju.0000079766.49550.94. [DOI] [PubMed] [Google Scholar]
- 6.Chesler EJ, Ritchie J, Kokayeff A, Lariviere WR, Wilson SG, Mogil JS. Genotype-dependence of gabapentin and pregabalin sensitivity: the pharmacogenetic mediation of analgesia is specific to the type of pain being inhibited. Pain. 2003;106:325–335. doi: 10.1016/S0304-3959(03)00330-0. [DOI] [PubMed] [Google Scholar]
- 7.Costigan M, Belfer I, Griffin RS, Dai F, Barrett LB, Coppola G, Wu T, Kiselycznyk C, Poddar M, Lu Y, Diatchenko L, Smith S, Cobos EJ, Zaykin D, Allchorne A, Shen PH, Nikolajsen L, Karppinen J, Mannikko M, Kelempisioti A, Goldman D, Maixner W, Geschwind DH, Max MB, Seltzer Z, Woolf CJ. Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1. Brain. 2010;133:2519–2527. doi: 10.1093/brain/awq195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crabbe JC, Phillips TJ, Kosobud A, Belknap JK. Estimation of genetic correlation: interpretation of experiments using selectively bred and inbred animals. Alcohol Clin Exp Res. 1990;14:141–151. doi: 10.1111/j.1530-0277.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 9.Devor M, Gilad A, Arbilly M, Yakir B, Raber P, Pisante A, Darvasi A. pain1: a neuropathic pain QTL on mouse chromosome 15 in a C3HxC58 backcross. Pain. 2005;116:289–293. doi: 10.1016/j.pain.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, Maixner W. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 11.Emin Erdal M, Herken H, Yilmaz M, Bayazit YA. Significance of the catechol-O-methyltransferase gene polymorphism in migraine. Brain Res Mol Brain Res. 2001;94:193–196. doi: 10.1016/s0169-328x(01)00219-4. [DOI] [PubMed] [Google Scholar]
- 12.Green AD, Young KK, Lehto SG, Smith SB, Mogil JS. Influence of genotype, dose and sex on pruritogen-induced scratching behavior in the mouse. Pain. 2006;124:50–58. doi: 10.1016/j.pain.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 13.Griffin RS, Costigan M, Brenner GJ, Ma CH, Scholz J, Moss A, Allchorne AJ, Stahl GL, Woolf CJ. Complement induction in spinal cord microglia results in anaphylatoxin C5a-mediated pain hypersensitivity. J Neurosci. 2007;27:8699–8708. doi: 10.1523/JNEUROSCI.2018-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hegmann JP, Possidente B. Estimating genetic correlations from inbred strains. Behav Genet. 1981;11:103–114. doi: 10.1007/BF01065621. [DOI] [PubMed] [Google Scholar]
- 15.Kuraishi Y, Nagasawa T, Hayashi K, Satoh M. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur J Pharmacol. 1995;275:229–233. doi: 10.1016/0014-2999(94)00780-b. [DOI] [PubMed] [Google Scholar]
- 16.Lacroix-Fralish ML, Mogil JS. Progress in genetic studies of pain and analgesia. Annu Rev Pharmacol Toxicol. 2009;49:97–121. doi: 10.1146/annurev-pharmtox-061008-103222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lariviere WR, Wilson SG, Laughlin TM, Kokayeff A, West EE, Adhikari SM, Wan Y, Mogil JS. Heritability of nociception. III. Genetic relationships among commonly used assays of nociception and hypersensitivity. Pain. 2002;97:75–86. doi: 10.1016/s0304-3959(01)00492-4. [DOI] [PubMed] [Google Scholar]
- 18.Meller ST, Gebhart GF. Intraplantar zymosan as a reliable, quantifiable model of thermal and mechanical hyperalgesia in the rat. Eur J Pain. 1997;1:43–52. doi: 10.1016/s1090-3801(97)90052-5. [DOI] [PubMed] [Google Scholar]
- 19.Minert A, Gabay E, Dominguez C, Wiesenfeld-Hallin Z, Devor M. Spontaneous pain following spinal nerve injury in mice. Exp Neurol. 2007;206:220–230. doi: 10.1016/j.expneurol.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Mogil JS. Pain genetics: past, present and future. Trends Genet. 2012;28:258–266. doi: 10.1016/j.tig.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Mogil JS, Adhikari SM. Hot and cold nociception are genetically correlated. J Neurosci. 1999;19:RC25. doi: 10.1523/JNEUROSCI.19-18-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mogil JS, Graham AC, Ritchie J, Hughes SF, Austin JS, Schorscher-Petcu A, Langford DJ, Bennett GJ. Hypolocomotion, asymmetrically directed behaviors (licking, lifting, flinching, and shaking) and dynamic weight bearing (gait) changes are not measures of neuropathic pain in mice. Mol Pain. 2010;6:34. doi: 10.1186/1744-8069-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mogil JS, Lichtensteiger CA, Wilson SG. The effect of genotype on sensitivity to inflammatory nociception: characterization of resistant (A/J) and sensitive (C57BL/6J) inbred mouse strains. Pain. 1998;76:115–125. doi: 10.1016/s0304-3959(98)00032-3. [DOI] [PubMed] [Google Scholar]
- 24.Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999;80:67–82. doi: 10.1016/s0304-3959(98)00197-3. [DOI] [PubMed] [Google Scholar]
- 25.Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. Heritability of nociception II. ‘Types’ of nociception revealed by genetic correlation analysis. Pain. 1999;80:83–93. doi: 10.1016/s0304-3959(98)00196-1. [DOI] [PubMed] [Google Scholar]
- 26.Nissenbaum J, Devor M, Seltzer Z, Gebauer M, Michaelis M, Tal M, Dorfman R, Abitbul-Yarkoni M, Lu Y, Elahipanah T, delCanho S, Minert A, Fried K, Persson AK, Shpigler H, Shabo E, Yakir B, Pisante A, Darvasi A. Susceptibility to chronic pain following nerve injury is genetically affected by CACNG2. Genome Res. 2010;20:1180–1190. doi: 10.1101/gr.104976.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pertovaara A, Wei H, Kalman J, Ruotsalainen M. Pain behavior and response propoerties of spinal dorsal horn neurosons following experimental diabetic neuropathy in the rat: modulation by nitecapone, a COMT inhibitor with antioxidant properties. Exp Neurol. 2001;167:425–434. doi: 10.1006/exnr.2000.7574. [DOI] [PubMed] [Google Scholar]
- 28.Roberson DP, Gudes S, Sprague JM, Patoski HA, Robson VK, Blasl F, Duan B, Oh SB, Bean BP, Ma Q, Binshtok AM, Woolf CJ. Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons. Nat Neurosci. 2013;16:910–918. doi: 10.1038/nn.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross SE. Pain and itch: insights into the neural circuits of aversive somatosensation in health and disease. Curr Opin Neurobiol. 2011;21:880–887. doi: 10.1016/j.conb.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Segall SK, Maixner W, Belfer I, Wiltshire T, Seltzer Z, Diatchenko L. Janus molecule I: dichotomous effects of COMT in neuropathic vs nociceptive pain modalities. CNS Neurol Disord Drug Targets. 2012;11:222–235. doi: 10.2174/187152712800672490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Segall SK, Nackley AG, Diatchenko L, Lariviere WR, Lu X, Marron JS, Grabowski-Boase L, Walker JR, Slade G, Gauthier J, Bailey JS, Steffy BM, Maynard TM, Tarantino LM, Wiltshire T. Comt1 genotype and expression predicts anxiety and nociceptive sensitivity in inbred strains of mice. Genes Brain Behav. 2010;9:933–946. doi: 10.1111/j.1601-183X.2010.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shir Y, Zeltser R, Vatine JJ, Carmi G, Belfer I, Zangen A, Overstreet D, Raber P, Seltzer Z. Correlation of intact sensibility and neuropathic pain-related behaviors in eight inbred and outbred rat strains and selection lines. Pain. 2001;90:75–82. doi: 10.1016/s0304-3959(00)00388-2. [DOI] [PubMed] [Google Scholar]
- 33.Smith SB, Crager SE, Mogil JS. Paclitaxel-induced neuropathic hypersensitivity in mice: responses in 10 inbred mouse strains. Life Sci. 2004;74:2593–2604. doi: 10.1016/j.lfs.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Sorge RE, Trang T, Dorfman R, Smith SB, Beggs S, Ritchie J, Austin JS, Zaykin DV, Vander Meulen H, Costigan M, Herbert TA, Yarkoni-Abitbul M, Tichauer D, Livneh J, Gershon E, Zheng M, Tan K, John SL, Slade GD, Jordan J, Woolf CJ, Peltz G, Maixner W, Diatchenko L, Seltzer Z, Salter MW, Mogil JS. Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat Med. 2012;18:595–599. doi: 10.1038/nm.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tammimaki A, Mannisto PT. Catechol-O-methyltransferase gene polymorphism and chronic human pain: a systematic review and meta-analysis. Pharmacogenetics and genomics. 2012;22:673–691. doi: 10.1097/FPC.0b013e3283560c46. [DOI] [PubMed] [Google Scholar]
- 36.Wall PD, Devor M, Inbal R, Scadding JW, Schonfeld D, Seltzer Z, Tomkiewicz MM. Autotomy following peripheral nerve lesions: experimental anaesthesia dolorosa. Pain. 1979;7:103–111. doi: 10.1016/0304-3959(79)90002-2. [DOI] [PubMed] [Google Scholar]
- 37.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 38.Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.