Abstract
The ultrastructure of light-grown Euglena gracilis var. bacillaris was examined by the techniques of thin sectioning and freeze-etching. Thin sectioning revealed the typical organelles previously observed in chemically fixed Euglena. In addition to confirming the observations on thin sections, the freeze-etch technique has revealed the presence in E. gracilis of a complex multilaminar pellicle, and an ordered arrangement to the paramylon granule. The chloroplast thylakoids are particulate and similar to those observed in higher plants.
Full text
PDF

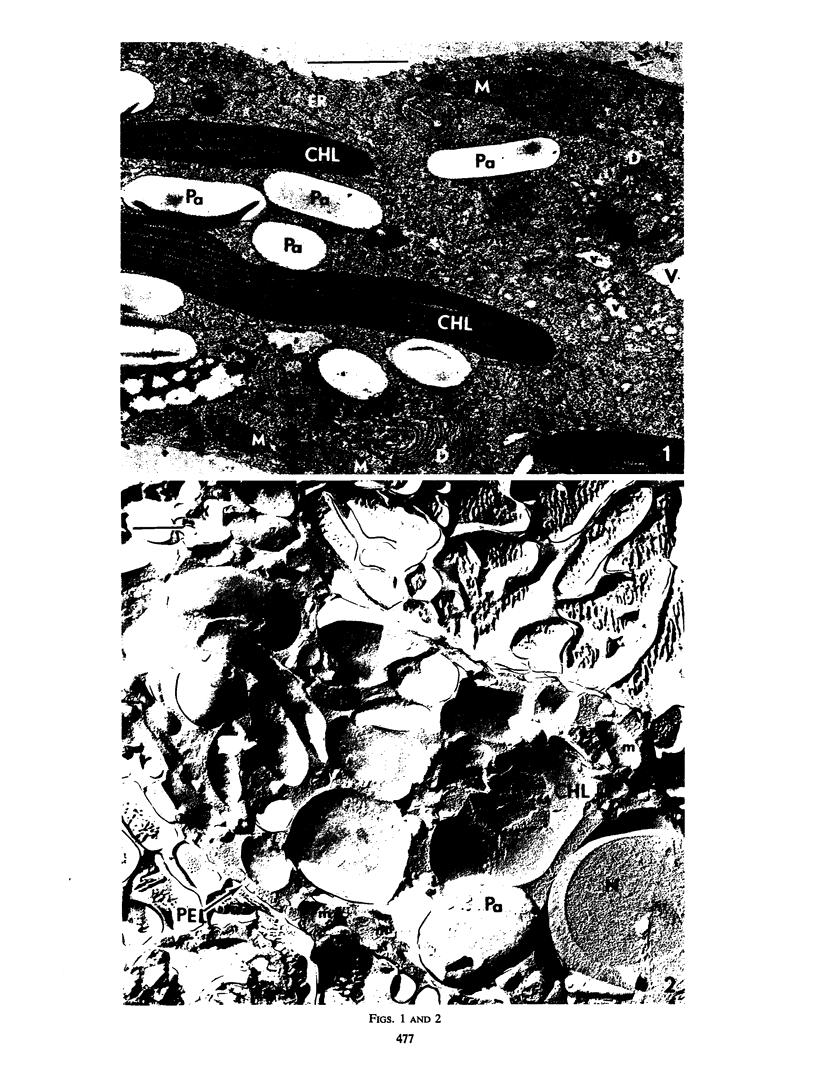






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott H. J., Walne P. L. Observations on the fine structure of the pellicle pores of Euglena granulata. Protoplasma. 1967;64(3):330–344. doi: 10.1007/BF01253711. [DOI] [PubMed] [Google Scholar]
- BRANDES D., BUETOW D. E., BERTINI F., MALKOFF D. B. ROLE OF LYSOSOMES IN CELLULAR LYTIC PROCESSES. I. EFFECT OF CARBON STARVATION IN EUGLENA GRACILIS. Exp Mol Pathol. 1964 Dec;90:583–609. doi: 10.1016/0014-4800(64)90036-x. [DOI] [PubMed] [Google Scholar]
- Ben-Shaul Y., Schiff J. A., Epstein H. T. Studies of Chloroplast Development in Euglena. VII. Fine Structure of the Developing Plastid. Plant Physiol. 1964 Mar;39(2):231–240. doi: 10.1104/pp.39.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton D., Park R. B. Subunits in chloroplast lamellae. J Ultrastruct Res. 1967 Aug;19(3):283–303. doi: 10.1016/s0022-5320(67)80222-3. [DOI] [PubMed] [Google Scholar]
- CLARKE A. E., STONE B. A. Structure of the paramylon from Euglena gracilis. Biochim Biophys Acta. 1960 Oct 21;44:161–163. doi: 10.1016/0006-3002(60)91534-1. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Leadbetter E. R. Comparative ultrastructure of selected aerobic spore-forming bacteria: a freeze-etching study. Bacteriol Rev. 1969 Jun;33(2):346–378. doi: 10.1128/br.33.2.346-378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Trüper H. G., Takács B. J. Fine structure of Ectothiorhodospira mobilis strain 8113 thylakoids: chemical fixation and freeze-etching studies. Arch Mikrobiol. 1968;62(2):111–128. doi: 10.1007/BF00410398. [DOI] [PubMed] [Google Scholar]
- Howell S. H., Moudrianakis E. N. Function of the "quantasome" in photosynthesis: structure and properties of membrane-bound particle active in the dark reactions of photophosphorylation. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1261–1268. doi: 10.1073/pnas.58.3.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREGER D. R., MEEUSE B. J. D. X-ray diagrams of Euglena-paramylon, of the acid-insoluble glucan of yeast cell walls and of laminarin. Biochim Biophys Acta. 1952 Dec;9(6):699–700. [PubMed] [Google Scholar]
- LEEDALE G. F., MEEUSE B. J., PRINGSHEIM G. STRUCTURE AND PHYSIOLOGY OF EUGLENA SPIROGYRA. 3-6.. Arch Mikrobiol. 1965 Jan 22;50:133–155. doi: 10.1007/BF00409124. [DOI] [PubMed] [Google Scholar]
- MOOR H. DIE GEFRIER-FIXATION LEBENDER ZELLEN UND IHRE ANWENDUNG IN DER ELEKTRONENMIKROSKOPIE. Z Zellforsch Mikrosk Anat. 1964 Apr 28;62:546–580. [PubMed] [Google Scholar]
- Moor H. Use of freeze-etching in the study of biological ultrastructure. Int Rev Exp Pathol. 1966;5:179–216. [PubMed] [Google Scholar]
- Park R. B., Pheifhofer A. O. The continued presence of quantasomes in ethylenediaminetetraacetate-washed chloroplast lamellae. Proc Natl Acad Sci U S A. 1968 May;60(1):337–343. doi: 10.1073/pnas.60.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remsen C. C., Watson S. W., Waterbury J. B., Trüper H. G. Fine structure of Ectothiorhodospira mobilis Pelsh. J Bacteriol. 1968 Jun;95(6):2374–2392. doi: 10.1128/jb.95.6.2374-2392.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remsen C., Lundgren D. G. Electron microscopy of the cell envelope of Ferrobacillus ferrooxidans prepared by freeze-etching and chemical fixation techniques. J Bacteriol. 1966 Dec;92(6):1765–1771. doi: 10.1128/jb.92.6.1765-1771.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rntzen C. J., Dilley R. A., Crane F. L. A comparison of chloroplast membrane surfaces visualized by freeze-etch and negative staining techniques; and ultrastructural characterization of membrane fractions obtained from digitonin-treated spinach chloroplasts. J Cell Biol. 1969 Oct;43(1):16–31. doi: 10.1083/jcb.43.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckenius W., Engelman D. M. Current models for the structure of biological membranes. J Cell Biol. 1969 Sep;42(3):613–646. doi: 10.1083/jcb.42.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weier T. E., Engelbreicht A. H., Harrison A., Risley E. B. Subunits in the membranes of chloroplasts of Phaseolus vulgaris, Pisum sativum, and Aspidistra sp. J Ultrastruct Res. 1965 Aug;13(1):92–111. doi: 10.1016/s0022-5320(65)80091-0. [DOI] [PubMed] [Google Scholar]
- Weier T. E., Stocking C. R., Shumway L. K. The photosynthetic apparatus in chloroplasts of higher plants. Brookhaven Symp Biol. 1966;19:353–374. [PubMed] [Google Scholar]