Abstract
The cellular identity of niche cells that regulate hematopoietic stem cell (HSC) self-renewal and differentiation has been debated for several years. Two recent studies in Nature (Ding and Morrison, 2013; Greenbaum et al., 2013), have shed light into the bone marrow stromal subsets making CXCL12, a chemokine critical for HSC maintenance.
The identification of niche cells in the bone marrow has been fraught with difficulties due, for instance, to the encasement of the marrow in bone, by the paucity of specific markers to separate stromal cells, and the infidelity of some genetic markers for mesenchymal lineages. Because of these challenges, there have been conflicting reports on the cellular identity of the HSC niche. For example, prior studies have suggested that the endosteal region, populated by osteoblasts, was a niche maintaining quiescent HSCs whereas other studies have suggested that most HSCs are found near blood vessels (Mercier et al., 2012; Frenette et al., 2013).
A hallmark of a putative stromal cell niche candidate is the regulated expression of specific factors mediating HSC maintenance. Among these factors is stromal cell-derived factor-1 (SDF1, now called CXCL12), a chemokine essential to maintain HSCs in adult BM (Tzeng et al., 2011). CXCL12-abundant reticular (CAR) cells, marked by green fluorescent protein (GFP) expression inserted in the Cxcl12 locus, are largely perivascular cells whereas endothelial cells and bone-lining osteoblasts express lower levels of GFP (Sugiyama and Nagasawa, 2012). Ablation of CAR cells leads to a reduction in the frequency of HSCs as well as lymphoid and erythroid progenitors. However, the identity and composition of CAR cells and their contributions as niche cells have been unclear.
In a recent issue of Nature, two companion papers from the laboratories of Drs. Sean Morrison and Daniel Link have applied an elegant approach by conditional deletion of Cxcl12 in various candidate niche cells (Ding and Morrison, 2013; Greenbaum et al., 2013). This approach allows them to evaluate the functional impact of CXCL12 synthesis by different niche components, and define distinct specialized niches for HSC maintenance, HSC retention, and for the generation of certain lymphoid progenitors.
Deletion of Cxcl12 in osteoblasts using Col2.3-cre mice reveals no alteration in HSC or myeloerythroid progenitor cell numbers. However, these mice show significantly lower levels of T- and B-cell reconstitution, and fewer early lymphoid progenitors in the bone marrow (Figure 1). These findings are consistent with the accumulation of early lymphoid progenitors adjacent to the endosteum (Ding and Morrison, 2013). In the article by Link and colleagues (Greenbaum et al., 2013), the contribution of osteolineage cells is further dissected by Cxcl12 deletion in mature osteoblasts (using Osteocalcin-cre transgenics) or in osteoprogenitors (using Osterix-cre). The authors also find that CXCL12 from mature osteoblasts as well as osteoblast progenitors is dispensable for HSC maintenance. However, conditional deletion of Cxcl12 in osteoprogenitors leads to mobilization of hematopoietic progenitor cells to the blood and spleen. Deletion of Cxcl12 in osteoprogenitors, but not mature osteoblasts, reduces the number of B-lymphoid progenitors, which is consistent with results following the deletion of CAR cells and supports a role for osteoprogenitors or/and CAR cells in B-lymphoid commitment (Omatsu et al., 2010).
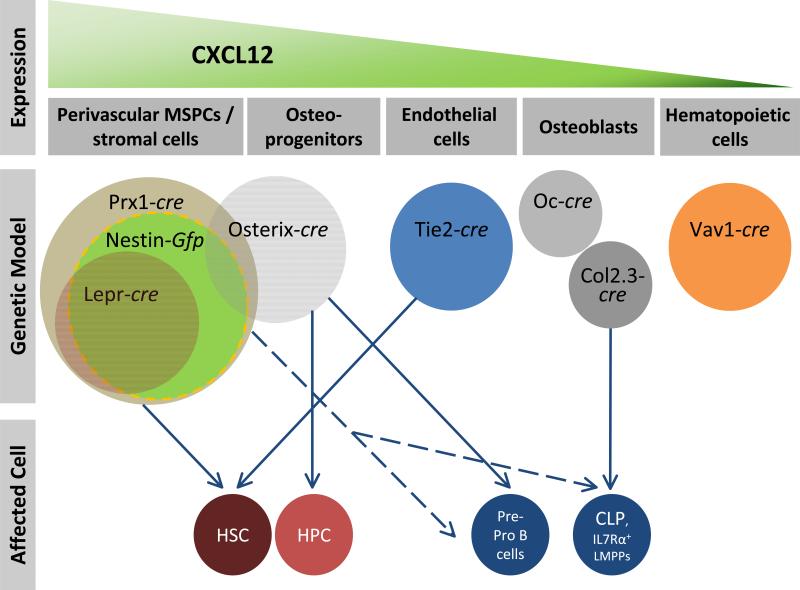
Figure 1. Distinct cellular sources and niches for CXCL12 in bone marrow.
Most CXCL12 is derived from perivascular stromal cells that can be marked by Prx1-cre, Lepr-cre or Nestin-Gfp that likely show significant overlap with each other. The highest levels of CXCL12 is secreted by the most immature mesenchymal stem / progenitor cells. Osteoblasts, marked by Osteocalcin (Oc), synthesize low amounts of CXCL12 that are not essential for normal hematopoiesis, whereas deletion of Cxcl12 in osteoblasts using Col2.3-cre leads to deficits in certain early lymphoid progenitors similar to Osterix-cre, suggesting a contribution of osteoprogenitors in the generation of lymphoid precursors. Endothelial cells, marked by Tie2-cre, also secrete CXCL12 and contribute to HSC maintenance. Although some hematopoietic cells express CXCL12, deletion in the hematopoietic system using Vav1-cre did not yield any phenotype. The effect of Prx1-targeted cells on lymphoid progenitors is indicated with a dashed line since it is likely to be derived from osteoprogenitors or perivascular stromal Prx1-cre-targeted cell fraction. The size of circles does not reflect the actual frequencies in the bone marrow and the overlap among different models is based on estimations. HSC, hematopoietic stem cell; HPC, hematopoietic progenitor cell; CLP, common lymphoid progenitor; LMMP, lymphoid-primed multipotent progenitor.
Endothelial cell-specific Cxcl12 deletion can be achieved using Tie2-cre mice, which reveal that endothelial cells synthesize a relatively modest amount of CXCL12 compared to other stromal cells. Consequently, modest defects in HSC numbers and competitive reconstitution activities are observed in these mice (Ding and Morrison, 2013; Greenbaum et al., 2013). No reductions in committed myeloid or lymphoid progenitors were documented, suggesting a restricted contribution of endothelial cell-derived CXCL12 to HSC maintenance (Figure 1).
Previous studies have shown that putative niche cells for HSC maintenance are marked by Nestin, an intermediate filament protein found in self-renewing mesenchymal stem cells (MSCs)(Frenette et al., 2013). In transgenic GFP reporter mice, Nes-GFP+ cells express very high levels of CXCL12 and contain all MSC activity in the bone marrow (Mendez-Ferrer et al., 2010). Since Nestin was first reported as a marker for neuroectoderm progenitors, transgenic reporters have thus far been generated and screened to label neural progenitors, and consequently, expression in the bone marrow is variable and depends on the transgenic strain. Ding and Morrison reported, surprisingly, no effect of Nes-cre on CXCL12 and HSC frequencies and numbers, suggesting that loxP recombination most likely did not occur in Nes-GFP+ cells. To delete Cxcl12 in multipotent mesenchymal progenitors, both companion studies used the Prx1-cre transgenic mice in which cre was driven by a transcription factor promoter expressed during limb bud mesoderm development. This model induces recombination in osteoblasts and the majority of PDGFRα+ stromal cells, but not in endothelial cells. In both studies, the authors observe a dramatic reduction in bone marrow HSCs and increased splenic HSCs, suggesting a substantial contribution of CXCL12 derived from perivascular stromal cells in HSC maintenance and retention in the bone marrow. Deletion of Cxcl12 in Prx1-cre-recombined cells also leads to reduced lymphoid progenitors, which reinforces the aforementioned contribution of osteolineage cells to the generation of early lymphoid progenitors.
Leptin receptor (Lepr)-driven cre leads to recombination in perivascular sinusoidal stromal cells that express high levels of stem cell factor (SCF), which was previously reported to be essential for HSC maintenance (Ding et al., 2012). Deletion of Cxcl12 by Lepr-cre significantly reduces Cxcl12 expression within the sinusoidal stromal compartment but it does not alter HSC and progenitor numbers in the bone marrow. However, it induces the mobilization of HSCs and progenitors to spleen and peripheral blood. These studies suggest a role for CXCL12 derived from Lepr-marked cells specifically in HSC retention rather than maintenance. Given that both SCF and CXCL12 expression in bone marrow are required to maintain HSCs, and that SCF and CXCL12 expressing stromal cells greatly (>94%) overlap (Ding and Morrison, 2013), the phenotypic difference with regards to HSC maintenance between Lepr-cre;ScfΔ/Δ and Lepr-cre;Cxcl12Δ/Δ mice is puzzling. Hence, the dramatic defect in HSC maintenance observed in the Prx1-cre;Cxcl12Δ/Δ model must arise from contributions of a non-endothelial Lepr-negative stromal cell.
To investigate further the nature of these cells, Greenbaum and colleagues evaluated whether Prx1-cre targeted PDGFRα+/Sca-1+ (PαS) cells, which have been suggested to comprise bone marrow MSCs (Morikawa et al., 2009). They found that Prx1-cre indeed targets 50% of PαS cells whereas Osterix-cre does not, which is consistent with the idea that Prx1-cre induce recombination in MSC-like cells. Furthermore, all colony-forming unit fibroblast (CFU-F) activity appears to be derived from Prx1-cre-targeted PαS cells that do not express Nestin. In fact, the frequency of CFU-F, a hallmark of MSCs, in Prx1-cre targeted PαS cells is much greater than that reported for Nes-GFP+ cells, suggesting that Prx1-cre indeed targets mesenchymal progenitors (Greenbaum et al., 2013). However, it is important to note that comparisons of Prx1-cre targeted PαS cells with other studies are prevented by meaningful (and underappreciated) differences in the methods of bone marrow harvest. For example, all MSC activity can be recovered from Nes-GFP+ cells when flushing the bone marrow core, a method that excludes bone stromal cells from the analysis (Mendez-Ferrer et al., 2010). Greenbaum and colleagues used a bone crushing method which incorporates bone stromal cells that exhibit much higher CFU-F content and are likely to be phenotypically and functionally different from those found in the bone marrow. Although further studies are needed to characterize these MSCs, it is likely that Prx1-cre also targets bone marrow Nes-GFP+ cells.
Overall, these two excellent papers are consistent with the idea that HSC maintenance and self-renewal are provided by an immature mesenchymal stem and progenitor (i.e. MSC), and show that the retention of HSCs and progenitors in bone marrow is provided by a distinct stromal niche marked by Lepr and Osterix. By identifying osteoprogenitor cells as a possible stromal constituent generating certain lymphoid precursors, these papers pave the way toward a systematic effort to match differentiated stromal niche subsets with their differentiating hematopoietic counterparts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013 doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal Stem Cell: Keystone of the Hematopoietic Stem Cell Niche and a Stepping-Stone for Regenerative Medicine. Annu Rev Immunol. 2013 Jan 3; doi: 10.1146/annurev-immunol-032712-095919. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013 doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier FE, Ragu C, Scadden DT. The bone marrow at the crossroads of blood and immunity. Nature Rev Immunol. 2012;12:49–60. doi: 10.1038/nri3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa S, Mabuchi Y, Niibe K, Suzuki S, Nagoshi N, Sunabori T, Shimmura S, Nagai Y, Nakagawa T, Okano H, et al. Development of mesenchymal stem cells partially originate from the neural crest. Biochem Biophys Res Com. 2009;379:1114–1119. doi: 10.1016/j.bbrc.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, Nagasawa T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387–399. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Nagasawa T. Bone marrow niches for hematopoietic stem cells and immune cells. Inflamm Allergy Drug Targets. 2012;11:201–206. doi: 10.2174/187152812800392689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng YS, Li H, Kang YL, Chen WC, Cheng WC, Lai DM. Loss of Cxcl12/Sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood. 2011;117:429–439. doi: 10.1182/blood-2010-01-266833. [DOI] [PubMed] [Google Scholar]