Abstract
Relative to monocytes, human macrophages are deficient in their ability to process and release IL-1β. In an effort to explain this difference, we used a model of IL-1β processing and release that is dependent upon bacterial escape into the cytosol. Fresh human blood monocytes were compared with monocyte-derived macrophages (MDM) for their IL-1β release in response to challenge with Francisella novicida. Although both cell types produced similar levels of IL-1β mRNA and intracellular pro-IL-1β, only monocytes readily released processed mature IL-1β. Baseline mRNA expression profiling of candidate genes revealed a remarkable deficiency in the pyrin gene, MEFV, expression in MDM compared with monocytes. Immunoblots confirmed a corresponding deficit in MDM pyrin protein. To determine whether pyrin levels were responsible for the monocyte/MDM difference in mature IL-1β release, pyrin expression was knocked down by nucleofecting small interfering RNA against pyrin into monocytes or stably transducing small interfering RNA against pyrin into the monocyte cell line, THP-1. Pyrin knockdown was associated with a significant drop in IL-1β release in both cell types. Importantly, M-CSF treatment of MDM restored pyrin levels and IL-1β release. Similarly, the stable expression of pyrin in PMA-stimulated THP-1-derived macrophages induces caspase-1 activation, associated with increased IL-1β release after infection with F. novicida. In summary, intracellular pyrin levels positively regulate MDM IL-1β responsiveness to Francisella challenge.
Living in close contact with viruses, bacteria, yeasts, and fungi, eukaryota have developed a complex system of pathogen sensing and defense. Key components of this system are pattern recognition receptors (PRR)3 that recognize very common and conserved danger-associated molecular patterns. Pathogen-associated molecular patterns (PAMPs) include components of bacterial and fungal cell walls, flagellar proteins, and nucleic acids (1). Stress-associated molecular patterns may recognize ATP, NAD, uric acid crystals, loss of intracellular potassium, aluminum salts, silica crystals, or asbestos (2–6).
Innate immunity evolved to use a very limited repertoire of PRR for efficient detection of the variety of pathogens by recognition of their common PAMPs. Three major families of the innate immunity PRR include transmembrane TLR, intracellular nucleotide binding and oligomerization domain-like receptors (NLR), and intracellular retinoid acid-inducible gene I-like receptors (1). Pathogen recognition by mononuclear cells initiates defense reaction by release of many inflammatory cytokines, including a key proinflammatory molecule IL-1β. Unlike many cytokines, IL-1β (and IL-18) processing and release are tightly regulated by an intracellular protein complex termed the inflammasome (7). This complex is assembled in response to PAMP (or danger-associated molecular patterns) by caspase recruitment domain (CARD)-CARD and pyrin domain (PYD)-PYD interactions between partner proteins: a NLR molecule (the intracellular bacterial sensor), caspase-1, and a key adaptor molecule ASC, providing both CARD and PYD (8). Inflammasome formation regulates the function of caspase-1, an enzyme that cleaves biologically inactive precursors to mature IL-1β and IL-18 (9). Although there are 23 human NLR family members (34 mouse NLR) (10), caspase-1 is a central part of every inflammasome. However, to date only three inflammasome structures have been described: the NLRP1 inflammasome (NLRP1, ASC, caspase-1, and caspase-5) (7), the NLRP3 inflammasome (NLRP3 or NLRP2, CARD8, ASC, caspase-1) (11), and the NLRC4 (IPAF) inflammasome (NLRC4, ASC, caspase-1) (12). It is likely that additional inflammasome platforms will be found to involve either other members of the NLR family or PYD- or CARD-containing proteins from other families. In support of this statement, it was recently found that the PYD-containing protein AIM2 recognizes cytosolic dsDNA, leading to activation of another caspase-1 inflammasome (13–16).
Francisella tularensis is a Gram-negative bacteria, the causative agent of the disease tularemia (17). Because of its high infectivity and potential as a biological weapon, F. tularensis has been placed in category A (organisms with the greatest potential for an adverse impact on public health if used in an act of terrorism), according to the Centers for Disease Control and Prevention (17, 18). In the mammalian host, Francisella resides in monocytes and macrophages for its growth and replication (17). Therefore, mononuclear phagocytes are important both as sites of bacterial replication and as sites of host defense against Francisella.
We previously showed that human monocytes, infected with Francisella, efficiently release IL-1β in a process that requires escape into the cytosol (19). Although innate immunity against Francisella is dependent on the ASC/caspase-1 axis (20), no specific pathogen receptor for Francisella has yet been determined. Mice, deficient in NLRP1, NLRP3, and NLRC4, still show caspase-1 activation and IL-1β release in response to Francisella infection, implying that other NLRs may serve as receptors for Francisella, inducing assembly of the Francisella-sensing inflammasome (21, 22).
In this study, we present data that suggest that pyrin, the product of MEFV gene, may be one such sensor. Although pyrin has a PYD, it is not a classic member of the NLR family. Furthermore, the role of pyrin in the development of inflammatory reaction is controversial. Several groups suggest an anti-inflammatory role for pyrin, showing that pyrin can inhibit IL-1β activation (23–25). In contrast, other groups provided evidence that pyrin is a proinflammatory protein, activating caspase-1-dependent IL-1β cleavage (26, 27). Our published (28) and present data support a proinflammatory role of pyrin. By modulating intracellular pyrin levels in mononuclear phagocytes in various stages of differentiation, we were able to regulate IL-1β release in response to Francisella infection.
Materials and Methods
Cells, bacterial strains, and reagents
Highly purified LPS from Escherichia coli strain 0111:B4 was purchased from Alexis Biochemicals. Francisella novicida, strain JSG2401;U112, was provided by J. Gunn (Ohio State University, Columbus, OH). Bacteria were grown on chocolate II agar (BD Biosciences) at 37°C, harvested, and resuspended in cell culture medium before adding to cells. Cells were purchased from American Type Culture Collection (THP-1, lot 385653 and HEK293-FT, lot 1154143) or isolated from blood of healthy volunteers. THP-1, monocytes, and MDM were cultured in RPMI 1640 (Mediatech), and packaging cell line HEK293-FT was cultured in high glucose DMEM (Invitrogen Life Technologies). All medium were supplemented with 10% heat-inactivated FBS (Atlanta Biologicals) and 1% penicillin-streptomycin (Invitrogen Life Technologies). Lentiviral constructs were made on the basis of pLenti6/V5 plasmid (Invitrogen Life Technologies). HEK293-FT cells were transfected with Lipofectamine 2000 (Invitrogen Life Technologies). Small interfering RNA (siRNA) was delivered either by lentivirus or as a synthetic siRNA (Dharmacon); transfected with Trans-IT-Jurkat kit, MIR 2122 (Mirus); or nucleofected with Y-01 program (Amaxa).
Construction of lentiviruses and generation of stable cell lines
The pLenti6/V5 TOPO vector (Invitrogen Life Technologies) was initially engineered to contain an enhanced GFP (EGFP) under control of CMV promoter, surrounded by additional polylinkers and designated pLenti-EGFP, as described earlier (29). Depending on the purpose of the experiment, EGFP was replaced with either yellow or red fluorescent proteins. To make a fusion protein, pyrin was amplified from cDNA by PCR and inserted at the C terminus of fluorescent protein. To express siRNA, we amplified the H1 promoter from human cDNA, and inserted a newly designed polylinker in front of the CMV promoter. Because the H1 promoter is driven by polymerase III, which has a very specific start and end site, this design avoids possible self targeting of siRNA, which happens if H1 promoter is placed after the CMV promoter. Oligonucleotides coding siRNA (CTAGCCC(sense)TTCAAGAGA(antisense)TTTTTGGAATT and GG G(antisense)TCTCTTGAAT(sense)AAAAACCTTTAAGC) were synthesized by Integrated DNA Technologies, annealed, and ligated into pLenti plasmid. To knock down pyrin, we designed two siRNA against pyrin sequences, as follows: siPyrin301 (cagggcagccattcaggaata) and siPyrin1682 (gaccactcctcaagagataaa). Each of these siRNA constructs suppressed pyrin levels by 70%. As a control siRNA, we targeted the EGFP sequence (aagctgaccctgaagttca) using in the pLenti plasmid expressing red fluorescent protein. All plasmids were verified by sequencing. Lentivirus was generated in the packaging cell line HEK293-FT (Invitrogen Life Technologies), transfected with pLenti and helper plasmids pCMVΔR8.2 and pMD.G (provided by K. Boris-Lawrie, Ohio State University, Columbus, OH). Cell culture medium containing virus was harvested at 48 and 72 h posttransfection and concentrated at 3200 × g for 30 min (Centricon C-20 columns, 100,000 MWCO; Millipore), resulting in the titer 1–2 × 107 TU/ml. Cells were transduced with virus at 2–5 multiplicity of infection (MOI) in the presence of 6 µg/ml polybrene. After lentiviral transduction, 10–15% cells expressed fluorescent protein. Stably transduced cells were selected with blasticidin (Invitrogen Life Technologies) for 10 days, followed by two rounds of flow sorting using FACSAria (BD Biosciences), resulting in 100% yield of stably transduced THP-1 cells.
Monocyte and macrophage isolation, maturation, and infection
Human monocytes were isolated from fresh blood or buffy coat (local Red Cross) by Histopaque-1077 (Sigma-Aldrich), followed by CD14 positive selection (Miltenyi Biotec). This procedure consistently led to a ≥98% pure population of CD14+ cells, confirmed by flow cytometry analysis. Purified monocytes were used in experiments immediately. For maturation into macrophages, monocytes were plated in 12-well plates for 5–7 days and either left unstimulated, or stimulated every other day with 20 ng/ml M-CSF (R&D Systems). THP-derived macrophages were generated by PMA (Fluka) treatment (0.5 µM for 3 h), washing, and plating into 12-well plate for 5–7 days.
All cells were infected with F. novicida at a MOI of 50, unless specified, for 8 or 15 h. In some experiments, cells were stimulated with 1 µg/ml LPS. Cell culture medium was used for detection of released cytokines, and cells were analyzed for protein and RNA.
In knockdown experiments for monocyte-derived macrophages (MDM), we used an IL-1/IL-8 ratio to control for variable viable cell numbers, which were noted to occur after MDM transfection with siRNA and infection with F. novicida. IL-8 served as the housekeeping control for inflammasome-independent effects on the cells.
Preparation of cell lysate, immunoblots, and ELISA
Cells were lysed in TN1 buffer (50 mM Tris-HCl (pH 8.0), 125 mM NaCl, 10 mM EDTA, and 1% Triton X-100) supplemented with complete protease inhibitor mixture (Sigma-Aldrich), 1 mM PMSF, and 100 µM N-(methoxysuccinyl)-Ala-Ala-Pro-Val chloromethyl ketone. The protein concentrations were determined using Bio-Rad DC protein Lowry assay (Bio-Rad). After SDS-PAGE gel separation, samples were transferred to a nitrocellulose membrane, probed with the Ab of interest, and developed by ECL (Amersham Biosciences). Rabbit polyclonal Abs against IL-1β and pyrin were developed in our laboratory, as described (28). Other Abs were as follows: rabbit polyclonal anti-caspase-1 (a gift from D. Miller, Merck Research Laboratories), monoclonal anti-EGFP (GE Healthcare), and monoclonal anti-actin (clone C4; MP Biomedicals).
Human IL-1β, TNF, and IL-8 in the cell culture medium were measured using the Immulite automated chemiluminometer (Diagnostic Products). Cytokine standards were run before each batched group of samples to verify accuracy. Mature IL-1β level was verified by a sandwich ELISA, developed in our laboratory (30).
Cell death detection by quantification of lactate dehydrogenase (LDH) release in cell culture medium
LDH release into cell culture medium was used as an indicator of cell death using NAD+ reduction assay, according to the manufacturer’s suggestions (Roche Applied Science). Cells were plated in 12-well plate at the density 1 × 106, and 50 MOI of F. novicida were added. Cell culture medium was collected 2, 8, and 15 h postinfection; clarified from floating bacteria by centrifugation; and used for LDH assay. To determine spontaneous cell death, referred as a negative control, we collected medium from cells incubated the same time without Francisella. To measure total LDH content in cells, referred as a positive control, cells were lysed by adding Triton X-100 (1% final concentration) to the well. LDH concentration in the medium was detected at OD 490 nm. Cell death was calculated by the following formula: (cytotoxicity (%) = (sample − negative control)/(positive control − negative control) × 100).
Caspase-1 activation assay
Caspase-1 activation was assayed according to the following method described by Kahlenberg and Dubyak (31): 50 million THP-1 cells were washed in 1× PBS and resuspended in 500 µl of buffer W (20 mM HEPES (pH 7.5), 10 mM KCl, 1.5 mM MgCl2, 1.0 mM EGTA, 1.0 mM EDTA) supplemented with 2 mM DTT and antiproteases. The cells were then pelleted at 300 × g for 1 min, and all but 50 µl of the buffer was removed. The cells were allowed to swell on ice for 10 min, and then lysed by 15 passages through a 22-g needle. Lysates were spun at 15,000 × g for 10 min, and the supernatant was removed to a fresh tube kept on ice. Protein concentrations were determined by Bradford assay (Bio-Rad). Lysates were shifted to 30°C for 1 h to facilitate proteolytic processing of native caspase 1. The reactions were stopped by adding equal volume of 4× SDS-PAGE buffer. Lysates were run on 14% polyacrylamide gels, transferred to polyvinylidene fluoride (Millipore), and probed with caspase 1 Ab.
Real-time PCR
Cells were lysed in TRIzol (Invitrogen Life Technologies), and total RNA was converted into cDNA by ThermoScript RT system (Invitrogen Life Technologies). Quantitative PCR was done in the ABI PRISM 7900 machine (Applied Biosystems) using SYBR Green PCR mix (Eurogentec North America). The target gene values were normalized to the values of two housekeeping genes, GAPDH and CAP-1, and expressed as relative copy number (RCN), as described previously (19, 32).
Statistical analysis
All data were expressed as mean ± SEM. Comparisons of groups for statistical difference were done using Student’s two-tailed t test. Significance was defined as p < 0.05.
Results
MDM have impaired inflammasome activation
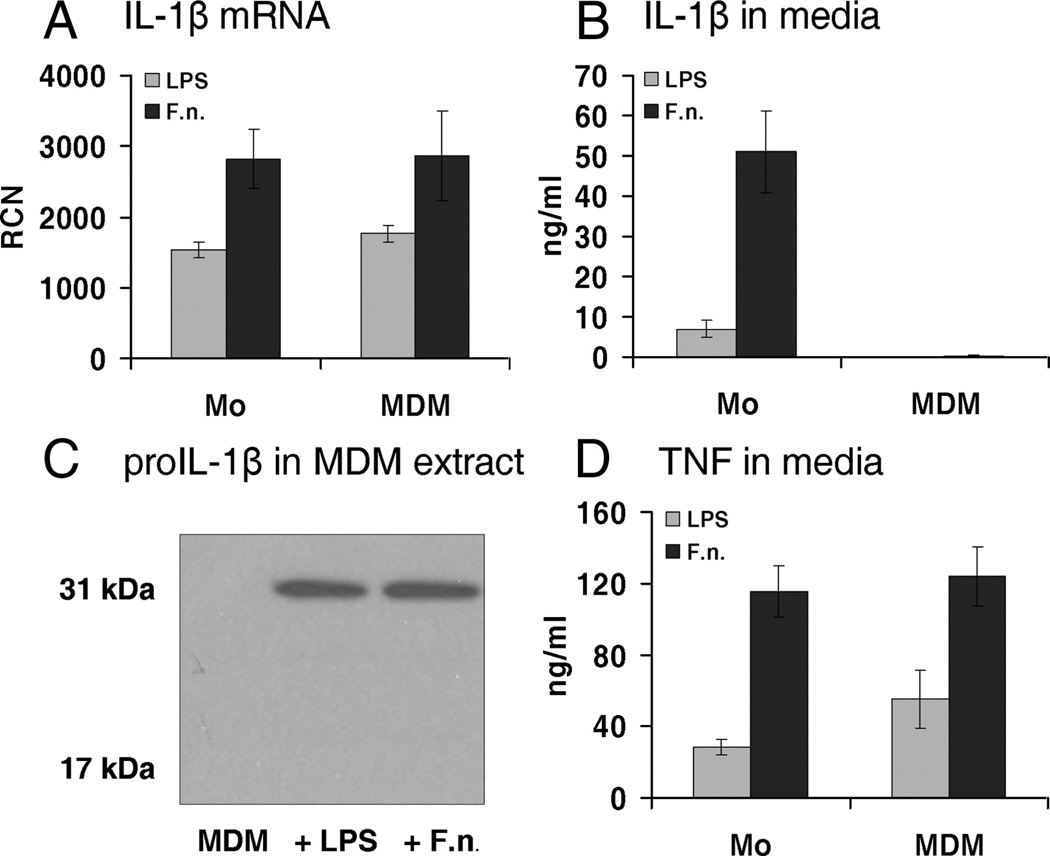
Because we had previously noted a difference in IL-1β processing and release in macrophages as compared with monocytes, we compared these two cell types for their response to an intracellular pathogen F. novicida. Similar to our prior observations with E. coli LPS (33, 34), monocytes and MDM differed in their response to Francisella (Fig. 1). MDM, treated with LPS or infected with F. novicida for 15 h, were defective in IL-1β release, as compared with fresh human monocytes (Fig. 1B). However, quantitative PCR (qPCR) analysis showed that MDM express IL-1β message with a level similar to monocytes (Fig. 1A), and immunoblots of MDM cellular extracts confirmed the production of pro-IL-1β (Fig. 1C). Importantly, there was no difference between monocytes and MDM in TNF release (Fig. 1D). Thus, MDM are capable of responding to Francisella stimulation, as evidenced by pro-IL-1β production and TNF release, but have a limitation in the inflammasome-dependent processing of IL-1β.
FIGURE 1.
Human MDM differ from monocytes in response to E. coli endotoxin and live infection by F. novicida. A, Monocytes and MDM respond equally well to E. coli LPS and live Francisella at mRNA level. B, MDM are unable to release IL-1β, in contrast to monocytes. C, MDM synthesize pro-IL-1β in response to E. coli LPS and F. novicida. A quantity amounting to 106 MDM was lysed in 100 µl of hypotonic lysis buffer and analyzed by immunoblot for IL-1β after no stimulation (MDM), and with the addition of E. coli LPS or F. novicida (F.n.). D, MDM release inflammasome-independent TNF, similar to monocytes. n = 5 for MDM and n = 9 for monocytes.
MEFV (pyrin) expression is suppressed in MDM as compared with fresh human monocytes and THP-1 cells
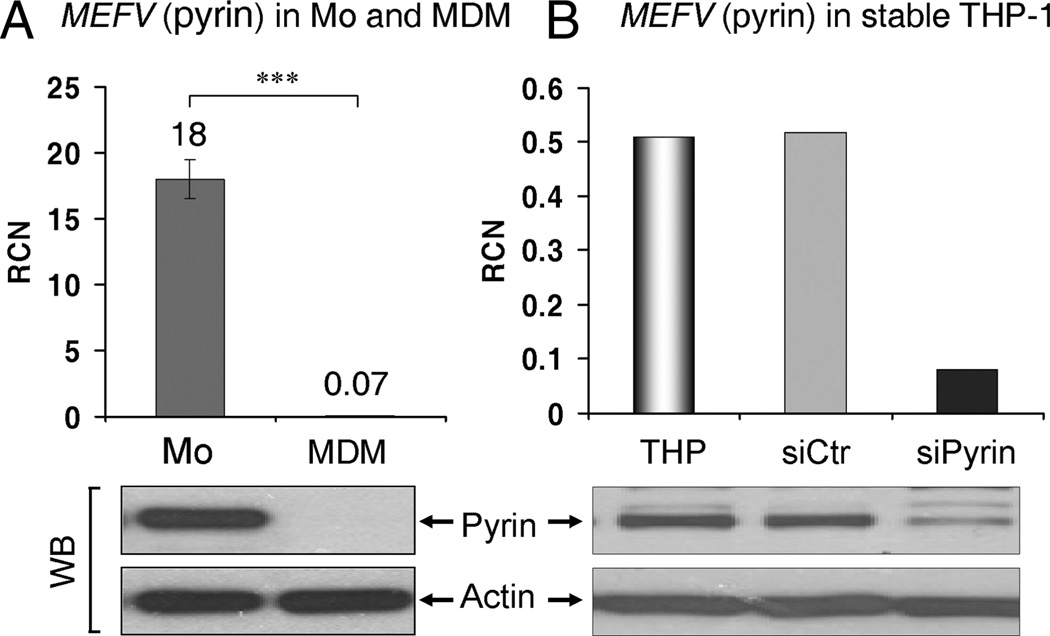
In an effort to explain how monocytes and macrophages are different in inflammasome-dependent pro-IL-1β processing, we used quantitative RT-PCR to screen TLR, NLR, cytokines, and NF-κB gene expression in monocytes and macrophages (supplemental Table I).4 Of note, there was a 250-fold decrease in MEFV (pyrin) mRNA level in macrophages, as compared with monocytes (18.0 ± 1.5 vs 0.07 ± 0.02 RCN) (Fig. 2A), whereas other genes of interest were less dramatically altered. Immunoblot of cell extracts confirmed the down-regulation of pyrin protein in MDM as compared with monocytes (Fig. 2A). The pyrin Ab specificity was confirmed by knockdown of pyrin in THP-1 cells (Fig. 2B). In agreement with the described difference in MEFV (pyrin) levels, human monocytes quickly lose pyrin mRNA (MEFV) expression during maturation: starting at 18 RCN in fresh monocytes, levels dropped to 8 RCN after 4 h, then to 2 RCN at 8 h and below 1 RCN by the next day.
FIGURE 2.
Difference in MEFV (pyrin) expression between human monocytes, MDM, and THP-1 cells. A, MDM are deficient in MEFV mRNA (measured by qPCR; bar graph) and intracellular pyrin protein (bottom; immunoblot), as compared with fresh human monocytes. ***, p < 0.0001; n = 9. B, THP-1 stably expressing siRNA against pyrin (siPyrin) show decreased MEFV mRNA (measured by qPCR) and intracellular pyrin levels (detected by immunoblot), as compared with untreated THP-1 and THP-1 stably expressing control siRNA (siCtr).
MEFV (pyrin) down-regulation leads to suppression of IL-1β release by human monocytes infected with F. novicida
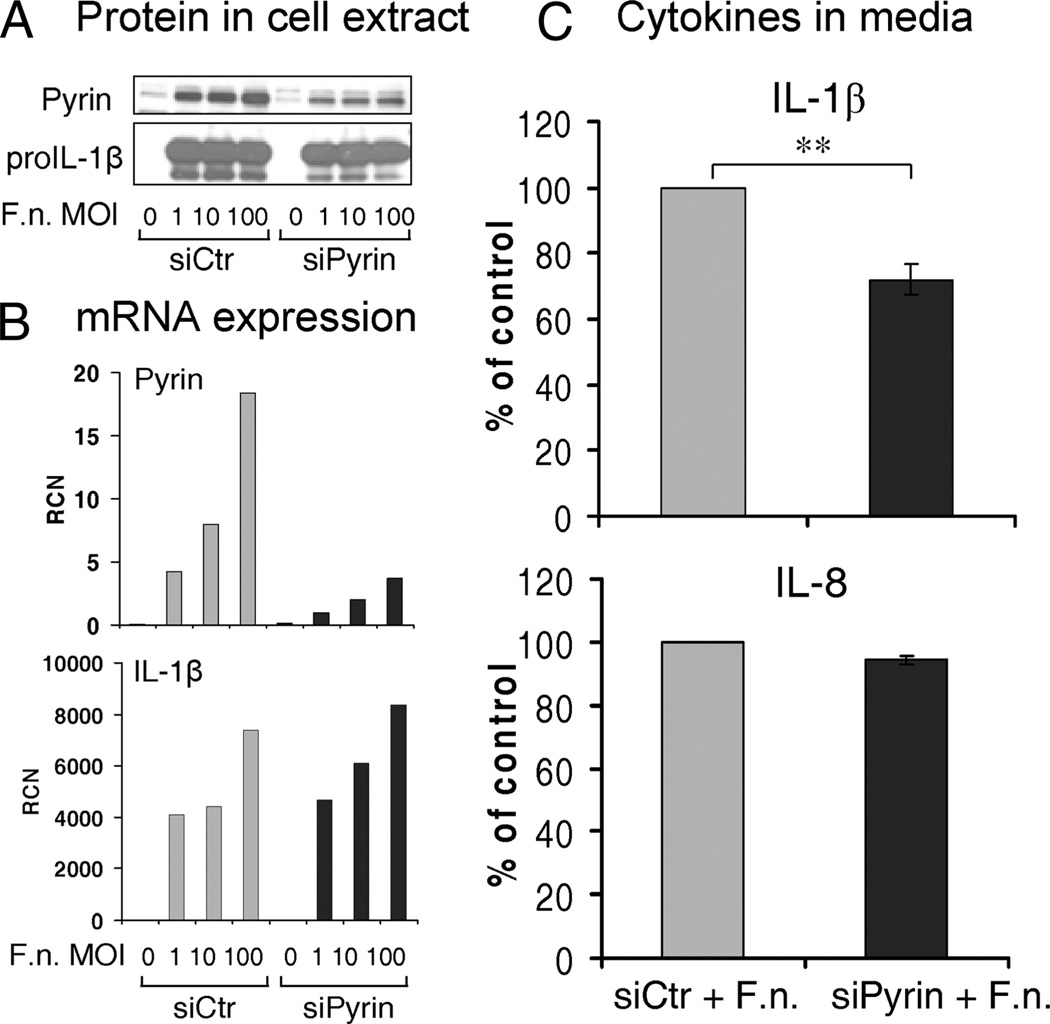
Based on the difference in pro-IL-1β processing and release between monocytes and MDM, and taking into account the dramatic decrease in pyrin levels in MDM, we asked whether mononuclear cell IL-1β release in response to Francisella infection is regulated by pyrin. To answer this question, we first suppressed pyrin expression in monocytes. Fresh human monocytes (5 × 106) were nucleofected with 100 pmol of control or pyrin siRNA. Then after overnight rest, monocytes were infected with F. novicida at three different MOI, as follows: 1, 10, and 100 for 8 h. Immunoblots of cell lysates (Fig. 3A) and qPCR (Fig. 3B) confirmed a specific decrease in pyrin expression. The decrease in pyrin expression in siPyrin-treated monocytes was observed for all three MOIs. Furthermore, there was no difference in pro-IL-1β expression upon Francisella infection between si control or siPyrin monocytes (Fig. 3, A and B). The decrease in the pyrin induction was associated with a significant reduction in IL-1β release (p = 0.004) for all three MOI (Fig. 3C). In contrast, IL-8 release, which is inflammasome independent, was not different between monocytes nucleofected with control siRNA or siPyrin (Fig. 3C).
FIGURE 3.
MEFV (pyrin) knockdown suppresses IL-1β release in human monocytes infected with F. novicida. Fresh human monocytes were nucleofected with 100 pmol of control or pyrin siRNA and incubated for 18 h before infection with F. novicida at three different MOI. Cells and their culture medium were collected 8 h after infection. Pyrin expression at both levels, protein by immunoblot (A) and mRNA by qPCR (B), is decreased in human monocytes nucleofected with siPyrin as compared with control siRNA (siCtr). In contrast, pro-IL-1β precursor expression is similar between siCtr and siPyrin monocytes, infected with Francisella. C, Cytokine release in cell culture medium. Human monocytes nucleofected with siPyrin show 28% reduction in IL-1β, but not in IL-8 release consistent for every experiment and every MOI used. However, because the actual cytokine release ranged from 1 to 20 ng/ml for IL-1β, the data are presented as percentage of the control value (monocytes nucleofected with control siRNA – siCtr) (p = 0.004; n = 5 independent experiments).
THP-1 cells with stable knockdown of pyrin expression are characterized by decreased IL-1β release, followed by Francisella infection
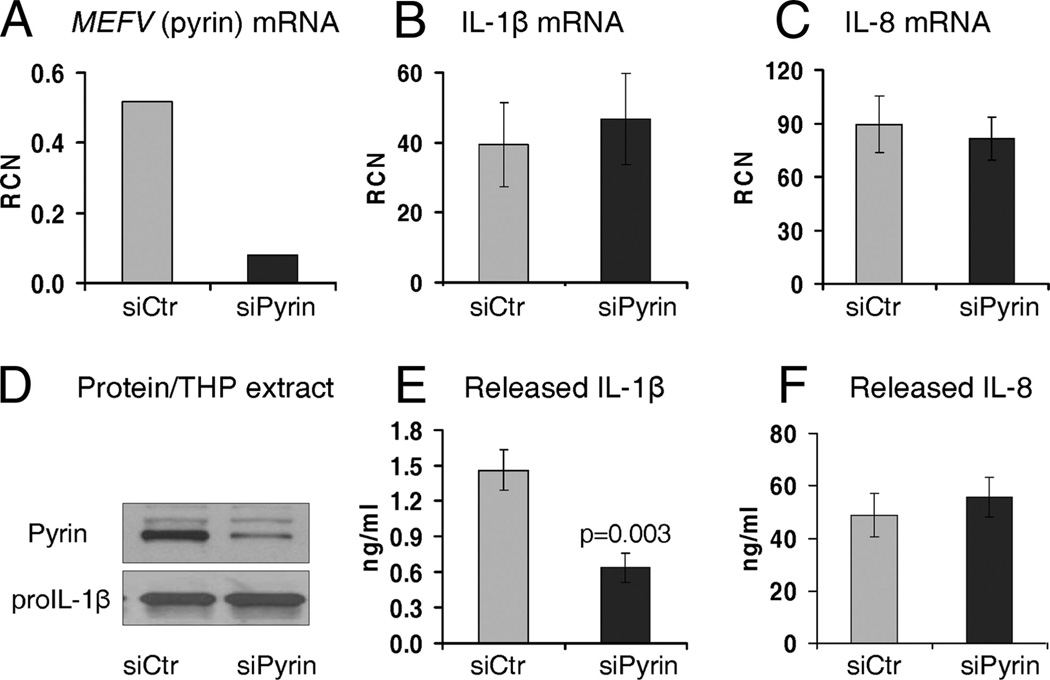
Because of the difficulties in genetically modulating primary mononuclear phagocytes, we turned to the promonocytic cell line, THP-1. THP-1 cells do express pyrin, but to a lesser extent than fresh human monocytes (Fig. 2B). Because these cells are able to process pro-IL-1β upon Francisella stimulation, we created cell lines stably expressing either control siRNA (siCtr) or siPyrin to study the possible role of pyrin in this process. We used a red fluorescent protein expressing lentiviral plasmid for the control siRNA and an EGFP-expressing plasmid for the siPyrin plasmid to allow selection of purified populations of cells by flow sorting. As shown in Fig. 2B, THP-1 cells stably expressing siRNA against pyrin have suppressed MEFV mRNA and pyrin protein levels, as compared with the parental THP-1 and THP-1 stably expressing control siRNA.
We next used these stable knockdowns of pyrin (Fig. 4, A and D) to analyze their response to F. novicida. There was no difference in IL-1β (Fig. 4B) and IL-8 (Fig. 4C) mRNA expression in response to the F. novicida infection between cells harboring either control siRNA (siCtr) or siPyrin. Inflammasome-independent IL-8 release was also similar between the two cell lines (Fig. 4F). However, in contrast, IL-1β release after Francisella infection was significantly reduced in THP-1 cells with stable pyrin knockdown (Fig. 4E). Thus, as seen with the transient siPyrin nucleofection of primary human monocytes, a stable decrease in pyrin levels is also associated with a decrease in IL-1β after F. novicida challenge.
FIGURE 4.
THP-1 cells stably expressing siRNA against MEFV (pyrin) show decrease in IL-1β release, followed by F. novicida infection. THP-1 cells stably expressing siPyrin show decreased MEFV (pyrin) mRNA (A) and intracellular pyrin (D) levels after infection with Francisella, as compared with control siRNA (siCtr) group. These stable THP-1 show a similar IL-1β (B) and IL-8 (C) mRNA synthesis after infection with F. novicida. However, following F. novicida infection, THP-1 cells with decreased levels of pyrin show decrease in mature IL-1β release (p = 0.003) (E). In contrast, in response to Francisella infection, inflammasome-independent IL-8 release is not affected by pyrin knockdown (F). To note, stable THP-1 not infected with F. novicida do not release IL-1β (<0.005 ng/ml) and IL-8 (<0.1 ng/ml), and therefore, are not included in the figure. Experiments represent n = 6 of independent experiments done in duplicates and triplicates.
M-CSF corrects MDM pyrin deficit and ability to process and release IL-1β in response to Francisella
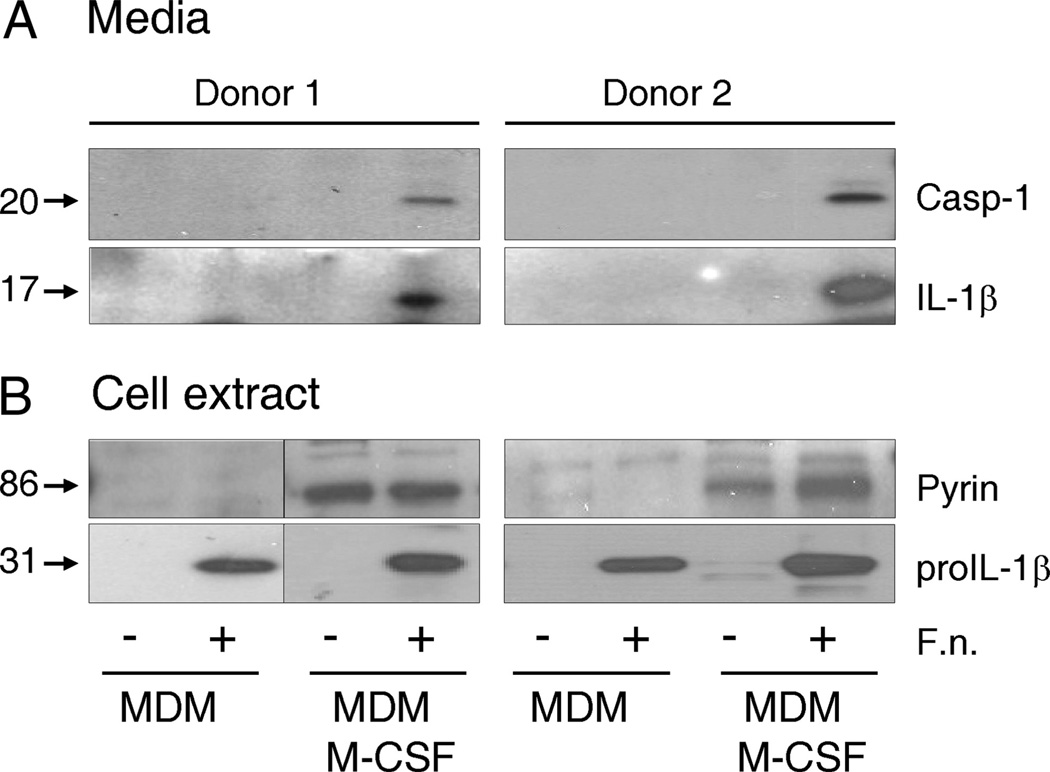
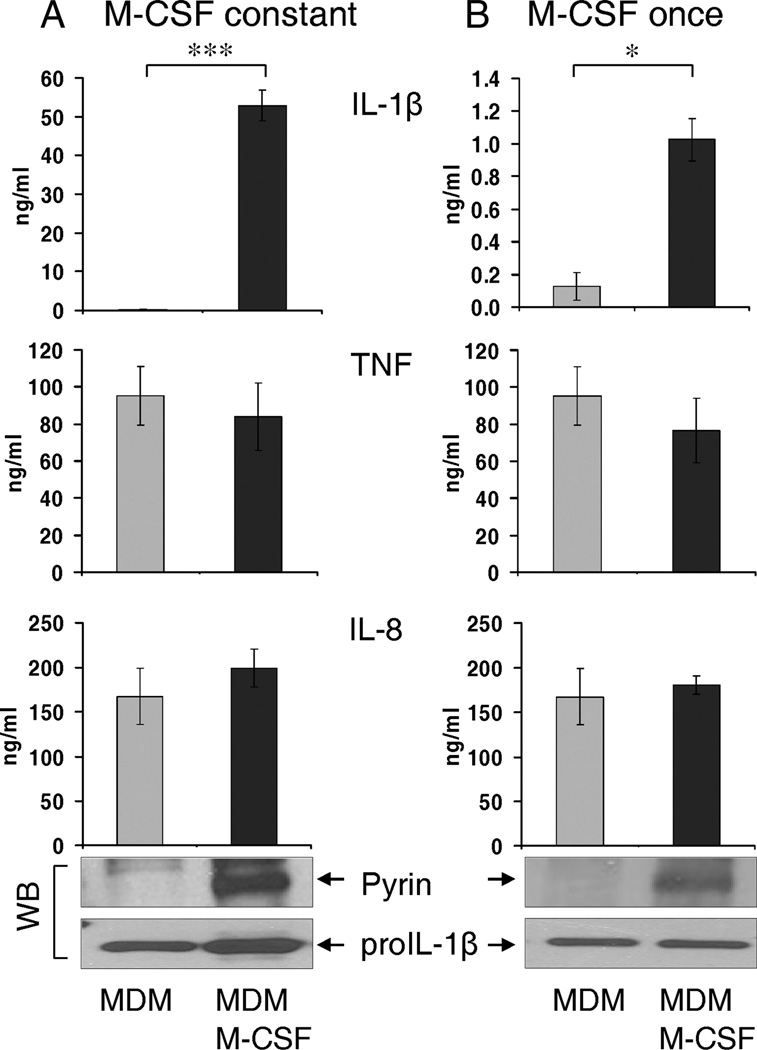
In an effort to refine our MDM model, we experimented with the use of M-CSF as an aid to MDM maturation. To our surprise, M-CSF-treated MDM maintained high levels of pyrin and efficiently released active caspase-1 and IL-1β (i.e., similar to fresh human monocytes) in response to Francisella infection (Fig. 5A). Fig. 5B shows that MDM matured with or without a M-CSF (20 ng/ml) contained correspondingly different levels of pyrin. However, even though both MDM types respond to F. novicida infection by synthesis of pro-IL-1β, only pyrin-containing MDM were able to release mature IL-1β (Fig. 6A). Of note, TNF and IL-8 release was not affected by M-CSF treatment.
FIGURE 5.
M-CSF induces pyrin expression and ability to respond to Francisella. Francisella infection activates an inflammasome only in pyrin-expressing MDM, as indicated by an active caspase-1 (p20) and mature IL-1β release into cell culture medium. MDM matured in the absence or constant presence of M-CSF (20 ng/ml) were infected with F. novicida for 15 h. Cell culture medium was collected and assayed for IL-1β and caspase-1 (A). Cells were lysed and immunoblotted for pro-IL-1β and pyrin (B). Two representative donors of six are shown.
FIGURE 6.
Human MDM treated with M-CSF express high levels of pyrin and release mature IL-1β in response to Francisella infection. A, MDM, matured in the absence of stimulating factors, are deficient in pyrin (immunoblot shown) and do not release mature IL-1β after infection with F. novicida. In contrast, MDM matured in the constant presence of M-CSF (20 ng/ml) for 5–7 days, maintained pyrin levels (detected by immunoblot) and efficiently release IL-1β after infection with Francisella for 15 h. B, Overnight stimulation of pyrin-deficient MDM with M-CSF (20 ng/ml) for 15 h induces pyrin levels (immunoblot shown) and restores ability to process and release IL-1β, followed by F. novicida infection for 15 h. M-CSF does not affect inflammasome-independent TNF and IL-8 release by human MDM (*, p < 0.01; ***, p < 0.0001; n = 6 for MDM and MDM + M-CSF constant and n = 4 for MDM + M-CSF once).
Because these MDM were matured in the presence of M-CSF, it is possible that these MDM represent a juvenile or monocytelike stage of development. Therefore, to verify the specific role of pyrin in IL-1β processing and release, we treated pyrin-deficient MDM with a single dose of M-CSF for 15 h, together with F. novicida infection. The delayed M-CSF dose restored both the pyrin levels and the ability of the MDM to process and release IL-1β, although with a lower magnitude as compared with the constant M-CSF presence (Fig. 6B). Similar results were also found with MDM matured in the presence of GM-CSF (data not shown). Thus, maintaining or inducing pyrin expression in MDM allows them to process and release IL-1β in response to the Francisella infection.
Knockdown of pyrin expression decreases IL-1β release by M-CSF-stimulated MDM
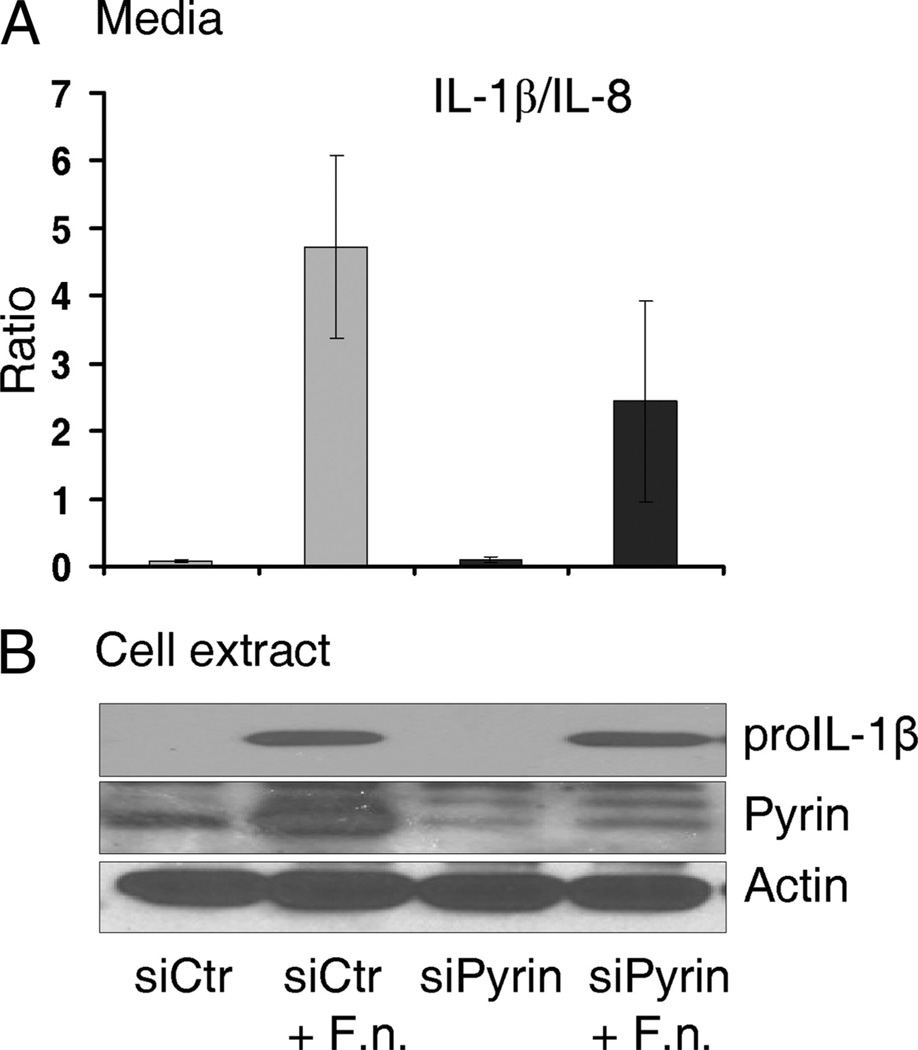
Because these MDM were matured in the presence of M-CSF, it is possible that M-CSF enhanced pyrin-independent regulators of the inflammasome. Therefore, to further verify that the M-CSF effect on IL-1β release is pyrin specific and not an indirect effect of the M-CSF stimulation, we applied a knockdown approach to M-CSF-stimulated MDM. Monocytes, derived to MDM for 7 days in the presence of 20 ng/ml M-CSF, were transfected with control (siCtr) or siPyrin siRNA for 16 h, followed by infection with 50 MOI of F. novicida for another 15 h. To control for differential cell losses between experimental groups, we normalized an inflammasome-dependent IL-1β by an inflammasome-independent IL-8 release, thus expressing released cytokines as IL-1/IL-8 ratio. Fig. 7A shows that MDM, transfected with siPyrin, release less IL-1β in response to Francisella infection, as compared with MDM transfected with control siRNA. Immunoblots of pro-IL-1β, pyrin, and actin are shown in Fig. 7B, indicating that Francisella induces pro-IL-1β and pyrin expression in MDM and that siPyrin suppresses pyrin levels. Importantly, pyrin levels correlate with IL-1β release.
FIGURE 7.
Pyrin knockdown decreases IL-1β release by M-CSF-stimulated MDM infected with F. novicida. MDM were matured in the presence of M-CSF (20 ng/ml) to maintain pyrin levels and transfected with either control siRNA (siCtr) or siPyrin (Dharmacon) with TransIT-Jurkat transfection reagent from Mirus. Next day, MDM were infected with 50 MOI of F. novicida for 15 h, cell medium was collected for released cytokine assay, and cells were lysed. A, Cytokine release into cell culture medium was expressed as IL1β/IL-8 ratio (see Materials and Methods). The released cytokines ranged between 3 and 30 ng/ml for IL-1β and 70–700 ng/ml for IL-8, depending on residual cell number. Results are the average of four independent experiments. B, Immunoblot of pro-IL-1β, pyrin, and actin expression in MDM (representative of four experiments).
Overexpressing pyrin in THP-1-derived macrophages increases their ability to release IL-1β in response to Francisella
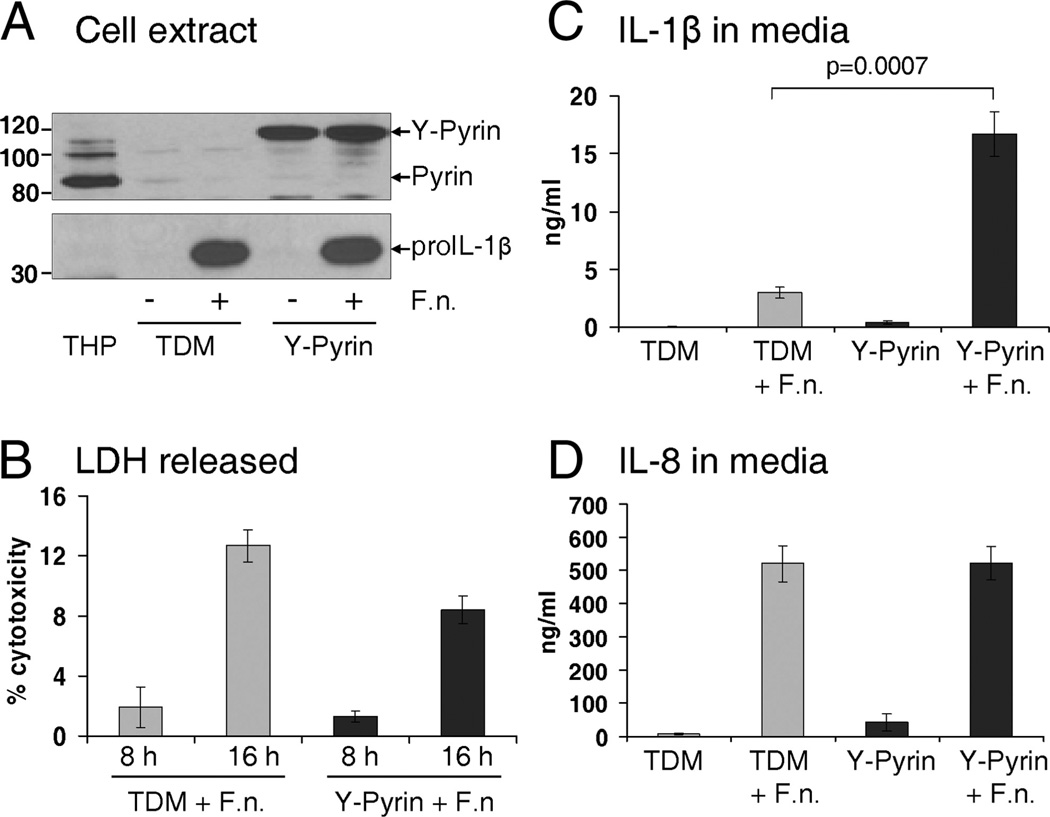
To provide a direct evidence of the link between pyrin expression and pro-IL-1β processing in MDM, we created THP-1 cells stably expressing either fluorescent protein only (THP-Red) or YFP-pyrin fusion protein. These stably transduced THP cells were stimulated with PMA for 3 h and then matured to macrophages for 5–7 days. These THP-derived macrophages (TDM) become adherent and developed a classical macrophage morphology. Consistent with our observations in MDM, TDM also lost pyrin expression with maturation. However, YFP-pyrin was readily expressed under the CMV promoter in these PMA-matured cells (Fig. 8A). Both control and pyrin-expressing TDM tolerated F. novicida infection (Fig. 8B). In agreement with our hypothesis, macrophages overexpressing YFP-pyrin show a significant increase in IL-1β release after stimulation with F. novicida for 15 h (p = 0.0006), reaching 16.7 ng/ml for 1 × 106 cells (Fig. 8C). At the same time, inflammasome- independent IL-8 release was similar between TDM expressing either fluorescent protein only or YFP-pyrin (Fig. 8D).
FIGURE 8.
TDM, overexpressing pyrin, show increase in IL-1β release after infection with F. novicida. THP-1, stably expressing either fluorescent protein (red) or YFP-pyrin, were stimulated with PMA (0.5 µM for 3 h), washed, and matured to TDM for 5 days. A, Endogenous pyrin, but not YFP-pyrin, is lost in THP during maturation to TDM. F. novicida infection induces pro-IL-1β production by TDM, having no effect on overexpression of YFP-pyrin. B, LDH release showed that TDM incubated with 50 MOI of F. novicida for 8 and 16 h were alive at the time of experiment. C, Infection with 50 MOI of F. novicida for 15 h showed significant upregulation of IL-1β in TDM expressing YFP-pyrin (p = 0.0006; n = 7 for YFP-pyrin and n = 4 for TDM). D, No difference in IL-8 release between TDM with or without overexpressed pyrin after Francisella challenge.
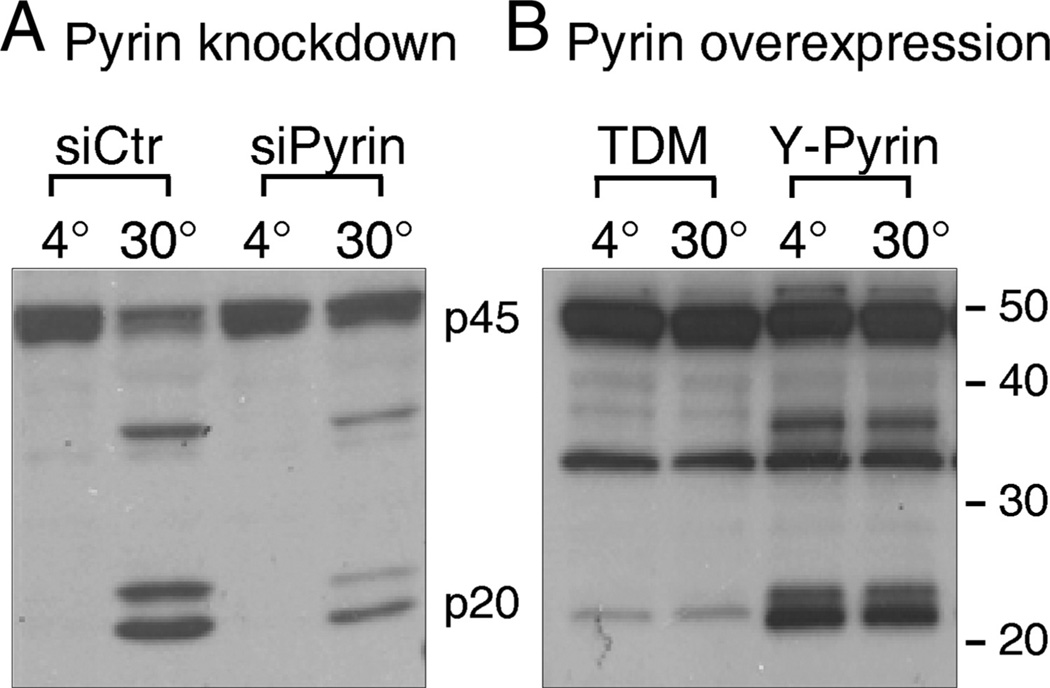
Caspase-1 activation positively correlates with pyrin levels
To further verify that IL-1β processing is dependent on pyrin levels, we performed an in vitro caspase-1 activation assay in cells with either suppressed or induced pyrin expression. THP-1 cells with suppressed pyrin expression (siPyrin) show a decrease in caspase-1 activation (Fig. 9A), which corresponds to suppression of IL-1β release (Fig. 4E). In contrast, TDM-overexpressing YFP-pyrin show a constitutive caspase-1 activation because p20 piece of an active caspase-1 was observed in cell lysate kept at 4°C (Fig. 9A). Of note, IL-1β release by these pyrin-containing TDM was significantly high as compared with pyrin-deficient cells (Fig. 8C).
FIGURE 9.
Caspase-1 activation positively correlates with pyrin expression in mononuclear cells. Cells, lysed in a hypotonic buffer, were subjected to an in vitro caspase-1 activation assay at 4°C and 30°C, as previously described (48). A, Caspase-1 activation is reduced in THP-1 with decreased pyrin level (siPyrin). B, Caspase-1 activation is enhanced in TDM, stably overexpressing pyrin fused with YFP (Y-Pyrin). p20 subunit of caspase-1, observed at 4°C, indicates that caspase-1 is constitutively active in TDM with Y-pyrin.
Discussion
Although Francisella efficiently activates monocytes to release IL-1β, the Francisella inflammasome remains uncharacterized. In this context, differences between human monocyte and MDM in IL-1β release after infection with Francisella have shed light on the nature of Francisella sensing. Screening for TLR, NLR, cytokines, and NF-κB proteins, we found similar levels of expression between monocytes and MDM for majority of these molecules (supplemental Table I).4 However, monocytes and MDM dramatically differ in expression of the pyrin gene, MEFV. Fresh human monocytes express high levels of pyrin, but pyrin levels fall with differentiation to MDM. Importantly, pyrin levels correlate with IL-1β release after infection with Francisella. Although fresh monocytes readily release the cytokine, this release is blunted in MDM. Suppression of pyrin expression in human monocytes or THP-1 cells decreases IL-1β release induced by F. novicida. Conversely, macrophages induced to express pyrin efficiently process and release IL-1β in response to Francisella compared with control transfections in which pyrin is absent.
Perhaps the most novel result of the present work is the striking effect that M-CSF has on the production of pyrin. To our knowledge, this report is the first recognition that pyrin levels are dependent upon a growth factor. Not only does M-CSF induce monocytes to maintain pyrin levels with differentiation, M-CSF treatment of matured macrophages can reinduce pyrin protein expression. However, we cannot exclude the possibility that M-CSF enhanced pyrin-independent regulators of the inflammasome. It is known that M-CSF is polyfunctional, inducing many genes and initiating numerous signaling pathways (35–37). Interestingly, elevated levels of M-CSF are often associated with TNF and IL-1β release at the sites of inflammation (35). However, that the induction of pyrin by M-CSF is at least in part responsible for the enhanced IL-1β processing and release is shown by the experiment with PMA-matured THP-1 cells (TDM). As TDM mature, they lose pyrin. However, the stable expression of recombinant pyrin in TDM greatly enhances their IL-1β-processing potential in response to Francisella.
Pyrin (also known as TRIM20) belongs to the tripartite motif (TRIM) family of proteins that number over 60 members, all of which share structural homology, and most contain a C-terminal B30.2 (SPRY) domain. Familial Mediterranean fever, a disease characterized by spontaneous bouts of inflammation and fever, has been linked to the mutations in the SPRY domain of pyrin (38).
At baseline, pyrin exists in an autoinhibited homotrimeric state, in which PYD is masked by direct interaction with its B-box. However, ligation of PSTPIP1 (proline-serine-threonine phosphatase-interacting protein-1) activates pyrin by unmasking its PYD, thus allowing it to interact with ASC (27). Importantly, the other half of the ASC molecule is composed of a CARD, which can interact directly with caspase-1 via caspase-1’s N-terminal CARD domain. Analogous to the NLRP3 inflammasome, a pyrin inflammasome, containing pyrin, ASC, and caspase-1, has been proposed (26). Also analogous to NLRP3 is the fact that pyrin can respond to more than one agonist. We have recently documented that pyrin is important in the LPS response as well (28).
Pyrin’s function in regulating inflammation, however, remains quite controversial. It was originally proposed to be an anti-inflammatory molecule because targeted disruption of pyrin in mice resulted in enhanced IL-1β release (23). One school of thought predicts that pyrin inhibits caspase-1 activation by virtue of its ability to sequester ASC away from caspase-1 via their PYDs (24, 25). It is well recognized that ASC is critical for caspase-1’s enzymatic activation (39, 40). Thus, this hypothesis states that the disease-associated mutations in the MEFV gene provide a loss of function. Hence, affected individuals have a decreased ability to sequester ASC away from caspase-1, allowing spontaneous processing and release of the caspase-1 substrates IL-1β and IL-18.
The contrary opinion, and the one that the present data support, is that pyrin functions as a unique sensor of pathogens, which has the capacity to enhance caspase-1 activity via a unique inflammasome format. We show a correlation between pyrin levels and caspase-1 activation and release, together with IL-1β. Although THP cells overexpressing recombinant pyrin demonstrate constitutively active caspase-1, we believe that our data clearly demonstrate that the effect of pyrin on caspase-1 is not simply a nonspecific increase in pyrin-mediated caspase activity. As shown in Fig. 5, M-CSF induces physiological pyrin levels that alone do not induce caspase-1 activity, but require the additional Francisella stimulus to induce caspase-1 activity.
Our previous experiments, done in primary human cells, as opposed to overexpression systems, also suggest that pyrin is proinflammatory (28). This is supported by work from the Alnemri laboratory (27). In this view of pyrin’s function, familial Mediterranean fever mutants are more likely gain-of-function mutations. In this context, it is intriguing that we recently found an association between the persistence of pyrin expression in monocytes and death from multiple organ failure in children (41). Taken together, it appears that pyrin plays an important role in general host defense.
We report in this study that IL-1β release after Francisella infection depends on pyrin levels. Based on the example that TRIM5α detects viral capsid proteins, thus preventing HIV infection in some primates (42, 43), one can speculate that Francisella may be recognized in a similar way by the SPRY domain of pyrin (TRIM20). If this is the case, Francisella binding to the SPRY may promote an interaction with the B-box, thus liberating pyrin’s PYD for engagement with ASC and caspase-1. Recently, it was shown that pyrin may be activated by Siva, a proapoptotic protein, via binding to SPRY domain and recruiting Siva to ASC specks (44). Pyrin may initiate ASC oligomerization into an active ASC-caspase-1 pyroptosome, resulting in the pyroptosis, a caspase-1-dependent cell death (27).
We have not directly addressed whether pyrin may be involved in regulating caspase-1 response to other organisms besides Francisella. However, we speculate that pyrin senses other pathogens as well. A case in point is Listeria monocytogenes. Both pyrin and ASC are associated with polymerized actin generated by L. monocytogenes. Furthermore, it has recently been demonstrated that pyrin’s B-box and coiled-coil region is required for the association with Listeria tails (45). Although the NLRP3 independence of Listeria is not uniformly accepted (5), L. monocytogenes is a Grampositive bacteria that shares many features with F. tularensis, i.e., NLRP3- and NLRC4-independent activation of caspase-1 (22), and necessity of type I IFN signaling for activation of caspase-1 inflammasome (46). Thus, Listeria is a candidate for pyrin interactions. In this context, it will be interesting to see whether alterations in pyrin levels have an influence on caspase-1-dependent IL-1β release induced by other intracellular pathogens.
The observed controversy in pyrin’s function may be explained in part by different experimental conditions. First, murine and human models may differ because murine pyrin is structurally dissimilar to human pyrin. Murine pyrin lacks the C-terminal B30.2 (SPRY) domain (47). This may partly explain why mice are very susceptible to the Francisella infection with high lethality, i.e., intracellular Francisella may not be adequately detected. Francisella induces high percentage of murine macrophage cell death several hours after internalization (20), which is different from our observations with human monocytes and macrophages. Second, human monocytes show a higher rate of caspase-1 activation than mouse macrophages, implying significant biochemical differences in the assembly and regulation of caspase-1 signaling complexes (48). This may explain why Francisella replicates in human and murine mononuclear cells, but induces only human cells to secrete proinflammatory cytokines (49). Third, it is conceivable that pyrin has more than one function. For example, pyrin may inhibit the NLRP3 inflammasome by the sequestration of NLRP3 from its activation (25). However, in contrast, specific stimuli, like Francisella, may activate a pyrin inflammasome that works independently of NLRs. Finally, there is also the possibility that pyrin’s own cleavage may be a determinant of inflammasome activity. As an example, CARD-only and PYD-only proteins are functional inhibitors of the inflammasome (50). Therefore, pyrin cleavage that can release its PYD domain from the rest of the molecule may induce another functional form of pyrin that has a modulatory effect on NF-κB function (51).
The discrepancy in IL-1β cleavage and release by monocytes, macrophages, THP-1 cells, and PMA stimulation in response to numerous stimuli was an issue for many investigators (52). According to Netea et al. (53), this difference is mostly dependent on the level of constitutive caspase-1 activation, which is regulated by the inflammasome components NLRP3 and ASC. Fresh human monocytes possess an active caspase-1 and require only one stimulus (LPS) for induction of IL-1β synthesis and release, whereas macrophages are unable to process and release IL-1β solely by TLR ligands and require a second ATP stimulation (53). In addition to that two-step signaling, we showed a critical role of pyrin in regulation of caspase-1 activity and IL-1β processing and release by human mononuclear cells.
In summary, pyrin is a complex molecule that can interact with a host of cytosolic proteins, including ASC, caspase-1, and PSTPIP1. We show in this study that pyrin is up-regulated in monocytes as compared with MDM, that its expression positively correlates with the ability of Francisella to activate an inflammasome, and, finally, that M-CSF can induce pyrin’s expression. Thus, the well-recognized difference between monocytes and macrophages for the processing and release of mature IL-1β may be due, at least in part, to the level of pyrin expression.
Supplementary Material
Acknowledgments
We thank Tim Eubank for assistance with figures.
Footnotes
This work was supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases Regional Center of Excellence for Bio-defense and Emerging Infectious Diseases Research (RCE) Program U54-AI-057153; by National Institutes of Health Grants HL40871 and HL76278; and by Davis Heart and Lung Research Institute intramural grant (to M.A.G.).
Abbreviations used in this paper: PRR, pattern recognition receptor; CARD, caspase recruitment domain; EGFP, enhanced GFP; LDH, lactate dehydrogenase; MDM, monocyte-derived macrophage; MOI, multiplicity of infection; NLR, nucleotide binding and oligomerization domain-like receptor; PAMP, pathogen-associated molecular pattern; PYD, pyrin domain; qPCR, quantitative PCR; RCN, relative copy number; siRNA, small interfering RNA; TDM, THP-derived macrophage; TRIM, tripartite motif.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 2.Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 3.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 5.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 6.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 8.Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- 9.Dinarello CA. Interleukin-1β, interleukin-18, and the interleukin-1β converting enzyme. Ann. NY Acad. Sci. 1998;856:1–11. doi: 10.1111/j.1749-6632.1998.tb08307.x. [DOI] [PubMed] [Google Scholar]
- 10.Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 12.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 13.Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, Hardy LL, Garceau V, Sweet MJ, Ross IL, et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323:1057–1060. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, Superti-Furga G. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 17.Ellis J, Oyston PC, Green M, Titball RW. Tularemia. Clin. Microbiol. Rev. 2002;15:631–646. doi: 10.1128/CMR.15.4.631-646.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rotz LD, Khan AS, Lillibridge SR, Ostroff SM, Hughes JM. Public health assessment of potential biological terrorism agents. Emerg. Infect. Dis. 2002;8:225–230. doi: 10.3201/eid0802.010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gavrilin MA, Bouakl IJ, Knatz NL, Duncan MD, Hall MW, Gunn JS, Wewers MD. Internalization and phagosome escape required for Francisella to induce human monocyte IL-1β processing and release. Proc. Natl. Acad. Sci. USA. 2006;103:141–146. doi: 10.1073/pnas.0504271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mariathasan S, Weiss DS, Dixit VM, Monack DM. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J. Exp. Med. 2005;202:1043–1049. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamkanfi M, Dixit VM. Inflammasomes: guardians of cytosolic sanctity. Immunol. Rev. 2009;227:95–105. doi: 10.1111/j.1600-065X.2008.00730.x. [DOI] [PubMed] [Google Scholar]
- 22.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Chae JJ, Komarow HD, Cheng J, Wood G, Raben N, Liu PP, Kastner DL. Targeted disruption of pyrin, the FMF protein, causes heightened sensitivity to endotoxin and a defect in macrophage apoptosis. Mol. Cell. 2003;11:591–604. doi: 10.1016/s1097-2765(03)00056-x. [DOI] [PubMed] [Google Scholar]
- 24.Chae JJ, Wood G, Masters SL, Richard K, Park G, Smith BJ, Kastner DL. The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1β production. Proc. Natl. Acad. Sci. USA. 2006;103:9982–9987. doi: 10.1073/pnas.0602081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papin S, Cuenin S, Agostini L, Martinon F, Werner S, Beer HD, Grutter C, Grutter M, Tschopp J. The SPRY domain of pyrin, mutated in familial Mediterranean fever patients, interacts with inflammasome components and inhibits proIL-1β processing. Cell Death Differ. 2007;14:1457–1466. doi: 10.1038/sj.cdd.4402142. [DOI] [PubMed] [Google Scholar]
- 26.Yu JW, Wu J, Zhang Z, Datta P, Ibrahimi I, Taniguchi S, Sagara J, Fernandes-Alnemri T, Alnemri ES. Cryopyrin and pyrin activate caspase-1, but not NF-κB, via ASC oligomerization. Cell Death Differ. 2006;13:236–249. doi: 10.1038/sj.cdd.4401734. [DOI] [PubMed] [Google Scholar]
- 27.Yu JW, Fernandes-Alnemri T, Datta P, Wu J, Juliana C, Solorzano L, McCormick M, Zhang Z, Alnemri ES. Pyrin activates the ASC pyroptosome in response to engagement by autoinflammatory PSTPIP1 mutants. Mol. Cell. 2007;28:214–227. doi: 10.1016/j.molcel.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seshadri S, Duncan MD, Hart JM, Gavrilin MA, Wewers MD. Pyrin levels in human monocytes and monocyte-derived macrophages regulate IL-1β processing and release. J. Immunol. 2007;179:1274–1281. doi: 10.4049/jimmunol.179.2.1274. [DOI] [PubMed] [Google Scholar]
- 29.Montague CR, Hunter MG, Gavrilin MA, Phillips GS, Goldschmidt-Clermont PJ, Marsh CB. Activation of estrogen receptor-α reduces aortic smooth muscle differentiation. Circ. Res. 2006;99:477–484. doi: 10.1161/01.RES.0000238376.72592.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wewers MD, Dare HA, Winnard AV, Parker JM, Miller DK. IL-1β-converting enzyme (ICE) is present and functional in human alveolar macrophages: macrophage IL-1β release limitation is ICE independent. J. Immunol. 1997;159:5964–5972. [PubMed] [Google Scholar]
- 31.Kahlenberg JM, Dubyak GR. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am. J. Physiol. 2004;286:C1100–C1108. doi: 10.1152/ajpcell.00494.2003. [DOI] [PubMed] [Google Scholar]
- 32.Fahy RJ, Exline MC, Gavrilin MA, Bhatt NY, Besecker BY, Sarkar A, Hollyfield JL, Duncan MD, Nagaraja HN, Knatz NL, et al. Inflammasome mRNA expression in human monocytes during early septic shock. Am. J. Respir. Crit. Care Med. 2008;177:983–988. doi: 10.1164/rccm.200703-418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wewers MD, Rennard SI, Hance AJ, Bitterman PB, Crystal RG. Normal human alveolar macrophages obtained by bronchoalveolar lavage have a limited capacity to release interleukin-1. J. Clin. Invest. 1984;74:2208–2218. doi: 10.1172/JCI111647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wewers MD, Winnard AV, Dare HA. Endotoxin-stimulated monocytes release multiple forms of IL-1β, including a proIL-1β form whose detection is affected by export. J. Immunol. 1999;162:4858–4863. [PubMed] [Google Scholar]
- 35.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 36.Hashimoto S, Suzuki T, Dong HY, Yamazaki N, Matsushima K. Serial analysis of gene expression in human monocytes and macrophages. Blood. 1999;94:837–844. [PubMed] [Google Scholar]
- 37.Douglass TG, Driggers L, Zhang JG, Hoa N, Delgado C, Williams CC, Dan Q, Sanchez R, Jeffes EW, Wepsic HT, et al. Macrophage colony stimulating factor: not just for macrophages anymore! A gateway into complex biologies. Int. Immunopharmacol. 2008;8:1354–1376. doi: 10.1016/j.intimp.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 38.No authors listed. Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. The International FMF Consortium. Cell. 1997;90:797–807. doi: 10.1016/s0092-8674(00)80539-5. [DOI] [PubMed] [Google Scholar]
- 39.Srinivasula SM, Poyet JL, Razmara M, Datta P, Zhang Z, Alnemri ES. The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J. Biol. Chem. 2002;277:21119–21122. doi: 10.1074/jbc.C200179200. [DOI] [PubMed] [Google Scholar]
- 40.Sarkar A, Duncan M, Hart J, Hertlein E, Guttridge DC, Wewers MD. ASC directs NF-κB activation by regulating receptor interacting protein-2 (RIP2) caspase-1 interactions. J. Immunol. 2006;176:4979–4986. doi: 10.4049/jimmunol.176.8.4979. [DOI] [PubMed] [Google Scholar]
- 41.Hall MW, Gavrilin MA, Knatz NL, Duncan MD, Fernandez SA, Wewers MD. Monocyte mRNA phenotype and adverse outcomes from pediatric multiple organ dysfunction syndrome. Pediatr. Res. 2007;62:597–603. doi: 10.1203/PDR.0b013e3181559774. [DOI] [PubMed] [Google Scholar]
- 42.Yap MW, Nisole S, Lynch C, Stoye JP. Trim5α protein restricts both HIV-1 and murine leukemia virus. Proc. Natl. Acad. Sci. USA. 2004;101:10786–10791. doi: 10.1073/pnas.0402876101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nisole S, Stoye JP, Saib A. TRIM family proteins: retroviral restriction and antiviral defense. Nat. Rev. Microbiol. 2005;3:799–808. doi: 10.1038/nrmicro1248. [DOI] [PubMed] [Google Scholar]
- 44.Balci-Peynircioglu B, Waite AL, Hu C, Richards N, Staubach-Grosse A, Yilmaz E, Gumucio DL. Pyrin, product of the MEFV locus, interacts with the proapoptotic protein, Siva. J. Cell. Physiol. 2008;216:595–602. doi: 10.1002/jcp.21435. [DOI] [PubMed] [Google Scholar]
- 45.Waite AL, Schaner P, Hu C, Richards N, Balci-Peynircioglu B, Hong A, Fox M, Gumucio DL. Pyrin and ASC co-localize to cellular sites that are rich in polymerizing actin. Exp. Biol. Med. 2009;234:40–52. doi: 10.3181/0806-RM-184. [DOI] [PubMed] [Google Scholar]
- 46.Henry T, Monack DM. Activation of the inflammasome upon Francisella tularensis infection: interplay of innate immune pathways and virulence factors. Cell Microbiol. 2007;9:2543–2551. doi: 10.1111/j.1462-5822.2007.01022.x. [DOI] [PubMed] [Google Scholar]
- 47.Chae JJ, Centola M, Aksentijevich I, Dutra A, Tran M, Wood G, Nagaraju K, Kingma DW, Liu PP, Kastner DL. Isolation, genomic organization, and expression analysis of the mouse and rat homologs of MEFV, the gene for familial Mediterranean fever. Mamm. Genome. 2000;11:428–435. doi: 10.1007/s003350010082. [DOI] [PubMed] [Google Scholar]
- 48.Kahlenberg JM, Dubyak GR. Differing caspase-1 activation states in monocyte versus macrophage models of IL-1β processing and release. J. Leukocyte Biol. 2004;76:676–684. doi: 10.1189/jlb.0404221. [DOI] [PubMed] [Google Scholar]
- 49.Bolger CE, Forestal CA, Italo JK, Benach JL, Furie MB. The live vaccine strain of Francisella tularensis replicates in human and murine macrophages but induces only the human cells to secrete proinflammatory cytokines. J. Leukocyte Biol. 2005;77:893–897. doi: 10.1189/jlb.1104637. [DOI] [PubMed] [Google Scholar]
- 50.Stehlik C, Dorfleutner A. COPs and POPs: modulators of inflammasome activity. J. Immunol. 2007;179:7993–7998. doi: 10.4049/jimmunol.179.12.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chae JJ, Wood G, Richard K, Jaffe H, Colburn NT, Masters SL, Gumucio DL, Shoham NG, Kastner DL. The familial Mediterranean fever protein, pyrin, is cleaved by caspase-1 and activates NF-κB through its N-terminal fragment. Blood. 2008;112:1794–1803. doi: 10.1182/blood-2008-01-134932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Netea MG, van de Veerdonk FL, Kullberg BJ, Van der Meer JW, Joosten LA. The role of NLRs and TLRs in the activation of the inflammasome. Expert Opin. Biol. Ther. 2008;8:1867–1872. doi: 10.1517/14712590802494212. [DOI] [PubMed] [Google Scholar]
- 53.Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1β in monocytes and macrophages. Blood. 2009;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.