Abstract
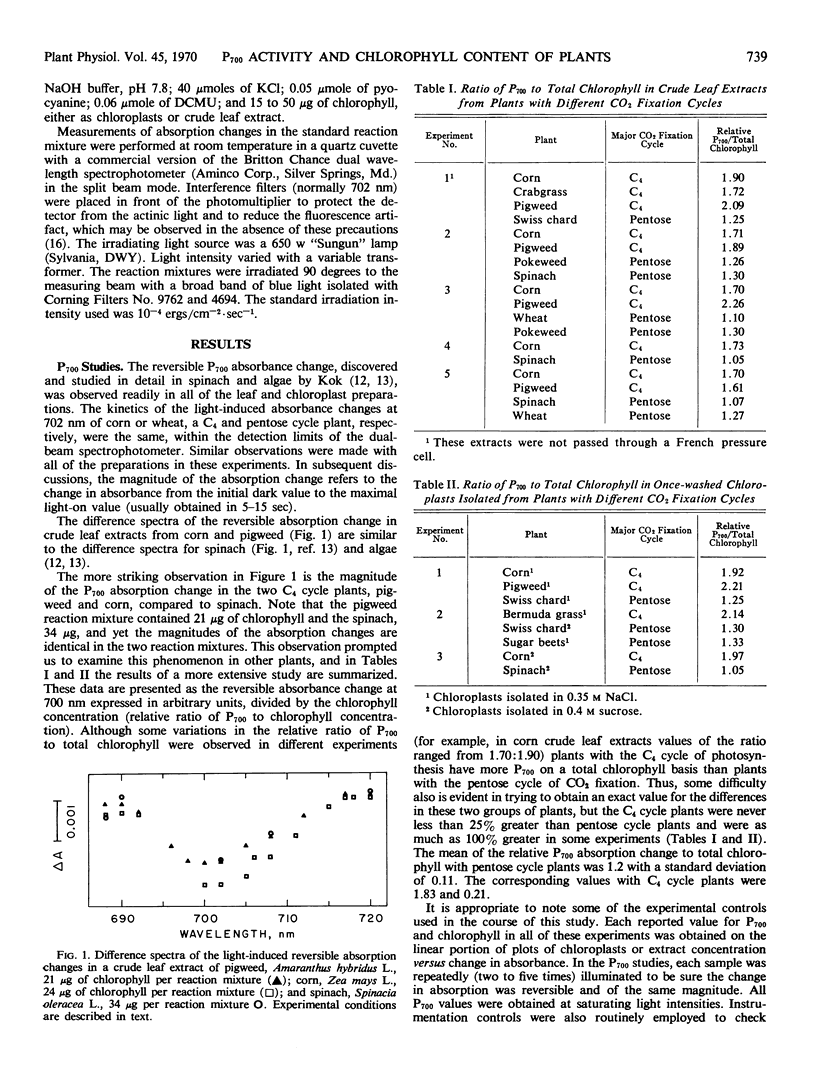
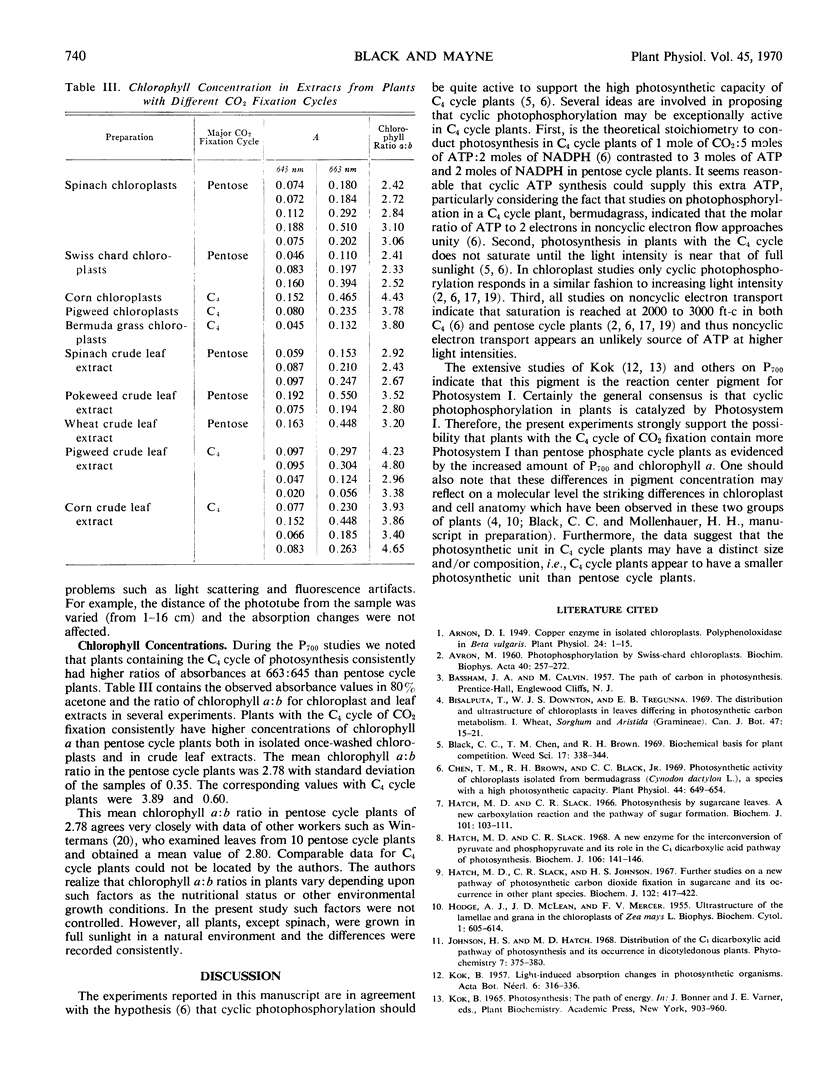
Representative plants containing either the reductive pentose phosphate cycle or the C4 dicarboxylic acid cycle of photosynthetic carbon dioxide fixation have distinctly different contents of P700 and chlorophylls a and b. With leaf extracts and isolated chloroplasts from C4 cycle plants, the mean value of the relative ratio of P700 to total chlorophyll was 1.83 and the mean value of the ratio of chlorophyll a to b was 3.89. The respective values in similar extracts and chloroplasts from pentose cycle plants are 1.2 and 2.78.
It seems likely that these results are indicative of a more active Photosystem I or a different size photosynthetic unit in C4 cycle plants than in the reductve pentose phosphate cycle plants.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. M., Brown R. H., Black C. C. Photosynthetic Activity of Chloroplasts Isolated From Bermudagrass (Cynodon dactylon L.), a Species With a High Photosynthetic Capacity. Plant Physiol. 1969 May;44(5):649–654. doi: 10.1104/pp.44.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. A new enzyme for the interconversion of pyruvate and phosphopyruvate and its role in the C4 dicarboxylic acid pathway of photosynthesis. Biochem J. 1968 Jan;106(1):141–146. doi: 10.1042/bj1060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R., Johnson H. S. Further studies on a new pathway of photosynthetic carbon dioxide fixation in sugar-cane and its occurrence in other plant species. Biochem J. 1967 Feb;102(2):417–422. doi: 10.1042/bj1020417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. Photosynthesis by sugar-cane leaves. A new carboxylation reaction and the pathway of sugar formation. Biochem J. 1966 Oct;101(1):103–111. doi: 10.1042/bj1010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortschak H. P., Hartt C. E., Burr G. O. Carbon Dioxide Fixation in Sugarcane Leaves. Plant Physiol. 1965 Mar;40(2):209–213. doi: 10.1104/pp.40.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. The reductive pentose phosphate cycle. I. Phosphoribulokinase and ribulose diphosphate carboxylase. Arch Biochem Biophys. 1957 Jul;69:300–310. doi: 10.1016/0003-9861(57)90496-4. [DOI] [PubMed] [Google Scholar]
- Slack C. R., Hatch M. D. Comparative studies on the activity of carboxylases and other enzymes in relation to the new pathway of photosynthetic carbon dioxide fixation in tropical grasses. Biochem J. 1967 Jun;103(3):660–665. doi: 10.1042/bj1030660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURNER J. F., BLACK C. C., GIBBS M. Studies on photosynthetic processes. I. The effect of light intensity on triphosphopyridine nucleotide reduction, adenosine triphosphate formation, and carbon dioxide assimilation in spinach chloroplasts. J Biol Chem. 1962 Feb;237:577–579. [PubMed] [Google Scholar]


