Abstract
Seasonal changes in numerous aspects of mammalian immune function arise as a result of the annual variation in environmental day length (photoperiod), but it is not known if absolute photoperiod or relative change in photoperiod drives these changes. This experiment tested the hypothesis that an individual’s history of exposure to day length determines immune responses to ambiguous, intermediate-duration day lengths. Immunological (blood leukocytes, delayed-type hypersensitivity reactions [DTH]), reproductive, and adrenocortical responses were assessed in adult Siberian hamsters (Phodopus sungorus) that had been raised initially in categorically long (15-h light/day; 15L) or short (9L) photoperiods and were subsequently transferred to 1 of 7 cardinal experimental photoperiods between 9L and 15L, inclusive Initial photoperiod history interacted with contemporary experimental photoperiods to determ.ine reproductive responses: 11L, 12L, and 13L caused gonadal regression in hamsters previously exposed to 15L, but elicited growth in hamsters previously in 9L. In hamsters with a 15L photoperiod history, photoperiods ≤ 11L elicited sustained enhancement of DTH responses, whereas in hamsters with a 9L photoperiod history, DTH responses were largely unaffected by increases in day length. Enhancement and suppression of blood leukocyte concentrations occurred at 13L in hamsters with photoperiod histories of 15L and 9L, respectively; however, prior exposure to 9L imparted marked hysteresis effects, which suppressed baseline leukocyte concentrations. Cortisol concentrations were only enhanced in 15L hamsters transferred to 9L and, in common with DTH, were unaffected by photoperiod treatments in hamsters with a 9L photoperiod history. Photoperiod history acquired in adulthood impacts immune responses to photoperiod, but manifests in a markedly dissimilar fashion as compared to the reproductive system. Prior photoperiod exposure has an enduring impact on the ability of the immune system to respond to subsequent changes in day length.
Keywords: delayed-type hypersensitivity (DTH), blood leukocyte, cortisol, neural-immune interactions, seasonality, reproduction
The annual change in day length (photoperiod) cues seasonal physiological adaptations in nature (Goldman, 2001). Siberian hamsters (Phodopus sungorus) are typical of many small rodents in their ability to respond to changes in day length with anticipatory physiological adaptations. For example, in the laboratory, short, winter-like photoperiods (e.g., <13 h of light per day; 13L) trigger gonadal regression and anestrus (Gorman and Zucker, 1998); in nature, inhibition of reproduction by photoperiod has been argued to be adaptive because it limits breeding to the fraction of the year when energetic conditions are most permissive for successful reproduction (Prendergast et al., 2009). Seasonal changes in numerous aspects of immune function have also been described in reproductively photoperiodic (Nelson and Demas, 1996) and nonphotoperiodic rodents (Prendergast et al., 2007). In Siberian hamsters, short days attenuate sickness behaviors (Bilbo et al., 2002b) and enhance lymphocyte blastogenesis (Yellon et al., 1999); circulating leukocyte concentrations, and T cell-dependent skin inflammatory responses (delayed-type hypersensitivity [DTH] responses) are greater in hamsters exposed to short days relative to those housed under long days, as are circulating concentrations of cortisol (Bilbo et al., 2002a). Other immune responses (e.g., primary and secondary antibody responses) are suppressed in short days (Drazen et al., 2000; Hadley et al., 2002). In common with photoperiodic responses in the reproductive system, immunological responses to photoperiod rely on the generation and entrainment of the circadian rhythm in nocturnal melatonin secretion (Gatien et al., 2005; Wen et al., 2007). The majority of diseases exhibit seasonal changes in prevalence, and the adaptive significance of seasonal changes in immune function has been argued to lie both in the anticipation of seasonally specific pathogens and in the attenuation of energetically costly immune responses at times of year when energetic resources are scarce (Nelson and Demas, 1996; Nelson, 2004).
Long (≥15L) and short (≤10L) photoperiods are customarily used in a categorical manner in laboratory studies of photoperiodism (Gorman and Zucker, 1998). In nature, however, animals are often exposed to a range of gradually increasing and decreasing photoperiods over the course of the annual cycle. Seasonal reproductive responses do not require exposure to very long or short day lengths (Gorman and Zucker, 1995a), but instead manifest in response to intermediate duration photoperiods (12L–14L) in a manner that is influenced by antecedent day lengths. Thus, a given intermediate photoperiod (e.g., 13L) elicits gonadal regression if preceded by longer days, or gonadal growth if preceded by shorter days (Duncan et al., 1985; Hoffman and Illnerova, 1986). Responses to intermediate day lengths and melatonin in this context allows appropriate seasonal responses through extrapolation of information about the direction of change in day length at times of year when photoperiods are intermediate (and therefore ambiguous) in duration.
It is not clear if the immune system, in common with the reproductive system, likewise relies on photoperiod history to engage seasonal phenotypic change. With few exceptions, effects of photoperiod on the immune system have been evaluated using only a few categorically long and short photoperiods, which presumably lie well above and below putative critical day lengths for eliciting reproductive responses. Postnatal exposure to 14L inhibited reproductive development in Siberian hamsters gestated in 16L, and accelerated development in those gestated in 8L; however, gestational photoperiod history alone was without effect on the immune system as evaluated in a broad array of assays, including leukocyte phenotypes, primary antibody responses, and DTH skin inflammatory responses (Prendergast et al., 2004a). The absence of clear effects of prenatal photoperiod history on immune function may reflect an inability of the photoperiod-responsive immune system to be impacted by any antecedent photoperiod information. Alternatively, 1) the intermediate photoperiod used (14L), while adequate to probe photoperiod history effects in the reproductive system, may lie sufficiently above or below the critical day length for triggering short-day immune responses (i.e., 14L is a categorical long or short day length for the immune system, thereby impervious to photoperiod history effects); or 2) maternal communication of photoperiod history cannot impact the immune system, but photoperiod history acquired by an individual in adulthood is still capable of affecting immunological responses to intermediate photoperiods. Either of these alternatives compels rejection of the hypothesis that photoperiod history does not affect immune responses to intermediate photoperiods.
This experiment tested the hypothesis that photoperiod history information acquired during adulthood influences immune responses to intermediate day lengths. Adult hamsters were exposed to categorically long (15L) or short (9L) photoperiods for intervals sufficient to impart photoperiod histories (Prendergast et al., 2000), and then were transferred to one of several intermediate photoperiods, representing all cardinal photoperiods between 9L and 15L, inclusive. This range of photoperiods allowed titration of the critical day lengths for induction of photoperiodic responses in the reproductive and immune systems. To compare reproductive and immune responses to changing photoperiods, pair-wise comparisons among data were complemented by multiple regressions with higher order contrasts. Blood leukocyte and cortisol concentrations were determined and hamsters were challenged with a novel antigen to assess memory T cell-dependent DTH responses.
MATERIALS AND METHODS
Animals
Male Siberian hamsters (Phodopus sungorus) were obtained from a breeding colony maintained on a light/dark cycle of 15L:9D (15L; lights-off at 1800 h CST). Hamster pups were weaned at 18 to 20 days of age and housed 1 to 4 per cage with same-sex siblings in polypropylene cages (28 × 17 × 12 cm) on wood shaving bedding (Harlan Sani-Chips, Harlan Inc., Indianapolis, IN). Ambient temperature of all experimental rooms was 20 ±0.5 °C; relative humidity was maintained at 53 ±2%. Food (Teklad Rodent Diet 8604, Harlan Inc.) and filtered tap water were provided ad libitum. Cotton nesting material was constantly available in the cage. All procedures conformed to the USDA Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Chicago.
Photoperiod treatments
At the time of weaning, hamsters were transferred to a 9L:15D photoperiod (lights-off at 1800 h CST; n = 70) or remained in their natal 15L photoperiod (n = 69). These initial photoperiod treatments (“initial” photoperiods) were maintained until hamsters reached adulthood (60–90 days of age), an interval that exceeds the duration of photoperiod exposure necessary and sufficient to impart a photoperiodic history that determines the reproductive response to intermediate day lengths (Prendergast et al., 2000). Following confirmation of reproductive responsiveness to initial photoperiods, hamsters from both postweaning photoperiods were transferred on week 0 to 1 of 7 photoperiods: 9L, 10L, 11L, 12L, 13L, 14L, or 15L (“experimental” photoperiods) for the remainder of the experiment. For all photoperiods, the time of lights-off remained constant (1800 h CST) to facilitate entrainment (Gorman et al., 1997). Testis volumes were determined at 3-week intervals between week 0 and week 12 (see the “Reproductive measurements” section, below). On week 12 blood samples were obtained for leukocyte and endocrine measures, and during weeks 13–15 skin immune function was assessed. In the text, photoperiod treatments are designated with a concatenated abbreviation consisting of [initial photoperiod] → [experimental photoperiod] (e.g., 15L → 13L).
Locomotor activity
Between weeks 6 and 12, home cage activity data were collected using passive infrared motion detectors (Coral Plus, Visonic, Bloomfield, CT) positioned 22 cm above the cage floor. Motion detectors registered activity when 3 of 27 zones were crossed. Activity triggered closure of an electronic relay, which was recorded by a PC running ClockLab software (Actimetrics, Evanston, IL).
The timing of activity was analyzed using ClockLab software according to methods described by Evans et al. (2004). In brief, a 24-h histogram was produced for each hamster by averaging activity counts in 5-min bins over a 7- to 10-day window between weeks 6 and 8. For each histogram, activity onset was defined as the point in the activity profile after 1400 h with average counts exceeding the daily overall mean level and sustained above the daily mean for at least 30 min. Activity offset was defined as the last point that exceeded the 24 h mean for 30 min ending up to 2 h after light onset. Activity offset was defined as the last time point exceeding this threshold. The duration of daily activity, α, was calculated as the interval between activity onset and activity offset (Evans et al., 2004).
Within all populations of Siberian hamsters there exist individuals that fail to entrain to decreasing photoperiods with species-typical expansion of nocturnal locomotor activity (α) and a corresponding expansion of nocturnal melatonin secretion and gonadal regression (Prendergast et al., 2001). Instead, such “nonresponder” (NR) hamsters exhibit large negative phase angles of entrainment and compressed α values in short days (typically <6 h in a 16-h scotophase; Puchalski and Lynch, 1986). Because reproductive and immunological responses to photoperiod are dependent on photoperiod-driven changes in melatonin (Carter and Goldman, 1983; Wen et al., 2007), we sought to exclude NR hamsters from this study. The absence of complete gonadal regression would be inadequate to identify NRs, because intermediate degrees of gonadal regression reflect normal responses to intermediate photoperiods (Duncan et al., 1985; Prendergast et al., 2000), thus NR hamsters were identified via abnormal entrainment to experimental photoperiods (Gorman et al., 1997; Prendergast and Freeman, 1999): individuals with α values that were >2 SD from the population mean α for a given photoperiod treatment. NR hamsters were excluded from all analyses.
Reproductive measurements
Hamsters were weighed weekly, and estimated testis volumes (ETVs) were determined on weeks 0, 3, 6, and 12. ETVs were obtained by measuring the length and width of the left testis through the scrotal skin with analog calipers while under light isoflurane anesthesia. In hamsters, ETV is positively correlated with testis weight, circulating testosterone, and spermatogenesis (Gorman and Zucker, 1995b; Schlatt et al., 1995).
Immune assays
Among the numerous and diverse measures of immune function affected by photoperiod in this species, we selected blood leukocyte concentrations and skin DTH reactions (see below) because these measures 1) exhibit high-amplitude changes following transfer from categorically long to short photoperiods, and 2) encompass a range of immune function, from the omnibus (blood leukocytes) to the highly specific (DTH) (Nelson and Prendergast, 2002). DTH reactions involve a rapid deployment of leukocytes out of the blood and infiltration into the epidermis and dermis (Dhabhar, 2000), where they provide defense against pathogens. This is a standard in vivo measure of T cell-mediated immunity (Turk, 1980).
Blood collection
On week 12, blood samples (500 μL) were collected 4 to 5 h before lights-off under light anesthesia from the right retroorbital sinus using heparinized Natelson collection tubes. Blood collections were performed in pseudo-random order, in a room separate from the general animal colonies, and following the procedure, hamsters were separated from the colony until all blood collections for the day were completed. Animal handling during the blood collection was kept to a minimum (<1 min). Following blood collection, hamsters were administered 0.5 mL of sterile 0.9% saline s.c. for rehydration. Blood samples were deposited into heparinized (50 units) microcentrifuge tubes, mixed gently, and a 25-μL aliquot of whole heparinized blood was removed for leukocyte determination. The remainder of the blood sample was kept on ice for <1 h and was centrifuged at 300 g for 30 min at 4 °C. Plasma was extracted and stored at −80 °C until assayed for cortisol.
Leukocyte counts
Leukocyte counts from a 25-μL aliquot of the whole blood sample were obtained by hemolysis with 3% acetic acid at a 1:20 dilution, and enumeration in duplicate on a hemacytometer at 400× magnification by an experimenter blind to treatment conditions. Lysed blood samples were kept at room temperature for <3 h before leukocyte counts were determined. Although distinct leukocyte subtypes are not identifiable with this method, this procedure reliably identifies photoperiod- and stress-induced changes in total leukocyte number in this, and other, rodent species (Bilbo et al., 2002a; Dhabhar et al., 1995; Wen et al., 2007). In studies of photoperiodic regulation of hamster leukocytogenesis, total leukocyte counts correlate positively with photoperiodic changes in specific leukocyte subtypes, including total lymphocytes, T cells, and NK cells (Bilbo et al., 2002a; Prendergast et al., 2004a, 2004b; Wen et al., 2007). The measure therefore provides for this species an omnibus indicator of treatment effects on the capacity for immunosurveillance in the blood (Dhabhar et al., 1995).
Induction of DTH reactions
DTH skin inflammatory responses were induced by application of the antigen 2,4-dinitro-1-fluorobenzene (DNFB; Sigma, St. Louis, MO) to the pinnae of each hamster after initial immunization (“sensitization”) by application of DNFB to the dorsum (Bilbo et al., 2002a). Sensitization was induced and DTH elicited as follows: on week 13, all hamsters (DNFB naïve) were anesthetized with iso-flurane vapor, and an area of 2 × 3 cm was shaved on the dorsum. Twenty-five microliters of DNFB (0.5% [wt/vol] in 4:1, acetone/olive oil vehicle) was applied to the dorsal skin on 2 consecutive days. Six days later, baseline thickness of both pinnae was measured in lightly anesthetized hamsters prior to induction of DTH by using a constant-loading dial micrometer (Mitutoyo, Tokyo, Japan). Immediately after baseline pinna measurements were obtained, 20 µL of DNFB (0.2% [wt/vol] in 4:1, acetone/olive oil) was applied to the skin of the dorsal surface of the right pinna. Left pinnae were treated with vehicle. Pinna thickness was measured every 24 h for the next 7 days. All measurements were performed by the same experimenter who was blind to treatment conditions. Pinna thickness values obtained on each day following challenge were expressed as a percentage of baseline thickness for statistical calculations. All DNFB treatments and pinna measurements were performed between 1400 and 1500 h, and all measurements were made on the same relative region of the pinna. This regimen of sensitization and challenge uses concentrations of DNFB that differ from those used in rats (Dhabhar and McEwen, 1999), but have been optimized for use in Siberian hamsters (Bilbo et al., 2002a).
Cortisol
Cortisol was measured from 100 μL of plasma in a single EIA (Correlate-EIA; Assay Designs, Ann Arbor, MI) that has been validated for this species (Demas et al., 2004) according to the manufacturer’s instructions. The cortisol EIA had a sensitivity of <57 pg/mL, an intra-assay coefficient of variation (CV) of <10.5%, and an interassay CV of <8.6%.
Testis weights
Hamsters were euthanized via inhalation of CO2 on week 15. Paired testis weight (PTW) for each hamster was determined to ±0.1 mg at autopsy.
Statistics
Raw data were analyzed using a 2 (initial postweaning photoperiod) × 7 (photoperiods from 9L to 15L, inclusive) factorial ANOVA. For longitudinal measures (testis size, DTH), a 2 × 7 repeated-measures ANOVA design was used. Pairwise comparisons were conducted using Fisher’s PLSD tests, where appropriate and warranted by a significant F-statistic.
To characterize the manner in which initial and experimental photoperiods affected reproduction and immune function, multiple regressions were performed on week 12 ETV values, week 12 blood leukocyte concentrations, day +1 and day +2 DTH responses, and cortisol concentrations. The 2 initial photoperiods were contrast coded, and orthogonal polynomial contrast codes were assigned for each of the 7 experimental photoperiods to represent linear, quadratic, and cubic effects. Significant correlations following contrast coding allow identification of whether the effect of increasing experimental photoperiods can be characterized by a linear, quadratic, or cubic function, and therefore allow insight into whether initial and experimental photoperiods affect reproduction and immune function in a similar manner. Multiple regression correlations (MRC) were then used to identify significant main effects for each photoperiod interval (initial, experimental) as specified by each polynomial code, as well as for interaction effects between initial and experimental photoperiods. All data are presented as mean values with SEM. For all analyses, differences were considered significant if p ≤ 0.05.
RESULTS
Reproductive responses to initial photoperiods
On week 0, testis sizes of all hamsters initially exposed to 9L were undeveloped (<200 mm3) and were significantly smaller than those of hamsters that were initially in 15L (15L: 714 ± 28 mm3; 9L: 99 + 2 mm3; F1,120 = 484, p < 0.0001).
Locomotor activity in experimental photoperiods
Following transfer from initial to experimental photoperiods on week 0, the majority of hamsters entrained to the experimental photoperiods with locomotor activity onset occurring around the time of dark onset, and an interval of sustained activity encompassing most of the dark phase (see Supplementary Online Material, Fig. S1).
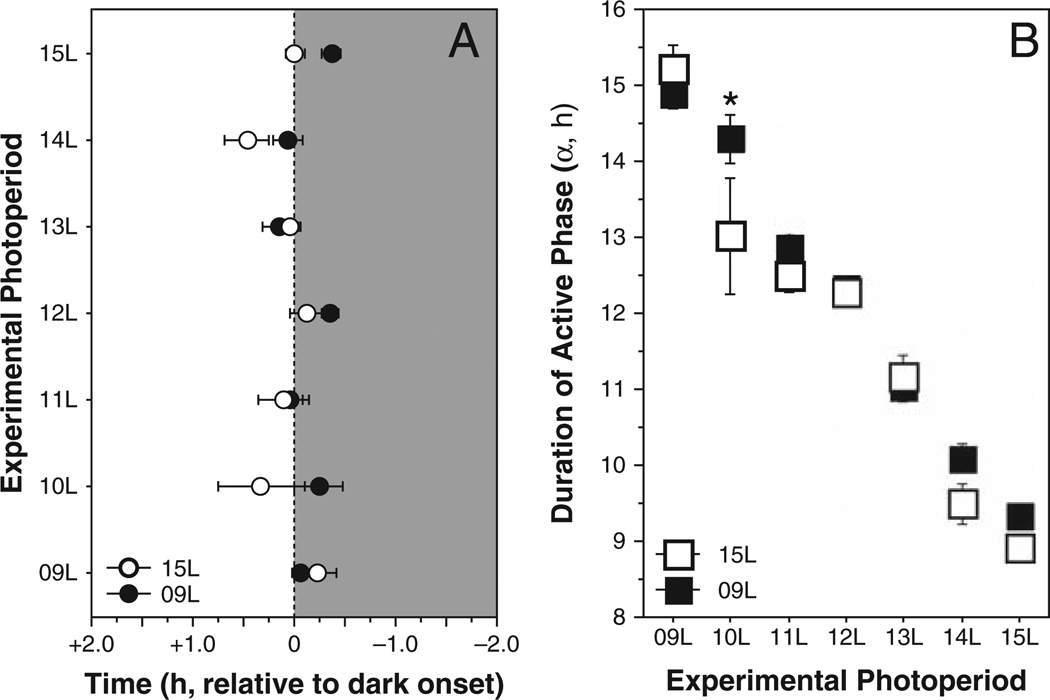
Among these hamsters, the timing of the onset of activity relative to lights-off did not vary according to initial postweaning photoperiod (F1,104 = 3.6, p > 0.05) or experimental photoperiod (F6,104 = 1.9, p > 0.05; Fig. 1A). In contrast, α values varied systematically as a function of both initial (F1,104 = 4.3, p < 0.05) and experimental (F6,104 = 112.1, p < 0.0001) photoperiod treatments (Fig. 1B). Mean values for α in each photoperiod are shown in Figure 1.
Figure 1.
Mean ± SEM steady-state (A) phase angle of entrainment and (B) duration of nocturnal locomotor activity (circadian α) of adult male Siberian hamsters raised in 15L (open symbols) or 9L (filled symbols; “initial” photoperiods) following transfer to 1 of 7 different photoperiods (“experimental” photoperiods) as indicated on the ordinate and abscissa, respectively. *p < 0.005 vs. 15L→10L.
A total of 17 hamsters were identified as “nonre-sponders” based on α values that were >2 SD below the mean α values in each photoperiod (9L: n = 2; 10L: n = 3; 11L: n = 3; 12L: n = 2; 13L: n = 4; 14L: n = 2; 15L: n = 1; Supplementary Online Material, Fig. S2); this proportion (17 of 139; 12%) is within a range predicted from meta-analyses of the incidence of nonresponsiveness in this species (Gorman and Zucker, 1997). Because nonresponders were few (n ≤ 3 per photoperiod group), no powerful statistical inferences could be made based on these individuals, and they were excluded from all subsequent analyses. Final sample sizes for each initial → experimental treatment group are indicated in Figure 2.
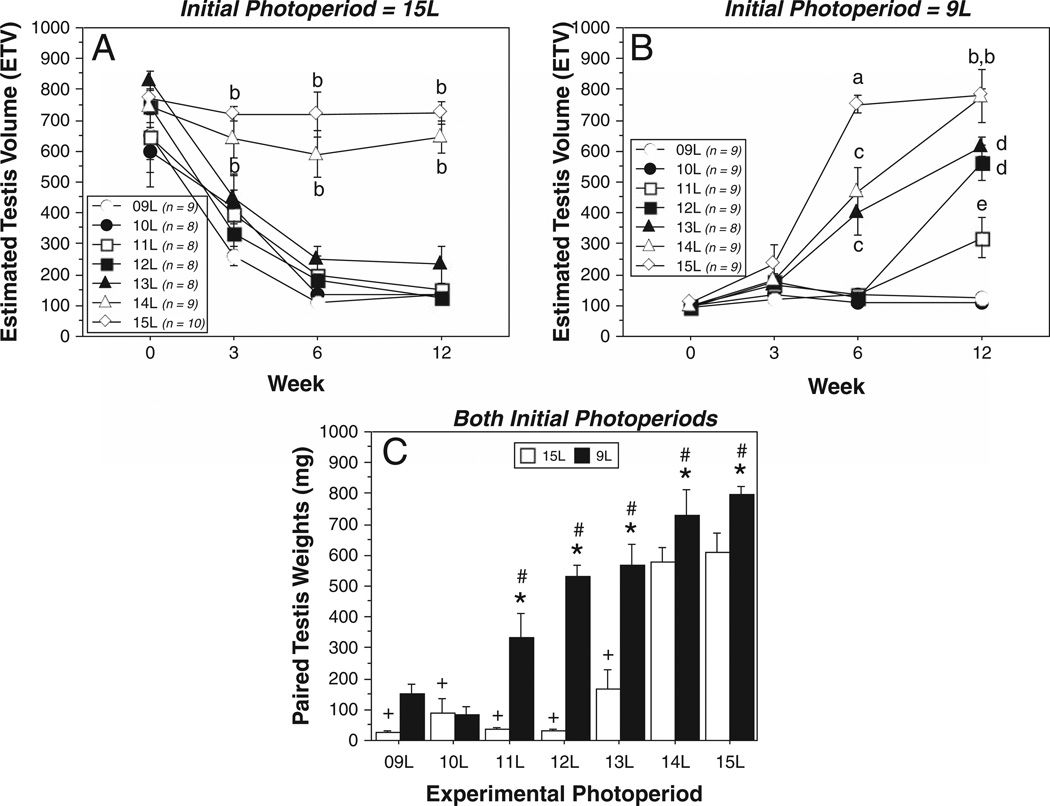
Figure 2.
Mean ± SEM estimated testis volumes (ETV) of male Siberian hamsters raised in (A) 15L or (B) 9L and transferred to 1 of 7 different experimental photoperiods. Final group sample sizes are indicated in the keys to panels A and B. Note nonlinearity of x-axis. (C) Mean + SEM paired testis weights at autopsy. In panels A and B: letters indicate p < 0.05 vs. aphotoperiods ≤14L, bphotoperiods ≤13L, cphotoperiods ≤12L, dphotoperiods ≤11L, ephotoperiods ≤10L. In panel C: *p < 0.05, 9L→ vs. 15L→ group, within experimental photoperiod. #p < 0.05 vs. 9L→9L value. +p < 0.05 vs. 15L→15L value.
Reproductive responses to experimental photoperiods
Both initial (F1,105 = 113.1, p < 0.0001) and experimental (F61,105 = 44.2, p < 0.0001) photoperiods affected reproductive responses to experimental photoperiods; initial and experimental photoperiod treatments also interacted to affect testis sizes (F1,105 = 113, p < 0.0001; F18,315 = 4.76, p < 0.0001; Fig. 2). Hamsters initially in 15L exhibited gonadal regression following transfer to photoperiods of 13 or fewer hours of light per day (p < 0.0001, all comparisons; Fig. 2A). In contrast, hamsters initially in 9L exhibited gonadal growth in response to photoperiods providing 11 or more hours of light per day (p < 0.0001, all comparisons; Fig. 2B). The rate of gonadal growth varied among photoperiods greater than 10L: the most rapid growth was evident in 9L→15L hamsters (p < 0.005 vs. 9L→10L, 9L→11L, 9L→12L, 9L→13L, all comparisons). Hamsters transferred from 9L to either 14L or 13L exhibited a monotonic pattern of gonadal growth between weeks 3 and 12, whereas growth only began after week 6 in 9L→12L and 9L→11L hamsters.
Analyses of testis weight (PTW) revealed similar main effects (initial: F1,105 = 73.8, p < 0.0001; experimental: F6,105 = 49.4, p < 0.0001) and interaction effects (F6,105 = 5.6, p < 0.0001) between initial and experimental photoperiod treatments (Fig. 2C). Among hamsters with a photoperiod history of 15L, experimental day lengths of 9L through 13L, inclusive, each elicited significant gonadal regression (p < 0.0001 vs. 15L→15L controls, all comparisons). PTWs were comparable among 15L→13L, 15L→12L, 15L→11L, 15L→10L, and 15L→9L groups (p > 0.05, all comparisons). Among hamsters with a photoperiod history of 9L, day lengths of 11L through 15L elicited significant gonadal growth (p ≤ 0.01 vs. 9L→9L controls, all comparisons), although testes of 9L→11L, 9L→ 12L, and 9L→13L groups weighed less than those of 9L→15L hamsters (p < 0.05, all comparisons).
In experimental photoperiods of 11L, 12L, and 13L, PTW of hamsters with a photoperiod history of 9L were significantly greater than those of hamsters with a photoperiod history of 15L (p < 0.0001, all comparisons).
Blood leukocytes
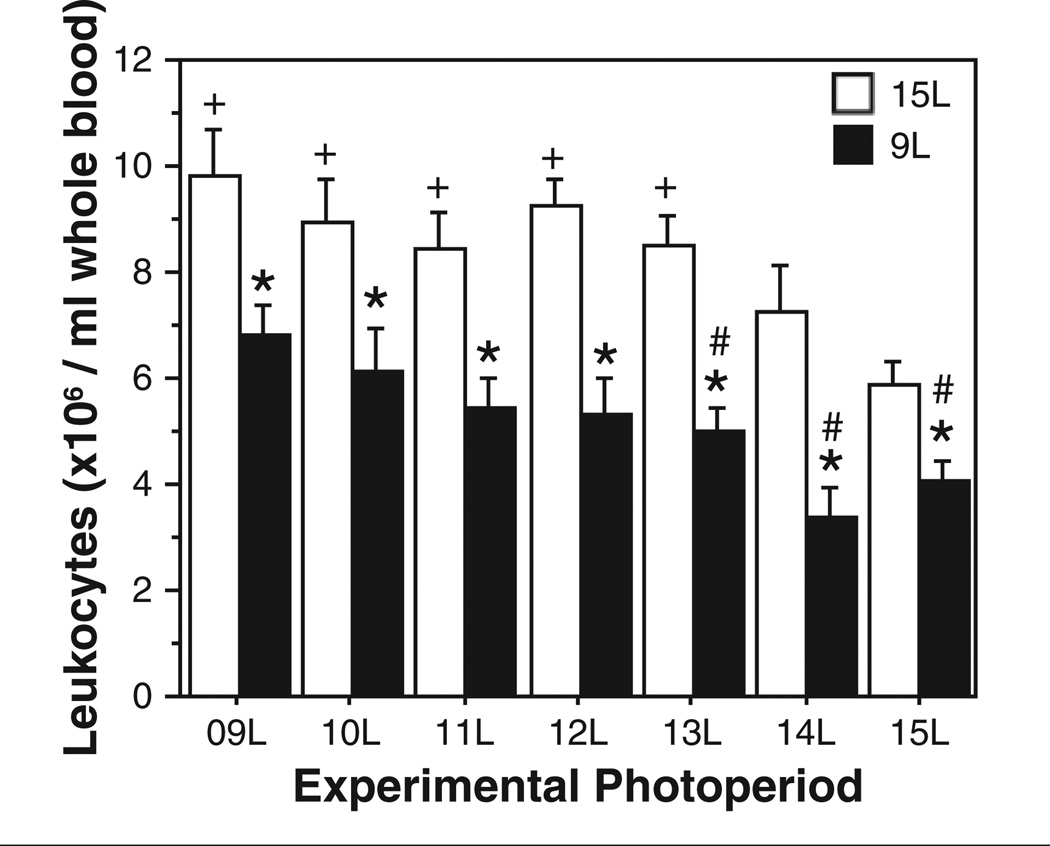
Initial (F1,107 = 7.5, p < 0.0001) and experimental (F6,107 = 85.2, p < 0.0001) photoperiods each affected blood leukocyte concentrations, but no interaction was evident (F6,107 = 0.7, p > 0.6; Fig. 3). Among hamsters initially in 15L, transfer to 14L tended to increase leukocyte concentrations (p = 0.09), and transfer to photoperiods <14L resulted in significant increases in blood leukocyte concentrations relative to 15L–15L controls (p < 0.005, all comparisons). Among hamsters initially in 9L, transfer to 10L or 11L did not yield a decrease in leukocyte concentrations, transfer to 12L tended to decrease leukocyte counts (p = 0.09), and transfer to photoperiods of 13L or greater caused significant decreases in leukocyte counts (p < 0.05, all comparisons). Within each experimental photoperiod, leukocyte concentrations were lower in hamsters with a photoperiod history of 9L relative to those with prior exposure to 15L (p < 0.05, all comparisons).
Figure 3.
Mean + SEM blood leukocyte concentrations of Siberian hamsters raised in 15L (open bars) or 9L (filled bars) following transfer to 1 of 7 different experimental photoperiods (indicated along the abscissa). *p < 0.05, 9L→ vs. 15L→ group, within experimental photoperiod. #p < 0.05 vs. 9L→9L value. +p < 0.05 vs. 15L→15L value.
DTH responses
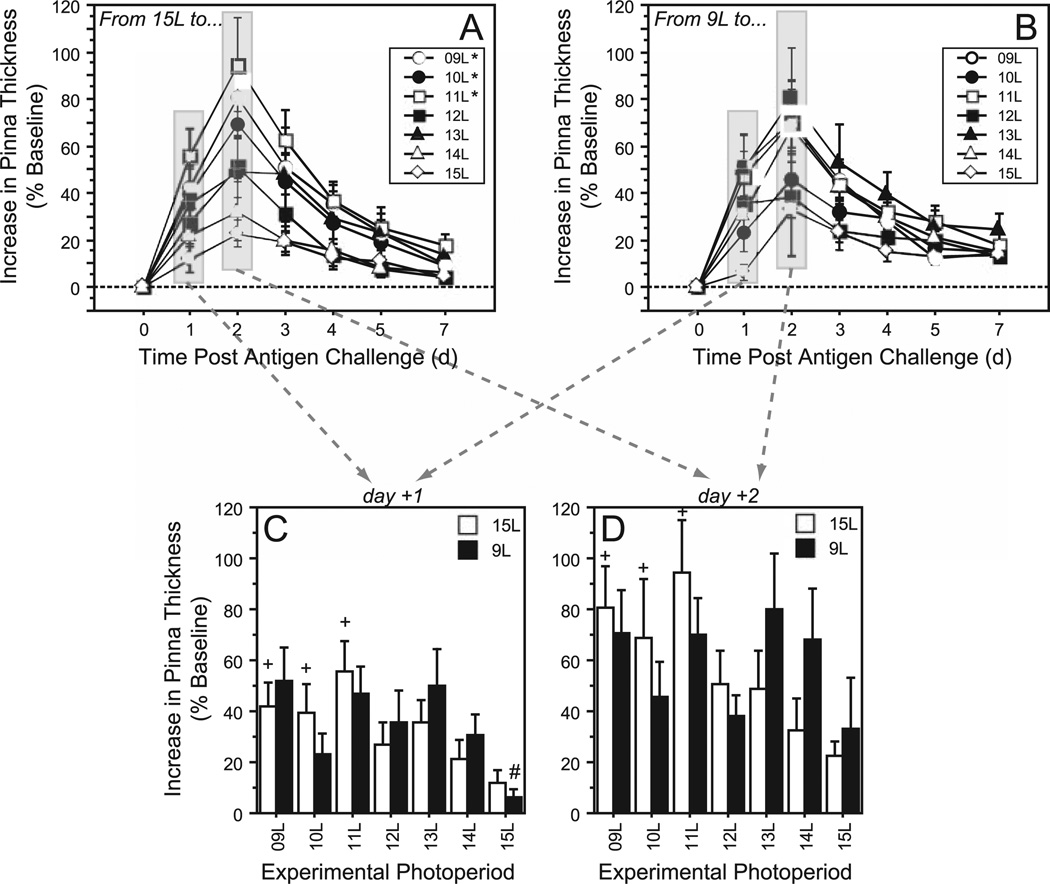
There was a significant main effect of the experimental photoperiod on the overall magnitude of DTH responses (F6,105 = 3.8, p < 0.005), and an interaction between experimental photoperiod and postchallenge day (F36,630 = 2.2, p < 0.0001), but there was no effect of photoperiod history on the response to experimental day lengths (F6,105 = 0.9, p > 0.4; Fig. 4). In none of the 7 experimental photoperiods was the pattern of DTH affected by initial photoperiod (p > 0.05, all comparisons). Among hamsters initially in 15L, the overall DTH response was enhanced in 11L (p < 0.001), 10L (p < 0.05), and 9L (p < 0.01) relative to 15L→15L (Fig. 4A); whereas among hamsters with a photoperiod history of 9L, the overall DTH response was not significantly affected by any of the experimental photoperiods (p > 0.05, all comparisons; Fig. 4B).
Figure 4.
Mean ± SEM delayed-type hypersensitivity (DTH) responses (percent increases in pinna thickness, compared to baseline) during the week after antigen (DNFB) challenge of Siberian hamsters raised in (A) 15L or (B) 9L and transferred to 1 of 7 different experimental photoperiods. Note nonlinearity of x-axis. Bar plots illustrate statistical comparisons of DTH magnitude (C) on the first day postchallenge (day +1) and (D) on the day of the peak DTH response (day +2) in each of the 7 experimental photoperiods (indicated along the abscissa). In panels A and B: *p < 0.05 vs. 15L→15L group (repeated-measures ANOVA). In panels C and D: #p < 0.05 vs. 9L→9L value. +p < 0.05 vs. 15L→15L value.
Pairwise comparisons on individual days postchallenge indicated that hamsters with a photoperiod history of 15L exhibited significant increases in ear inflammation following transfer to 11L, 10L, and 9L (p < 0.05 vs. 15L→15L, all comparisons) on day +1 (Fig. 4C) and day +2 (Fig. 4D). Enhancement of DTH in 15L→11L and 15L→9L persisted through day +4. In marked contrast, hamsters with a photoperiod history of 9L exhibited suppression of the DTH response only following transfer to 15L, and only on day +1 (p < 0.05 vs. 9L→9L). Again, in none of the 7 experimental photoperiods was there an effect of initial photoperiod on DTH (p > 0.05, all 7 days, all pairwise comparisons). Last, ears treated with the vehicle did not exhibit significant inflammation on any day post-challenge (data not shown).
Cortisol
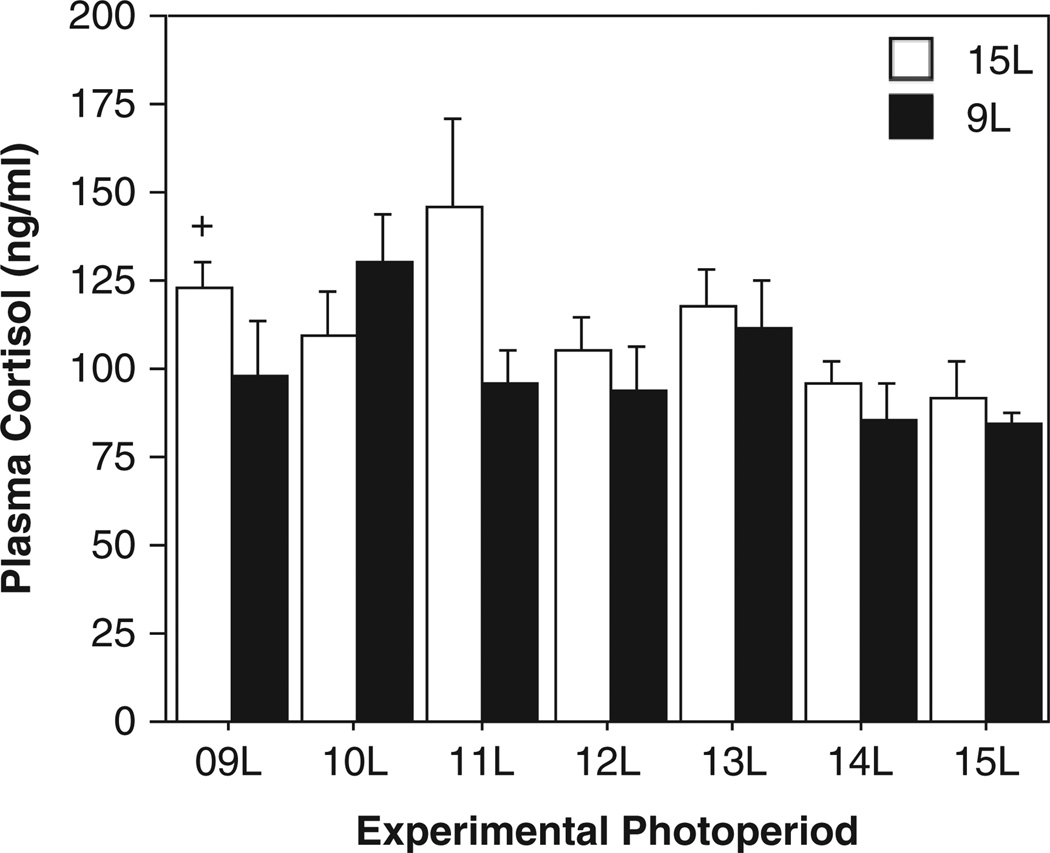
Experimental photoperiods (F6,104 = 2.3, p < 0.05) affected cortisol responses, but initial photoperiod was without effect on this measure (F1,104 = 3.7, p > 0.05), and there was no interaction between initial and experimental photoperiods (F6,104 = 1.4, p > 0.2; Fig. 5). Hamsters with a 15L photoperiod history exhibited a significant increase in cortisol following transfer to 9L (p < 0.05). Among hamsters with a 9L photoperiod history, none of the experimental photoperiods resulted in significant changes in cortisol concentrations (p > 0.10 vs. 9L→9L, all comparisons). Initial photoperiod did not affect cortisol concentrations in any of the 7 experimental photoperiods (p > 0.05, all comparisons).
Figure 5.
Mean + SEM plasma cortisol concentrations of Siberian hamsters raised in 15L (open bars) or 9L (filled bars) following transfer to 1 of 7 different experimental photoperiods (indicated along the abscissa). +p < 0.05 vs. 15L→15L value.
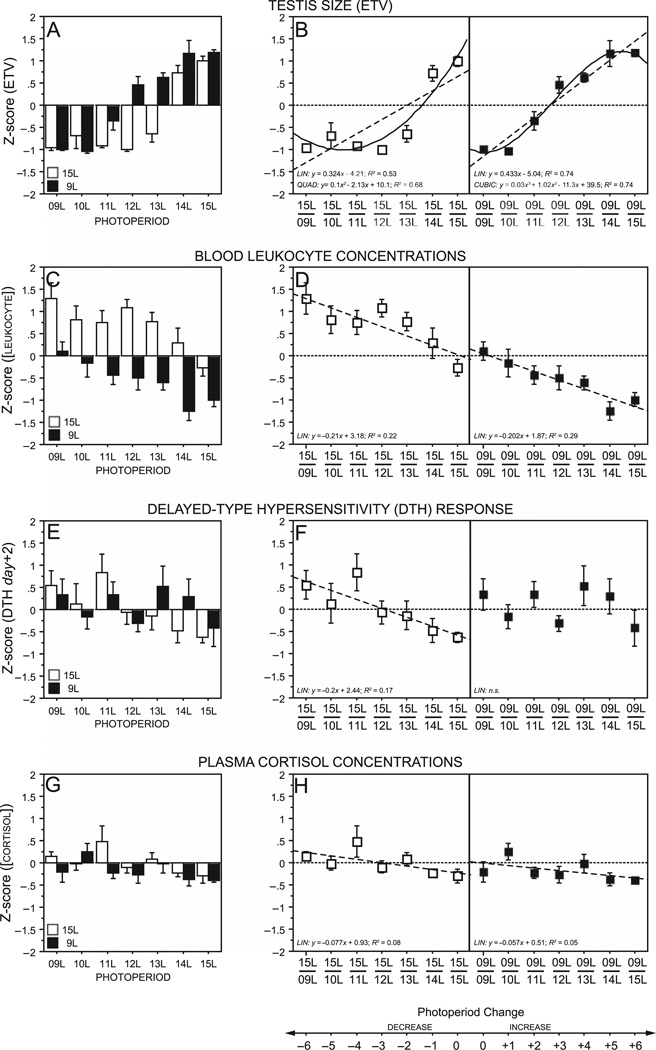
Responses to experimental day lengths: linear, quadratic, and cubic contrasts. Multiple regressions indicated significant simple effects of initial photoperiod (|t| = 4.96, p < 0.0001) on week 12 testis size, such that hamsters with a 9L photoperiod history, on average, had larger week 12 ETV relative to those with a 15L photoperiod history. Significant effects of linear (|t| =15.5, p < 0.0001), quadratic (|t| = 2.89, p < 0.0048), and cubic (|t| = 2.65, p < 0.0093) contrasts on the pattern of ETV responses to experimental photoperiod were also evident, and these effects were qualified by interactions between initial and experimental photoperiods (initial × linear: |t| = 1.84, p < 0.069; initial × quadratic: |t| = 4.47, p < 0.0001; initial × cubic: |t| = 2.19, p < 0.031), indicating that the manner in which linear, quadratic, or cubic functions explain the pattern of ETV responses to increasing experimental photoperiods depends on the initial photoperiod treatment (Fig. 6A, B). Among hamsters initially in 15L, there were significant effects of linear (|t| = 10.0, p < 0.0001) and quadratic (|t| = 5.39, p < 0.0001) contrasts, but the cubic contrast was not significant (|t| = 0.33; p > 0.74); in contrast, among hamsters initially in 9L, there were significant effects of linear (|t| = 11.9, p < 0.0001) and cubic (|t| = 3.36, p < 0.0015) contrasts, but the quadratic (|t| = 1.08, p > 0.28) was not significant (Fig. 6A).
Figure 6.
Mean ± SEM reproductive (week 12 ETV [A, B]), immunological (blood leukocyte concentrations [C, D], day +2 DTH [E, F]), and endocrine (cortisol [G, H]) responses to initial (15L = open symbols; 9L = filled symbols) and experimental (indicated along abscissae) photoperiods. Data in panels A, C, E, and G are raw values transformed into Z-scores to illustrate relative response magnitudes for each dependent variable. Data in panels B, D, F, and H depict Z-scored values accompanied only by significant (p < 0.05) linear (LIN; dashed lines) and higher order (quadratic = QUAD; cubic = CUBIC; solid lines) contrast effects obtained in the multiple regression correlation analyses. Net photoperiod change is indicated below the abscissae of figures in the right column.
For blood leukocytes, multiple regression analyses indicated a significant simple effect of initial photoperiod (|t| = 9.18, p < 0.0001; Fig. 6C, D); hamsters initially in 15L had significantly greater concentrations of blood leukocytes relative to those initially in 9L. Significant effects of the linear contrast were evident in the overall analysis (|t| = 6.25, p < 0.0001), indicating that leukocyte concentrations decreased in a linear fashion with increasing photoperiod, but the quadratic and cubic contrasts were not significant (|t| < 0.93, p > 0.35, both comparisons), nor were any interaction effects (|t| < 1.43, p > 0.15, all comparisons).
Regression analyses were performed on day +1 and day +2 DTH because these were the 2 postchallenge days on which significant effects of photoperiod were detected. On day +1, no effect of initial photoperiod history was evident (|t| > 0.39, p > 0.68); the linear contrast was significant (|t| = 3.78, p < 0.0004), but neither the higher order simple contrasts (|t| < 1.88, p > 0.06, both comparisons) nor the interaction contrasts (|t| < 1.82, p > 0.07) were significant (data not depicted in Fig. 6). A similar outcome was obtained on day +2, at the peak of the DTH response (Fig. 6E): overall photoperiod history effects were absent (|t| < 0.23, p > 0.81), and the linear contrast was significant (|t| = 2.95, p < 0.004), but the quadratic and cubic contrasts were not (|t| < 0.94, p > 0.34, both comparisons). The significant linear effect of experimental day length on peak (day +2) DTH was qualified by an interaction effect of photoperiod history (|t| = 1.92, p = 0.057): there was a strongly significant linear effect of photoperiod on DTH of hamsters initially in 15L (|t| = 3.66, p < 0.0005; Fig. 6F), but no effect was evident in hamsters with a 9L photoperiod history (|t| < 0.69, p > 0.49; Fig. 6F).
The effect of initial photoperiod on plasma cortisol concentrations was marginally significant (|t| = 1.94, p = 0.05), and the linear contrast was significant (|t| = 2.63, p < 0.0099), but the higher order contrasts (|t| < 1.30, p > 0.19, both comparisons) and the interaction contrasts (|t| < 0.76, p > 0.46, all comparisons) were not significant (Fig. 6G, H).
DISCUSSION
This experiment investigated whether photoperiod history impacts immunological responses to changes in day length in a manner similar to the well-established effects of photoperiod history described in the reproductive system. In adult male hamsters, antecedent day lengths determined reproductive responses to a range of experimental intermediate photoperiods: day lengths from 11L to 13L were responded to as reproductively inhibitory by hamsters with a long-day photoperiod history of 15L, but stimulated reproductive development in hamsters with a short-day history of 9L. Photoperiod history did not affect phase angles of entrainment to any experimental photoperiods, and did not affect circadian α in any photoperiod except 10L. Because the duration of circadian α is proportional to the duration of nocturnal melatonin secretion (Elliott and Tamarkin, 1994; Yellon and Truong, 1998), we may reasonably infer from records of locomotor activity that pineal melatonin secretion—the neuroendocrine representation of photoperiod for the reproductive and immune systems—was comparable in nearly all groups of hamsters within a given experimental photoperiod (Fig. 1), with the exception of 10L in which α was shorter in 15L–10L vs. 9L–10L hamsters. However, mean α exceeded 13 h in both 15L–10L and 9L–10L hamsters, and these groups were comparable in all reproductive, immunological, and endocrine measures. Although hamsters initially in 9L had been in short days for a prolonged (>20 weeks) interval, photorefractoriness had not yet occurred, as evidenced by sustained involuted gonads in 9L→9L hamsters on week 12. These data replicate and extend prior work demonstrating that photoperiod history dictates reproductive responses to intermediate day lengths by altering the response to intermediate-duration melatonin signals (Duncan et al., 1985; Prendergast et al., 2000).
The present data indicate that photoperiod history effects are also evident in the immune system. In all 7 experimental photoperiods, blood leukocyte concentrations were lower in hamsters with a short-day relative to a long-day photoperiod history (Bilbo et al., 2002a). In a strict sense, this can be interpreted as a photoperiod history effect, but one that manifests itself categorically differently than in the reproductive system. In this case, photoperiod history imparted hysteresis effects, altering the baseline values from which experimental photoperiods subsequently exerted their effects. The marked similarity in the linear effects of photoperiod change on leukocyte concentrations resulted in this measure of immunity responding primarily to photoperiod change, rather than to absolute photoperiod duration (Fig. 6C, D). Photoperiod history did not alter the critical day length for this measure; indeed, the threshold day lengths for enhancement (in hamsters initially in 15L) and suppression (in hamsters initially in 9L) of blood leukocytes were identical, 13L. It is not known why (from an ecological perspective) or how (from a mechanistic perspective) blood leukocyte concentrations respond primarily to direction of change in photoperiod over absolute photoperiod.
In common with blood leukocyte concentrations, DTH responses were augmented by experimental short-day photoperiods (compare with Bilbo et al., 2002a). In hamsters initially in 15L, day lengths shorter than 12L enhanced DTH. In contrast, among hamsters initially in 9L, DTH was marginally attenuated by increases in day length, but this only occurred in 15L, and was only evident on day +1. Although the ANOVA did not indicate a significant effect of initial photoperiod on DTH, there was a significant interaction between initial and experimental photoperiods. Moreover, there was a significant linear effect of photoperiod on DTH in 15L→ hamsters, but no such effect was evident in 9L→ hamsters, and there was an interaction between initial and experimental photoperiods on the linear regression (Fig. 6). Thus, whereas 15L hamsters exhibited a markedly linear DTH response to decreasing photoperiod, hamsters with a 9L photoperiod history were resistant to any subsequent photoperiodic modulation of DTH. Again, this is interpreted as evidence of a photoperiod history effect, but one that manifests as an elimination of the ability of longer days to suppress DTH in hamsters with a 9L history, rather than a shift in the critical day length for induction of the long-and short-day phenotypes in this trait. This effect resembles an earlier report in which hamsters receiving prenatal and brief postnatal exposure to 8L were resistant to suppression of DTH by adult exposure to 16L (Weil et al., 2006).
Together, the data indicate that prior photoperiod history affects leukocyte and DTH responses to photoperiod, and thereby compel rejection of the hypothesis that photoperiod history information is not retained by the immune system. However, a “photoperiodic memory,” formally similar to that which impacts hamster seasonal reproductive responses by shifting the critical day length (Hoffman, 1982; Prendergast et al., 2000), does not adequately characterize immunological photoperiod history effects. Rather, in the immune system, antecedent day lengths either 1) alter the baseline values against which contemporary day lengths subsequently affect the trait (blood leukocytes), or 2) impart hysteresis effects that markedly attenuate responsiveness to subsequent photoperiod manipulations (DTH responses).
Earlier work indicated that photoperiod history information acquired in utero did not affect adult immune responses to intermediate photoperiods (Prendergast et al., 2004a). Hamsters housed postnatally in 14L exhibited long-day-like blood leukocyte phenotypes and DTH responses, independent of whether they were gestated in 16L or 8L photoperiods (Prendergast et al., 2004a). The effects of photoperiod history on immune function identified in the present report evidently are not communicated to offspring in utero. However, perinatal photoperiods have been reported to engage select changes in immune function: hamsters exposed to 8L from early gestation through weaning exhibited greater DTH responses as adults relative to hamsters that were gestated and reared in 16L, independent of post-weaning photoperiod (Weil et al., 2006). Such enduring effects of exposure to short days are also evident in adult hamsters, however (Prendergast et al., 2004b). In the interpretation of photoperiod history effects, it is important to differentiate between hysteresis effects of prior photoperiod exposure and latent effects of photoperiod that are only revealed when animals are challenged with ambiguous (intermediate) day lengths. The present report permits the inference that antecedent information acquired exclusively in adulthood does not impact the response to intermediate photoperiods by the immune system in a manner similar to that of the reproductive system, but rather imparts hysteresis effects or renders the immune response largely unresponsive to changes in photoperiod.
Immunological photoperiodism is commonly investigated in adult animals following transfer from a natal long-day photoperiod into a categorically short (≤10L) photoperiod (Nelson, 2004; Prendergast et al., 2009). The present data indicate that the immunomodulatory consequences of transfer from long to short days occur at day lengths substantially greater than 10L, and in a trait-specific manner: 11L was sufficient to fully impart the short-day phenotype in DTH responses, and exposure to 13L yielded leukocyte counts that were comparable to those of hamsters housed in 9L. Thus, categorically short days are not required to instate photoperiodic enhancements of immune function. Moreover, photoperiodic changes in immunity do not occur at a uniform threshold day length, but rather occur in a trait-specific manner across a range of day lengths. In nature, as day lengths decrease from the summer solstice, one would predict that seasonal immunoenhancement of blood leukocyte concentrations would precede changes in skin immune function.
This study provides a novel quantitative analysis of the effect of photoperiod history on the response to experimental day lengths. Multiple regression analyses indicated significant linear effects of increasing experimental photoperiod, which obtained independent of photoperiod history for most dependent variables, except DTH. However, higher order effects were evident only in the reproductive system, further distinguishing reproductive from immunological responses to photoperiod (Prendergast et al., 2008), but also suggesting that photoperiod history introduces an asymmetry in the effect of photoperiod change on the reproductive system. There was a strong quadratic effect of experimental photoperiod in hamsters initially in 15L, indicating that as day lengths decrease from 15L, there is a nonlinear acceleration in the impact of photoperiod change on testis size, which reaches an asymptote after 13L. In contrast, only the cubic effect was significant for hamsters initially in 9L, indicating that stimulatory responses to increasing photoperiods are best characterized by a step function, with no response to 10L, progressive increases in 11L through 14L, and an asymptote after 14L. The mechanisms that introduce this asymmetric response to photoperiod change are not known, but presumably lie in the melatonin-responsive reproductive neuroen-docrine system, and are absent in the immune system.
This study also permits insights into the role of 2 major endocrine mechanisms that have been proposed to participate in photoperiodic changes in immune function: adrenal glucocorticoids and gonadal steroids. Cortisol was largely unaffected by intermediate photoperiods; only 15L→9L hamsters exhibited a significant increase in cortisol (compare with Bilbo et al., 2002a). Conflicting results have been reported on the effects of categorically long and short days on circulating cortisol in this species (Bilbo and Nelson, 2003; Weil et al., 2007; Yellon, 2007; Zysling and Demas, 2007). Nevertheless, in hamsters with a history of 15L, photoperiod enhanced DTH and blood leukocytes, respectively, at photoperiods 2 h and 4 h longer than those required to increase plasma cortisol. Although these observations are only correlational, they are consistent with the hypothesis that a marked change in cortisol production is not necessary to mediate effects of photoperiod on these immune measures.
Both gonadal hormone-dependent and gonadal hormone-independent processes contribute to the short-day enhancement of blood leukocytes (Prendergast et al., 2008), but DTH responses are unaffected by photoperiodic changes in gonadal hormone secretion (Prendergast et al., 2005). Testis size is a reliable proxy for circulating testosterone in this species (Schlatt et al., 1995). In the present study, leukocytes followed a gonadal hormone-dependent pattern in hamsters with a history of 15L exposure to 13L was sufficient to trigger both gonadal regression and increases in leukocyte numbers—but not in hamsters with a history of 9L. In 9L→ hamsters, transfer to photoperiods sufficient to induce substantial gonadal growth (11L, 12L) was without effect on leukocyte concentrations, indicating that activation of gonadal activity is not sufficient to impart a long-day-like immunophenotype in this trait. A history of exposure to elevated testosterone in 15L→ hamsters may render the immune system more responsive to changes in circulating gonadal steroids. Also, in 15L→ hamsters, DTH was only enhanced in photoperiods ≤11L, consistent with a gonadal hormone-independent effects of photoperiod reported previously for this measure of immune function (Prendergast et al., 2005).
In conclusion, photoperiod history effects were evident in both reproductive and immunological responses to experimental photoperiod change. In the reproductive system, photoperiod history shifted the threshold day length for induction of the long-and short-day phenotypes and introduced asymmetric, nonlinear responses to incremental photoperiod change. In the immune system, in contrast, photoperiod history effects manifest as a change in the baseline values against which photoperiod caused linear enhancement or suppression of leukocyte concentrations, and a categorical attenuation of DTH responsiveness to photoperiod. Although reproductive and immunological responses to photoperiod share formal similarities, the reproductive and immune systems appear to rely on prior photoperiod information in fundamentally different ways.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Jerome Galang, Sean Bradley, August Kampf-Lassin, Sally Cochrane, Rebecca Ouwenga, Priyesh Patel, Jimmy Dooley, Justin Aschenbener, and Curtis Wilkerson for technical assistance. Joshua Correll provided expert statistical assistance and comments on a draft of the manuscript. This work was supported by grant R01-AI67406 from the National Institute of Allergy and Infectious Disease (BJP).
Footnotes
Supplementary online material for this article is available from the journal’s Web site: http://jbr.sagepub.com/ supplemental.
REFERENCES
- Bilbo SD, Dhabhar FS, Viswanathan K, Saul A, Yellon SM, Nelson RJ. Short day lengths augment stress-induced leukocyte trafficking and stress-induced enhancement of skin immune function. Proc Natl Acad Sci U S A. 2002a;99:4067–4072. doi: 10.1073/pnas.062001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Drazen DL, Quan N, He L, Nelson RJ. Short day lengths attenuate the symptoms of infection in Siberian hamsters. Proc Biol Sci. 2002b;269:447–454. doi: 10.1098/rspb.2001.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Nelson RJ. Sex differences in photoperiodic and stress-induced enhancement of immune function in Siberian hamsters. Brain Behav Immun. 2003;17:462–472. doi: 10.1016/s0889-1591(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Carter DS, Goldman BD. Antigonadal effects of timed melatonin infusion in pinealectomized male Djungarian hamsters (Phodopus sungorus sungorus): Duration is the critical parameter. Endocrinology. 1983;113:1261–1267. doi: 10.1210/endo-113-4-1261. [DOI] [PubMed] [Google Scholar]
- Demas GE, Johnson C, Polacek KM. Social interactions differentially affect reproductive and immune responses of Siberian hamsters. Physiol Behav. 2004;83:73–79. doi: 10.1016/j.physbeh.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Acute stress enhances while chronic stress suppresses skin immunity. The role of stress hormones and leukocyte trafficking. Ann N Y Acad Sci. 2000;917:876–893. doi: 10.1111/j.1749-6632.2000.tb05454.x. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci U S A. 1999;96:1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Effects of stress on immune cell distribution. Dynamics and hormonal mechanisms. J Immunol. 1995;154:5511–5527. [PubMed] [Google Scholar]
- Drazen DL, Kriegsfeld LJ, Schneider JE, Nelson RJ. Leptin, but not immune function, is linked to reproductive responsiveness to photoperiod. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1401–R1407. doi: 10.1152/ajpregu.2000.278.6.R1401. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Goldman BD, Di Pinto MN, Stetson MH. Testicular function and pelage color have different critical daylengths in the Djungarian hamster, Phodopus sungorus sungorus . Endocrinology. 1985;116:424–430. doi: 10.1210/endo-116-1-424. [DOI] [PubMed] [Google Scholar]
- Elliott JA, Tamarkin L. Complex circadian regulation of pineal melatonin and wheel-running in Syrian hamsters. J Comp Physiol A. 1994;174:469–484. doi: 10.1007/BF00191713. [DOI] [PubMed] [Google Scholar]
- Evans JA, Elliott JA, Gorman MR. Photoperiod differentially modulates photic and nonphotic phase response curves of hamsters. Am J Physiol Regul Integr Comp Physiol. 2004;286:R539–R546. doi: 10.1152/ajpregu.00456.2003. [DOI] [PubMed] [Google Scholar]
- Gatien ML, Hotchkiss AK, Dhabhar FS, Nelson RJ. Skeleton photoperiods alter delayed-type hyper-sensitivity responses and reproductive function of Siberian hamsters (Phodopussungorus) J Neuroendocrinol. 2005;17:733–739. doi: 10.1111/j.1365-2826.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- Goldman BD. Mammalian photoperiodic system: Formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J Biol Rhythms. 2001;16:283–301. doi: 10.1177/074873001129001980. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Freeman DA, Zucker I. Photoperiodism in hamsters: Abrupt versus gradual changes in day length differentially entrain morning and evening circadian oscillators. J Biol Rhythms. 1997;12:122–135. doi: 10.1177/074873049701200204. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Zucker I. Seasonal adaptations of Siberian hamsters. II. Pattern of change in daylength controls annual testicular and body weight rhythms. Biol Reprod. 1995a;53:116–125. doi: 10.1095/biolreprod53.1.116. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Zucker I. Testicular regression and recrudescence without subsequent photorefractoriness in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 1995b;269:R800–R806. doi: 10.1152/ajpregu.1995.269.4.R800. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Zucker I. Environmental induction of photononresponsiveness in the Siberian hamster, Phodopus sungorus . Am J Physiol. 1997;272:R887–R895. doi: 10.1152/ajpregu.1997.272.3.R887. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Zucker I. Mammalian photoperiodism: New perspectives from the use of simulated natural photoperiods. In: Touitou Y, editor. Biological Clocks: Mechanisms and Applications. Amsterdam: Elsevier; 1998. pp. 195–204. [Google Scholar]
- Hadley AR, Tran LT, Fagoaga OR, Nehlsen-Cannarella SL, Yellon SM. Sex differences in photoperiod control of antigen-specific primary and secondary humoral immunity in Siberian hamsters. J Neuroimmunol. 2002;128:39–48. doi: 10.1016/s0165-5728(02)00144-3. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. The critical photoperiod in the Djungarian hamster Phodopus sungorus . In: Aschoff J, Daan S, Gross G, editors. Vertebrate Circadian Systems: Structure and Physiology. New York: Springer; 1982. pp. 297–304. [Google Scholar]
- Hoffmann K, Illnerovà H. Photoperiodic effects in the Djungarian hamster. Rate of testicular regression and extension of pineal melatonin pattern depend on the way of change from long to short photoperiods. Neuroendocrinology. 1986;43:317–321. doi: 10.1159/000124562. [DOI] [PubMed] [Google Scholar]
- Nelson RJ. Seasonal immune function and sickness responses. Trends Immunol. 2004;25:187–192. doi: 10.1016/j.it.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Demas GE. Seasonal changes in immune function. Q Rev Biol. 1996;71:511–548. doi: 10.1086/419555. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Prendergast BJ. Form over function? J Biol Rhythms. 2002;17:406–409. doi: 10.1177/074873002237134. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Baillie SR, Dhabhar FS. Gonadal hormone-dependent and -independent regulation of immune function by photoperiod in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294:R384–R392. doi: 10.1152/ajpregu.00551.2007. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Bilbo SD, Dhabhar FS, Nelson RJ. Effects of photoperiod history on immune responses to intermediate day lengths in Siberian hamsters (Phodopus sungorus) J Neuroimmunol. 2004a;149:31–39. doi: 10.1016/j.jneuroim.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Bilbo SD, Nelson RJ. Photoperiod controls the induction, retention, and retrieval of antigen-specific immunological memory. Am J Physiol Regul Integr Comp Physiol. 2004b;286:R54–R60. doi: 10.1152/ajpregu.00381.2003. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Bilbo SD, Nelson RJ. Short day lengths enhance skin immune responses in gonadect-omised Siberian hamsters. J Neuroendocrinol. 2005;17:18–21. doi: 10.1111/j.1365-2826.2005.01273.x. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Freeman DA. Pineal-independent regulation of photo-nonresponsiveness in the Siberian hamster (Phodopus sungorus) J Biol Rhythms. 1999;14:62–71. doi: 10.1177/074873099129000452. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Gorman MR, Zucker I. Establishment and persistence of photoperiodic memory in hamsters. Proc Natl Acad Sci U S A. 2000;97:5586–5591. doi: 10.1073/pnas.100098597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Kampf-Lassin A, Yee JR, Galang J, McMaster N, Kay LM. Winter day lengths enhance T lymphocyte phenotypes, inhibit cytokine responses, and attenuate behavioral symptoms of infection in laboratory rats. Brain Behav Immun. 2007;21:1096–1108. doi: 10.1016/j.bbi.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Kriegsfeld LJ, Nelson RJ. Photoperiodic polyphenisms in rodents: Neuroendocrine mechanisms, costs, and functions. Q Rev Biol. 2001;76:293–325. doi: 10.1086/393989. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Zucker I, Nelson RJ. Seasonal rhythms of mammalian behavioral neuroendocrinology. In: Pfaff D, Etgen A, Fahrbach S, Moss R, Rubin R, editors. Hormones, Brain, and behavior. 2nd Edition. Vol. 1. San Diego: Academic Press; 2009. pp. 507–538. [Google Scholar]
- Puchalski W, Lynch GR. Evidence for differences in the circadian organization of hamsters exposed to short day photoperiod. J Comp Physiol A. 1986;159:7–11. doi: 10.1007/BF00612490. [DOI] [PubMed] [Google Scholar]
- Schlatt S, De Geyter M, Kliesch S, Nieschlag E, Bergmann M. Spontaneous recrudescence of spermatogenesis in the photoinhibited male Djungarian hamster, Phodopus sungorus . Biol Reprod. 1995;53:1169–1177. doi: 10.1095/biolreprod53.5.1169. [DOI] [PubMed] [Google Scholar]
- Turk JL. Delayed Hypersensitivity Research Monographs in Immunology. Amsterdam: Elsevier; 1980. [Google Scholar]
- Weil ZM, Pyter LM, Martin LB, 2nd, Nelson RJ. Perinatal photoperiod organizes adult immune responses in Siberian hamsters (Phodopus sungorus) Am J Physiol Regul Integr Comp Physiol. 2006;290:R1714–R1719. doi: 10.1152/ajpregu.00869.2005. [DOI] [PubMed] [Google Scholar]
- Weil ZM, Workman JL, Nelson RJ. Housing condition alters immunological and reproductive responses to day length in Siberian hamsters (Phodopus sungorus) Horm Behav. 2007;52:261–266. doi: 10.1016/j.yhbeh.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen JC, Dhabhar FS, Prendergast BJ. Pineal-dependent and -independent effects of photoperiod on immune function in Siberian hamsters (Phodopus sungorus) Horm Behav. 2007;51:31–39. doi: 10.1016/j.yhbeh.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellon SM. Melatonin mediates photoperiod control of endocrine adaptations and humoral immunity in male Siberian hamsters. J Pineal Res. 2007;43:109–114. doi: 10.1111/j.1600-079X.2007.00448.x. [DOI] [PubMed] [Google Scholar]
- Yellon SM, Fagoaga OR, Nehlsen-Cannarella SL. Influence of photoperiod on immune cell functions in the male Siberian hamster. Am J Physiol. 1999;276:R97–R102. doi: 10.1152/ajpregu.1999.276.1.R97. [DOI] [PubMed] [Google Scholar]
- Yellon SM, Truong HN. Melatonin rhythm onset in the adult Siberian hamster: Influence of photoperiod but not 60-Hz magnetic field exposure on melatonin content in the pineal gland and in circulation. J Biol Rhythms. 1998;13:52–59. doi: 10.1177/074873098128999916. [DOI] [PubMed] [Google Scholar]
- Zysling DA, Demas GE. Metabolic stress suppresses humoral immune function in long-day, but not short-day, Siberian hamsters (Phodopus sungorus) J Comp Physiol B. 2007;177:339–347. doi: 10.1007/s00360-006-0133-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.