Abstract
BACKGROUND
Hepatocellular carcinoma (HCC) is a concern among individuals with human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS).
METHODS
The authors analyzed population-based registry linkage data from the US HIV/AIDS Cancer Match Study (1980–2009) to examine the risk and trends of HCC among individuals with AIDS. Standardized incidence ratios (SIRs) were used to measure HCC risk relative to the general population, and Poisson regression was used to calculate incidence rate ratios (RR) comparing incidence among individuals with AIDS. People with AIDS were categorized according to their HIV risk group into high and low hepatitis C virus (HCV) prevalence groups based on their HIV transmission risk category.
RESULTS
Among 615,150 individuals with AIDS, HCC risk was elevated almost 4 times compared with the risk in the general population (N = 366; SIR, 3.8; 95% confidence interval, 3.5–4.3). Although HCC incidence increased steadily across calendar periods (Ptrend < .0001; adjusted for sex and age), the excess risk in individuals with AIDS compared with the general population remained somewhat constant (SIRs range, 3.5–3.9) between the monotherapy/dual therapy era (1990–1995) and the recent highly active antiretroviral therapy era (2001–2009). In a multivariate model adjusting for sex, race/ethnicity, and attained calendar period, HCC incidence increased with advancing age (Ptrend < .0001) and was associated with HIV risk groups with a known higher prevalence of HCV (adjusted RR, 2.2; 95% confidence interval, 1.8–2.8).
CONCLUSIONS
HCC incidence in individuals with AIDS has increased over time despite improved HIV treatment regimens, likely reflecting prolonged survival with chronic liver disease. The high incidence in older adults suggests that this cancer will increase in importance with aging of the HIV-infected population.
Keywords: hepatocellular carcinoma, human immunodeficiency virus, acquired immunodeficiency syndrome, epidemiology, hepatitis C virus
INTRODUCTION
Hepatocellular carcinoma (HCC) is characterized by aggressive growth, frequent metastases, and recurrences after treatment, and it is a major source of cancer-related morbidity and mortality.1 HCC arises as a long-term sequela of chronic liver disease and cirrhosis, frequently as a result of persistent inflammation associated with chronic hepatitis B virus (HBV) and/or hepatitis C virus (HCV) infections. HCC is of special concern in individuals who are living with human immunodeficiency virus (HIV) or acquired immunodeficiency syndrome (AIDS) because of frequent HBV and HCV coinfection and accelerated progression of viral hepatitis to chronic liver disease and cirrhosis.2–6 Excessive alcohol consumption, obesity, diabetes, and nonalcoholic steatohepatitis (NASH) are other risk factors for chronic liver disease and HCC that have a higher or increasing burden among HIV-infected populations.7–9
The introduction of highly active antiretroviral therapy (HAART) in 1996 led to a dramatic decline among HIV-infected individuals in the risk of Kaposi sarcoma and non-Hodgkin lymphoma, two AIDS-defining cancers with viral etiologies. In the era of widespread HAART use, non-AIDS–defining cancers (which include HCC) are now as common as AIDS-defining cancers among individuals with AIDS in the United States.10 HAART would be expected to improve immune control of HBV and HCV, thereby minimizing liver damage and reducing the risk of HCC. Alternatively, HCC incidence may rise in HIV-infected individuals over time, because prolonged survival as a result of improved antiretroviral therapy (ART) increases the duration of chronic liver disease.11
We used data from the US HIV/AIDS Cancer Match (HACM) Study to examine the epidemiology of HCC and other hepatobiliary cancers in individuals with AIDS. We compared the risk of these cancers in individuals who had AIDS with the risk in the general population, evaluated risk factors for HCC, and examined trends in HCC incidence over time in individuals with AIDS.
MATERIALS AND METHODS
The HACM Study is a linkage study of population-based HIV/AIDS and cancer registries in 14 US areas (available at: www.hivmatch.cancer.gov; [accessed May 30, 2012]).12,13 The study was approved by the institutional review boards of participating registries. All 14 HACM HIV/AIDS registries provided data on individuals with AIDS (615,150 individuals with AIDS diagnosed during 1980–2009; approximately 60% of the total US AIDS population), with cancers ascertained in cancer registries from 1980 to 2009. We included individuals with AIDS who contributed follow-up time at risk beginning at 4 months after the date of AIDS onset or the start of cancer registry coverage (whichever came later) until death, the end of cancer registry coverage, or 120 months after AIDS onset (whichever came first). Twelve HACM registries also provided data on individuals with HIV infection in the absence of AIDS (153,349 individuals reported between 1982 and 2009). In a secondary analysis, using data from these registries, we evaluated cancer risk in individuals who were AIDS-free at the time of HIV registration. All analyses were restricted to individuals in the most common racial/ethnic groups (non-Hispanic white, non-Hispanic black, Hispanic) to allow comparison with the general population. Although HCC is infrequent in younger age groups, we included all age groups to also account for the rare cases arising at younger ages, especially among HIV-infected hemophiliacs.
By using cancer registry data, we identified incident cases of hepatobiliary cancers, a heterogeneous grouping of cancers of the liver and biliary tract. On the basis of a validated classification that used refined cancer registry topography and morphology codes for hepatobiliary cancers,14 we grouped these cancers into 5 distinct categories: HCC (the dominant histologic type of liver cancer), cholangiocarcinoma (cancer of the intrahepatic and extrahepatic bile ducts), other liver and intrahepatic bile duct cancers (except cholangiocarcinoma), other extrahepatic bile duct cancers (except cholangiocarcinoma), and gallbladder cancer.
We calculated standardized incidence ratios (SIRs) with exact 95% confidence intervals (CIs) to compare the risk of HCC and other hepatobiliary cancers in individuals with AIDS versus the risk in the general population. SIRs were defined as the ratio of observed cancer cases in individuals with AIDS to expected cancer cases, in which expected cases were estimated by applying general population cancer rates to the AIDS population in strata defined by sex, age, race/ethnicity, calendar year, and registry. We used Poisson regression to estimate incidence rate ratios (RRs) comparing HCC incidence among subgroups of individuals with AIDS. SIRs and RRs were estimated across categories of demographic characteristics, attained calendar year as a proxy for ART regimens (1980–1989, pretherapy era; 1990–1995, monotherapy/dual therapy era; 1996–2000, early HAART era; and 2001–2009, recent HAART era), and CD4 count at AIDS onset (HIV targets CD4 positive T-cells, so the CD4 count is a marker of remaining immune competence). The presented RRs are adjusted for age and sex and also were derived from a multivariate model. P values for trend (Ptrend) by age group and attained calendar period were estimated by treating categories as ordinal variables in the model.
In addition, we calculated SIRs and used Poisson regression to compare HCC incidence in HIV-infected individuals with and without AIDS based on data from the 12 registries that provided this information. These analyses considered HIV-infected individuals (who were initially reported without AIDS) and classified their subsequent follow-up according to whether they remained AIDS-free or had developed AIDS. We also conducted a sensitivity analysis to correct for potential migration of individuals out of the cancer registry areas by applying a 7% correction factor to expected counts in the period of 5 to 10 years after AIDS diagnosis.15
The HACM Study does not collect information on HCV status. Therefore, to indirectly assess the effect of HCV coinfection on HCC risk, individuals with AIDS were grouped into HIV transmission risk categories with differing HCV prevalence.16,17 The “high” HCV prevalence group (estimated HCV prevalence, >60%16,17) included hemophiliacs and injection drug users (IDUs) (including those who were also men who have sex with men [MSM]), and the “low” HCV prevalence group (estimated HCV prevalence, <15%16,17) included heterosexuals, non-IDU MSM, and those with other or unknown HIV risk factors. Finally, trends in HCC incidence rates over time were estimated for the general US population and the AIDS population, standardized by age, sex, and race to the 2000 HACM AIDS population.
RESULTS
Table 1 lists the follow-up time contributed by individuals with AIDS and those with HIV infection in the absence of AIDS. A majority of the population was male, and non-Hispanic blacks were the most common racial/ethnic group among individuals with AIDS. Most of the person-time was contributed by individuals ages 30 to 49 years. Among the individuals with AIDS, 77.4% of the person-time came from the 1996 to 2000 (the early HAART era) and 2001 to 2009 (the recent HAART era) calendar periods. In the analyses that were restricted to individuals who were first reported without AIDS, an even larger proportion of the follow-up was in those years, reflecting the dates when registries typically began HIV registration.
Table 1.
Follow-Up Contributed by Individuals With Acquired Immunodeficiency Syndrome (AIDS) or With Human Immunodeficiency Virus (HIV) Infection in the Absence of AIDS
| All Participating Registriesa |
Registries With Prospective HIV Datab | |||||
|---|---|---|---|---|---|---|
| Individuals with AIDS |
Individuals with HIV but not AIDS |
Individuals With AIDS |
||||
| Variable | Person-Years | % | Person-Years | % | Person-Years | % |
| Sex | ||||||
| Males | 1,724,200 | 79.7 | 416,586 | 70.0 | 95,219 | 69.0 |
| Females | 438,051 | 20.3 | 178,242 | 30.0 | 42,731 | 31.0 |
| Attained age, y | ||||||
| 0–29 | 196,300 | 9.1 | 107,581 | 18.1 | 13,918 | 10.1 |
| 30–39 | 808,503 | 37.4 | 208,312 | 35.0 | 46,278 | 33.5 |
| 40–49 | 797,085 | 36.9 | 191,856 | 32.3 | 53,968 | 39.1 |
| 50–59 | 281,434 | 13.0 | 69,248 | 11.6 | 19,347 | 14.0 |
| ≥60 | 78,929 | 3.7 | 17,831 | 3.0 | 4439.0 | 3.2 |
| Race/ethnicity | ||||||
| Non-Hispanic white | 839,409 | 38.8 | 201,111 | 33.8 | 39,102 | 28.3 |
| Non-Hispanic black | 887,996 | 41.1 | 290,627 | 48.9 | 72,463 | 52.5 |
| Hispanic | 434,846 | 20.1 | 103,090 | 17.3 | 26,384 | 19.1 |
| HIV transmission risk group | ||||||
| Non-IDU MSM | 965,669 | 44.7 | 211,373 | 35.5 | 43,794 | 31.7 |
| IDU | 504,988 | 23.4 | 114,626 | 19.3 | 38,729 | 28.1 |
| IDU-MSM | 142,052 | 6.6 | 31,891 | 5.4 | 10,069 | 7.3 |
| Hemophiliac | 7887 | 0.4 | 1137 | 0.2 | 415.0 | 0.3 |
| Heterosexual | 295,835 | 13.7 | 111,297 | 18.7 | 27,771 | 20.1 |
| Other/unknown | 245,820 | 11.4 | 124,505 | 20.9 | 17,171 | 12.4 |
| Attained calendar year: ART era | ||||||
| 1980–1989: Pre-ART era | 61,315 | 2.8 | 170 | 0 | 75.0 | 0.1 |
| 1990–1995: Monotherapy/dual therapy era | 427,123 | 19.8 | 46,218 | 7.8 | 5295.0 | 3.8 |
| 1996–2000: Early HAART era | 826,484 | 38.2 | 136,631 | 23.0 | 35,384 | 25.7 |
| 2001–2009: Recent HAART era | 847,329 | 39.2 | 411,808 | 69.2 | 97,195 | 70.5 |
Abbreviations: ART, antiretroviral therapy; HAART, highly active antiretroviral therapy; IDU, injection drug users; MSM, men who have sex with men.
Follow-up was restricted to 4 to 120 months after AIDS onset.
Follow-up was restricted to individuals who were reported initially with HIV infection but without AIDS. Person-time is categorized according to whether the individual remained without AIDS or had developed AIDS. Person-time in individuals with AIDS was restricted to the 4 to 120 months after AIDS onset.
Among individuals with AIDS, we identified 366 individuals with HCC between 1980 and 2009. Of the 192 patients who had disease stage reported, 79 (41.1%) had locally invasive HCC, and the remaining patients had cancers with regional involement (n = 51; 26.6%) or distant metastases (n = 62; 32.3%). The risk of HCC was elevated 4-fold compared with the general population (SIR, 3.8; 95% CI, 3.5–4.3) (Table 2). The risk of other liver and intrahepatic bile duct cancers (except cholangiocarcinoma) also was elevated (n = 27; SIR, 3.3; 95% CI, 2.2–4.7). However, the risk was not elevated for cholangiocarcinoma, other extrahepatic bile duct cancers (except cholangiocarcinoma), or gallbladder cancer (Table 2). In a sensitivity analysis assessing the impact of outmigration from the registry area, SIR estimates were similar (results not shown).
Table 2.
Incidence of Hepatobiliary Cancers in Individuals With Acquired Immunodeficiency Syndrome (AIDS), 1980–2009a
| Cancer Histology Typeb | Observed Cases |
Incidence Rate per 100,000 Person-Years |
SIR [95% CI] |
|---|---|---|---|
| Hepatocellular carcinoma | 366 | 16.9 | 3.8 [3.5–4.3] |
| Cholangiocarcinoma (cancer of the intrahepatic and extrahepatic bile duct) | 22 | 1.02 | 1.4 [0.9–2.1] |
| Other liver and intrahepatic bile duct cancers (except cholangiocarcinoma) | 27 | 1.25 | 3.3 [2.2–4.7] |
| Other extrahepatic bile duct cancers (except cholangiocarcinoma) | 0 | 0.00 | 0.0 [0–4.5] |
| Gallbladder cancer | 11 | 0.51 | 1.4 [0.7–2.5] |
Abbreviations: CI, confidence interval; SIR, standardized incidence ratio.
This analysis included patients who were diagnosed 4 to 120 months after their AIDS diagnosis.
Hepatobiliary cancers were classified according to the International Classification of Diseases for Oncology (ICD-O3; 3rd edition) topography and morphology codes described elsewhere (for a detailed listing of the cancers included in these categories, please refer to the article by Altekruse SF, Devesa SS, Dickie LA, McGlynn KA, Kleiner DE. Histological classification of liver and intrahepatic bile duct cancers in SEER registries. J Registry Manag. 2011;38:201–205.14).
An assessment of risk factors for HCC among individuals with AIDS is provided in Table 3. The results adjusted for sex and age were similar to those from a multivariate model that included sex, attained age, race/ethnicity, HIV risk group by HCV prevalence, and attained calendar period. In the multivariate model (Table 3), HCC incidence was twice as high in males compared with females (RR, 2.5; 95% CI, 1.7–3.6). Incidence increased with age (Ptrend < .0001): individuals aged ≥50 years had 8-fold to 10-fold higher incidence than those ages 0 to 39 years. No significant difference in HCC risk was observed by race/ethnicity. HCC risk was twice as high in the high HCV prevalence groups as in the low HCV prevalence groups (RR, 2.2; 95% CI, 1.8–2.8). Nonetheless, HCC risk was elevated in individuals with AIDS compared with the general population for all HIV transmission risk groups, with the highest incidence rates observed among hemophiliacs (88.8 per 100,000 person-years). HCC incidence increased across calendar periods (Ptrend < .0001), with the rate 2.7 times higher in the years 2001 to 2009 (the recent HAART era) compared with the years 1990 to 1995 (monotherapy/dual therapy era) (Table 3).
Table 3.
Hepatocellular Carcinoma Incidence in Individuals With Acquired Immunodeficiency Syndrome (AIDS), 1980–2009
| Variable | Observed Cases |
Incidence per 100,000 Person-Years |
RR [95% CI]a |
RR [95% CI]b |
SIR [95% CI] |
|---|---|---|---|---|---|
| Sex | |||||
| Males | 333 | 19.3 | 2.2 [1.5–3.2] | 2.5 [1.7–3.6] | 3.7 [3.3–4.1] |
| Females | 33 | 7.5 | Reference | Reference | 6.8 [4.7–9.5] |
| Attained age, y | |||||
| 0–29 | 3 | 1.5 | 0.3 [0.1–1.1] | Referencec | 8.6 [1.8–25] |
| 30–39 | 41 | 5.1 | Reference | Referencec | 8.5 [6.1–12] |
| 40–49 | 150 | 18.8 | 3.6 [2.6–5.2] | 3.4 [2.4–4.8] | 4.3 [3.7–5.1] |
| 50–59 | 133 | 47.3 | 9.1 [6.4–13] | 8.2 [5.8–12.0] | 3.3 [2.7–3.9] |
| ≥60 | 39 | 49.4 | 9.6 [6.2–15] | 10.0 [6.5–16.0] | 2.6 [1.9–3.6] |
| p-trend <.0001 | p-trend <.0001 | ||||
| Race/ethnicity | |||||
| Non-Hispanic white | 142 | 16.9 | Reference | Reference | 4.6 [3.8–5.4] |
| Non-Hispanic black | 165 | 18.6 | 1.3 [1.0–1.6] | 1.0 [0.8–1.2] | 3.3 [2.8–3.8] |
| Hispanic | 59 | 13.6 | 1.0 [0.7–1.3] | 0.9 [0.6–1.2[ | 4.3 [3.3–5.6] |
| HIV transmission risk group | |||||
| Non-IDU MSM | 152 | 15.7 | Reference | 3.6 [3.1–4.2] | |
| IDU | 140 | 27.7 | 1.9 [1.5–2.4] | 5.6 [4.7–6.6] | |
| IDU-MSM | 26 | 18.3 | 1.4 [0.9–2.1] | 4.4 [2.9–6.4] | |
| Hemophiliac | 7 | 88.8 | 8.2 [3.8–17.0] | 40 [16.0–83.0] | |
| Heterosexual | 20 | 6.8 | 0.6 [0.4–1.0] | 2.0 [1.2–3.1] | |
| Other/unknown | 21 | 8.5 | 0.6 [0.4–0.9] | 1.7 [1.1–2.7] | |
| HIV risk groups by HCV prevalence | |||||
| Groups with high HCV prevalence (IDU, IDU-MSM, hemophiliacs) | 173 | 26.4 | 2.1 [1.7–2.6] | 2.2 [1.8–2.8] | 5.6 [4.8–6.5] |
| Groups with low HCV prevalence (non-IDU MSM, heterosexuals, others/unknown) | 193 | 12.8 | Reference | Reference | 3.0 [2.6–3.5] |
| Attained calendar period | |||||
| 1980–1989 (pre-ART era) | 4 | 6.5 | 1.0 [0.4–2.9] | 1.1 [0.4–3.1] | 5.7 [1.6–15] |
| 1990–1995 (monotherapy/dual therapy era) | 30 | 7.0 | Reference | Reference | 3.5 [2.4–5.1] |
| 1996–2000 (early HAART era) | 118 | 14.3 | 1.7 [1.2–2.6] | 1.8 [1.2–2.6] | 3.8 [3.1–4.5] |
| 2001–2009 (recent HAART era) | 214 | 25.3 | 2.5 [1.7–3.7] | 2.7 [1.8–4.0] | 3.9 [3.4–4.5] |
| p-trend <.0001 | p-trend <.0001 | ||||
| CD4 cell count at AIDS diagnosis, cells/µLd | |||||
| 0–99 | 78 | 15.1 | Reference | 3.2 [2.5–4.0] | |
| 100–199 | 135 | 22.1 | 1.4 [1.1–1.9] | 4.7 [4.0–5.6] | |
| 200–349 | 33 | 17.2 | 1.1 [0.7–1.6] | 3.4 [2.3–4.8] | |
| ≥350 | 22 | 23.6 | 1.5 [1.0–2.5] | 5.0 [3.1–7.5] | |
| p-trend = 0.13 | |||||
| AIDS statuse | |||||
| Individuals with HIV but not AIDS | 60 | 10.1 | Reference | 2.4 [1.8–3.1] | |
| Individuals with AIDS | 36 | 26.1 | 2.4 [1.2–3.6] | 5.0 [3.5–6.9] |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HAART, highly active antiretroviral therapy; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IDU, injection drug users; MSM, men who have sex with men; RR, incidence rate ratio; SIR: standardized incidence ratio.
All RR estimates are adjusted for sex and age, except the RR for sex (which was adjusted for age) and age (which was adjusted for sex).
All RR estimates are from a multivariate model that included terms for sex, age, race/ethnicity, HIV risk groups by HCV prevalence, and attained calendar period. These RRs were not calculated for individual HIV risk groups, CD4 cell count strata, or strata according to AIDS status.
For this analysis, the groups ages 0 to 29 years and ages 30 to 39 years were combined into a single reference group of individuals ages 0 to 39 years.
Ninety-eight individuals with hepatocellular carcinoma were missing data on CD4 cell counts.
This analysis was restricted to individuals who initially were reported as positive for HIV infection but without AIDS and was based on data from 12 registries that provided information on these individuals.
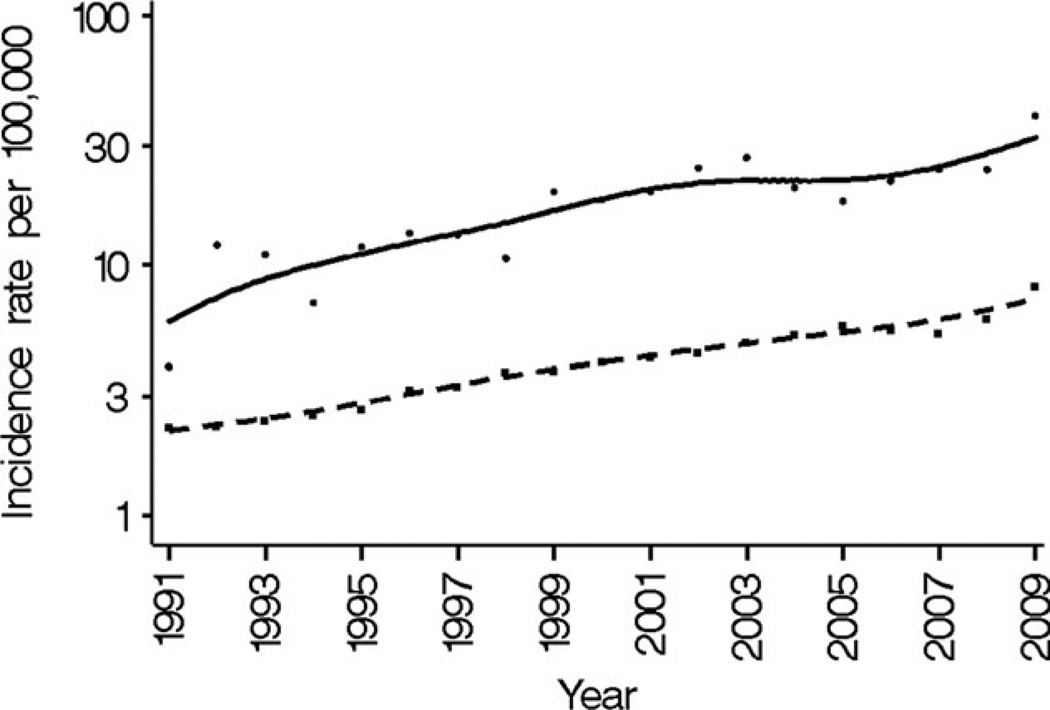
For the years 1991 to 2009, this increase in HCC incidence over time paralleled the trend in the US general population, as illustrated in Figure 1. Consequently, SIRs comparing HCC risk in individuals with AIDS with the general population did not change between the monotherapy/dual therapy era (1990–1995) and the recent HAART era (2001–2009), ranging between 3.5 and 3.9 (Table 3).
Figure 1.
Hepatocellular carcinoma (HCC) incidence is illustrated in individuals with acquired immunodeficiency syndrome (AIDS) and in the general US population for the years 1991 to 2009. Results for the AIDS population are indicated by round points and a solid line (for smoothed fit), and results for the general population are indicated by square points and a dashed line. Incidence rates were standardized according to age, sex, and race to the 2000 human immunodeficiency virus (HIV)/AIDS Cancer Match (HACM) AIDS population. Cases before 1990 were too few and were not included in the graph.
HCC rates did not differ according to CD4 cell counts recorded at the time of AIDS onset (Ptrend = .13). However, in an analysis limited to HIV-infected individuals who were followed beginning with HIV registration, HCC incidence was higher among those with AIDS than among those without AIDS (RR, 2.4; 95% CI, 1.2–3.6) (Table 3).
DISCUSSION
The current population-based study demonstrates that HCC incidence has steadily increased among individuals with AIDS in the United States over the past 3 decades. Individuals with AIDS have a 4-fold higher HCC risk than the general population, and the magnitude of this relative excess has remained relatively unchanged over time, including in the HAART era. Although risk also was elevated for other liver and intrahepatic bile duct cancers (except cholangiocarcinoma), HCC was by far the most common and the most important hepatobiliary cancer from a clinical and public health perspective.
It is believed that the rising incidence of HCC in the US general population is related to trends in HCV incidence and prevalence.1,18,19 HCV incidence in the US general population increased from the 1960s until the mid-1980s and then declined with the introduction of widespread HCV screening of blood in 1992.20,21 The resultant pool of over 3 million individuals with chronic HCV infection in the United States has experienced a decades-long increase in the risk of HCV-associated chronic liver disease and cirrhosis. In our study, HCC risk was 2-fold higher in individuals with AIDS who likely were HCV-infected (ie, hemophiliacs and IDUs: HCV prevalence, >60%–85%) than in those who likely were uninfected (ie, non-IDU MSMs, heterosexuals, and others: HCV prevalence, <15%).16,17,22 HCC risk was especially high among the relatively small group of hemophiliacs with AIDS, reflecting the near-universal acquisition of HCV through repeated transfusions of blood products before widespread HCV screening.21 Most hemophiliacs likely acquired HCV infection during childhood, which probably contributed to their high risk for HCC.
We could not evaluate the contribution of HBV, alcohol use, or other medical conditions to HCC risk, because these conditions are not recorded by HIV registries, and their prevalence does not vary substantially across HIV risk groups. HBV prevalence in the US general population is lower than that for HCV,1,23 and HBV plays a smaller, although not insignificant,24 role than HCV in the development of cirrhosis and HCC among individuals with AIDS in the United States. With declining alcohol consumption during the past 3 decades, alcohol abuse also contributes less to the burden of HCC in the US general population.25 However, alcohol abuse is more common in HIV-infected individuals.26,27 Finally, other chronic diseases, such as obesity, type II diabetes mellitus, metabolic syndrome, and NASH, are emerging as HCC risk factors in the US general population.1,28,29 With increased longevity, HIV-infected individuals will develop a growing burden of these conditions.30–32 In addition, lipodystrophy syndrome, which is consequent to the receipt of certain ART regimens, may be accompanied by insulin resistance, increasing the risk for NASH and, consequently, for cirrhosis and HCC.33
HIV-associated immunosuppression may be important in modulating the hepatotropic virus-driven progression of liver disease. Among HCV-infected individuals, coinfection with HIV is associated with increased risk of chronic liver disease and HCC.34,35 Supporting a role for immunosuppression, we observed higher HCC incidence in individuals who had progressed to AIDS than in HIV-infected individuals without AIDS (Table 3). In contrast to recent studies,5,36,37 the CD4 count was not associated with HCC risk, but our analyses were limited to a single CD4 count measurement at AIDS onset.
Nonetheless, if immunosuppression plays a role in the development of HCC, then a decline in HCC incidence may have been expected with the introduction of HAART in 1996. In fact, incidence rates increased during this period (Fig. 1). It is possible that the hepatotoxicity of ART drugs counterbalanced the benefits conveyed by improved immune function.38 Alternatively, improved survival in HIV-infected individuals may have led to increased HCC risk by allowing individuals to live for an extended period with chronic liver disease.6
In the future, HIV-infected individuals with chronic HCV/HBV coinfection may face an increasing risk of HCC.10 Our trend analyses demonstrated increasing HCC rates over time. Because this trend was age-adjusted, the increase in the crude incidence over time is even greater. Although approximately half of the HCC cases in individuals with AIDS occurred in middle-aged adults (ages 30–49 years), the risk in older adults (aged ≥50 years) was almost 10 times higher than in those ages 30 to 39 years. Increasing HCC rates in the future, when applied to the aging HIV-infected population, would greatly increase the number of HCC cases overall, as well the proportion of cases among older adults.
Our study’s strengths include its large size, the inclusion of all HIV risk groups, and coverage of major geographic centers of the US AIDS epidemic over its entire duration. The main limitations were the lack of individual-level data on HCC risk factors and our reliance on an indirect measure of HCV risk by grouping individuals into categories with different HCV prevalence.39 Our study also was limited in the number of hepatobiliary cancer cases other than HCC, which prevented us from examining the epidemiology of these rare cancers in detail.
The rising burden of HCC in HIV-infected individuals highlights the importance of additional research on this malignancy. One unanswered question is whether the early initiation of appropriate antiviral therapies for HCV/HBV along with HAART can reduce HCC incidence. More work also needs to be undertaken to disentangle the effect of HAART on chronic liver disease and HCC.11 Some ART drugs, such as lamivudine and tenofovir, have anti-HBV activity that may contribute to the control of HBV-related liver disease. Optimal clinical surveillance methods for HCC in HIV-infected populations need further investigation, for example, characterizing the utility of risk scoring algorithms or noninvasive imaging methods in HCV/HBV-coinfected patients.40,41 Cost-benefit and cost-effectiveness analyses also will be needed to evaluate the role of surveillance in linking at-risk individuals to early initiation of newer antiviral therapies and identifying appropriate individuals with cirrhosis for liver transplantation.42,43
In the interim, clinical and public health prevention efforts should continue to focus on reducing the risk of chronic liver disease among HIV-infected individuals, through counseling on risk factor modification and HBV vaccination when applicable and by offering both HAART and antiviral treatments for HCV/HBV.8,34 Limitations in health care access for the treatment of viral hepatitis because of lack of health insurance, psychiatric illness, or active drug or alcohol use, also need to be addressed to mitigate the downstream risk of HCC in HIV-infected populations.44,45
Acknowledgments
The authors thank the HIV/AIDS and cancer registry staff in the following states and metropolitan areas for contributing data to the study: 14 registries for data on individuals with AIDS (the state registries of California, Colorado, Connecticut, Florida, Georgia, Illinois, Maryland, Massachusetts, Michigan, New Jersey, and Texas; and registries in the metropolitan areas of New York, NY; Seattle, WA; and Washington, DC) and 12 registries for data on individuals with HIV infection in the absence of AIDS (the state registries of Colorado, Connecticut, Florida, Georgia, Illinois, Maryland, Michigan, New Jersey, and Texas; and registries in the metropolitan areas of Los Angeles, CA; Seattle, WA; and Washington, DC). The authors also thank Tim McNeel (Information Management Systems) for database management.
FUNDING SOURCES
This work was supported by the Intramural Research Program of the National Cancer Institute at the National Institutes of Health.
Footnotes
This article is US Government work and, as such, is in the public domain in the United States of America.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis. 2011;15:223–243. vii–x. doi: 10.1016/j.cld.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engels EA, Frisch M, Lubin JH, Gail MH, Biggar RJ, Goedert JJ. Prevalence of hepatitis C virus infection and risk for hepatocellular carcinoma and non-Hodgkin lymphoma in AIDS. J Acquir Immune Defic Syndr. 2002;31:536–541. doi: 10.1097/00126334-200212150-00012. [DOI] [PubMed] [Google Scholar]
- 3.Puoti M, Rossotti R, Garlaschelli A, Bruno R. Hepatocellular carcinoma in HIV hepatitis C virus. Curr Opin HIV AIDS. 2011;6:534–538. doi: 10.1097/COH.0b013e32834bd2b7. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Dieguez M, Montes ML, Pascual-Pareja JF, et al. The natural history of liver cirrhosis in HIV-hepatitis C virus-coinfected patients. AIDS. 2011;25:899–904. doi: 10.1097/QAD.0b013e3283454174. [DOI] [PubMed] [Google Scholar]
- 5.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:2551–2559. doi: 10.1158/1055-9965.EPI-11-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giordano TP, Kramer JR, Souchek J, Richardson P, El-Serag HB. Cirrhosis and hepatocellular carcinoma in HIV-infected veterans with and without the hepatitis C virus: a cohort study, 1992–2001. Arch Intern Med. 2004;164:2349–2354. doi: 10.1001/archinte.164.21.2349. [DOI] [PubMed] [Google Scholar]
- 7.Bongiovanni M, Tordato F. Steatohepatitis in HIV-infected subjects: pathogenesis, clinical impact and implications in clinical management. Curr HIV Res. 2007;5:490–498. doi: 10.2174/157016207781662407. [DOI] [PubMed] [Google Scholar]
- 8.Joshi D, O’Grady J, Dieterich D, Gazzard B, Agarwal K. Increasing burden of liver disease in patients with HIV infection. Lancet. 2011;377:1198–1209. doi: 10.1016/S0140-6736(10)62001-6. [DOI] [PubMed] [Google Scholar]
- 9.Pol S, Lebray P, Vallet-Pichard A. HIV infection and hepatic enzyme abnormalities: intricacies of the pathogenic mechanisms. Clin Infect Dis. 2004;38(suppl 2):S65–S72. doi: 10.1086/381499. [DOI] [PubMed] [Google Scholar]
- 10.Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103:753–762. doi: 10.1093/jnci/djr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sulkowski M. Hepatocellular carcinoma in HIV-infected patients comes of age: the convergence of epidemiology and treatment effectiveness. J Hepatol. 2009;50:655–658. doi: 10.1016/j.jhep.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20:1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 13.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 14.Altekruse SF, Devesa SS, Dickie LA, McGlynn KA, Kleiner DE. Histological classification of liver and intrahepatic bile duct cancers in SEER registries. J Registry Manag. 2011;38:201–205. [PMC free article] [PubMed] [Google Scholar]
- 15.Simard EP, Pfeiffer RM, Engels EA. Spectrum of cancer risk late after AIDS onset in the United States. Arch Intern Med. 2010;170:1337–1345. doi: 10.1001/archinternmed.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US Adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34:831–837. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 17.Sulkowski MS, Thomas DL. Hepatitis C in the HIV-infected person. Ann Intern Med. 2003;138:197–207. doi: 10.7326/0003-4819-138-3-200302040-00012. [DOI] [PubMed] [Google Scholar]
- 18.Shaw JJ, Shah SA. Rising incidence and demographics of hepatocellular carcinoma in the USA: what does it mean? Expert Rev Gastroenterol Hepatol. 2011;5:365–370. doi: 10.1586/egh.11.20. [DOI] [PubMed] [Google Scholar]
- 19.Wasley A, Grytdal S, Gallagher K. Surveillance for acute viral hepatitis—United States, 2006. MMWR Surveill Summ. 2008;57:1–24. [PubMed] [Google Scholar]
- 20.Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Empirically calibrated model of hepatitis C virus infection in the United States. Am J Epidemiol. 2002;156:761–773. doi: 10.1093/aje/kwf100. [DOI] [PubMed] [Google Scholar]
- 21.Goedert JJ, Chen BE, Preiss L, Aledort LM, Rosenberg PS. Reconstruction of the hepatitis C virus epidemic in the US hemophilia population, 1940–1990. Am J Epidemiol. 2007;165:1443–1453. doi: 10.1093/aje/kwm030. [DOI] [PubMed] [Google Scholar]
- 22.Chak E, Talal AH, Sherman KE, Schiff ER, Saab S. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090–1101. doi: 10.1111/j.1478-3231.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- 23.Wasley A, Kruszon-Moran D, Kuhnert W, et al. The prevalence of hepatitis B virus infection in the United States in the era of vaccination. J Infect Dis. 2010;202:192–201. doi: 10.1086/653622. [DOI] [PubMed] [Google Scholar]
- 24.Thio CL, Seaberg EC, Skolasky R, Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360:1921–1926. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 25.Parry CD, Patra J, Rehm J. Alcohol consumption and non-communicable diseases: epidemiology and policy implications. Addiction. 2011;106:1718–1724. doi: 10.1111/j.1360-0443.2011.03605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samet JH, Cheng DM, Libman H, Nunes DP, Alperen JK, Saitz R. Alcohol consumption and HIV disease progression. J Acquir Immune Defic Syndr. 2007;46:194–199. doi: 10.1097/QAI.0b013e318142aabb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baum MK, Rafie C, Lai S, Sales S, Page JB, Campa A. Alcohol use accelerates HIV disease progression. AIDS Res Hum Retroviruses. 2010;26:511–518. doi: 10.1089/aid.2009.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanyal A, Poklepovic A, Moyneur E, Barghout V. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin. 2010;26:2183–2191. doi: 10.1185/03007995.2010.506375. [DOI] [PubMed] [Google Scholar]
- 29.Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463–471. doi: 10.1002/hep.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crum-Cianflone N, Roediger MP, Eberly L, et al. Increasing rates of obesity among HIV-infected persons during the HIV epidemic [serial online] PLoS One. 2010;5:e10106. doi: 10.1371/journal.pone.0010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Satlin MJ, Hoover DR, Glesby MJ. Glycemic control in HIV-infected patients with diabetes mellitus and rates of meeting American Diabetes Association management guidelines. AIDS Patient Care STDS. 2011;25:5–12. doi: 10.1089/apc.2010.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sterling RK, Contos MJ, Smith PG, et al. Steatohepatitis: risk factors and impact on disease severity in human immunodeficiency virus/hepatitis C virus coinfection. Hepatology. 2008;47:1118–1127. doi: 10.1002/hep.22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feeney ER, Mallon PW. Insulin resistance in treated HIV infection. Best Pract Res Clin Endocrinol Metab. 2011;25:443–458. doi: 10.1016/j.beem.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Mallet V, Vallet-Pichard A, Pol S. The impact of human immunodeficiency virus on viral hepatitis. Liver Int. 2011;31(suppl 1):135–139. doi: 10.1111/j.1478-3231.2010.02394.x. [DOI] [PubMed] [Google Scholar]
- 35.Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562–569. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 36.Clifford GM, Rickenbach M, Polesel J, et al. Influence of HIV-related immunodeficiency on the risk of hepatocellular carcinoma. AIDS. 2008;22:2135–2141. doi: 10.1097/QAD.0b013e32831103ad. [DOI] [PubMed] [Google Scholar]
- 37.Guiguet M, Boue F, Cadranel J, Lang JM, Rosenthal E, Costagliola D. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009;10:1152–1159. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- 38.Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA. 2000;283:74–80. doi: 10.1001/jama.283.1.74. [DOI] [PubMed] [Google Scholar]
- 39.Tarwater PM, Mellors J, Gore ME, et al. Methods to assess population effectiveness of therapies in human immunodeficiency virus incident and prevalent cohorts. Am J Epidemiol. 2001;154:675–681. doi: 10.1093/aje/154.7.675. [DOI] [PubMed] [Google Scholar]
- 40.Park LS, Tate JP, Justice AC, et al. FIB-4 index is associated with hepatocellular carcinoma risk in HIV-infected patients. Cancer Epidemiol Biomarkers Prev. 2011;20:2512–2517. doi: 10.1158/1055-9965.EPI-11-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehta SH, Buckle GC. Assessment of liver disease (noninvasive methods) Curr Opin HIV AIDS. 2011;6:465–471. doi: 10.1097/COH.0b013e32834b55c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cooper C, Kanters S, Klein M, et al. Liver transplant outcomes in HIV-infected patients: a systematic review and meta-analysis with synthetic cohort. AIDS. 2011;25:777–786. doi: 10.1097/QAD.0b013e328344febb. [DOI] [PubMed] [Google Scholar]
- 43.Poordad F, Khungar V. Emerging therapeutic options in hepatitis C virus infection. Am J Manag Care. 2011;17(suppl 4):S123–S130. [PubMed] [Google Scholar]
- 44.Fleming CA, Craven DE, Thornton D, Tumilty S, Nunes D. Hepatitis C virus and human immunodeficiency virus coinfection in an urban population: low eligibility for interferon treatment. Clin Infect Dis. 2003;36:97–100. doi: 10.1086/344907. [DOI] [PubMed] [Google Scholar]
- 45.Mehta SH, Lucas GM, Mirel LB, et al. Limited effectiveness of antiviral treatment for hepatitis C in an urban HIV clinic. AIDS. 2006;20:2361–2369. doi: 10.1097/QAD.0b013e32801086da. [DOI] [PubMed] [Google Scholar]