Abstract
The effect of ethylene on the uptake, distribution, and metabolism of indoleacetic acid (IAA)-1-14C, IAA-2-14C, and naphthaleneacetic acid (NAA)-1-14C in cotton stem sections (Gossypium hirsutum L., var. Stoneville 213) was studied. Stem sections excised from plants pretreated with ethylene for 15 hours transported significantly less 14C-IAA and 14C-NAA than control sections. Concomitant features of the reduction of 14C-IAA transport were an increase in decarboxylation and a trend toward a reduction in total uptake. With 14C-NAA, however, total uptake was significantly increased, and decarboxylation was unaffected.
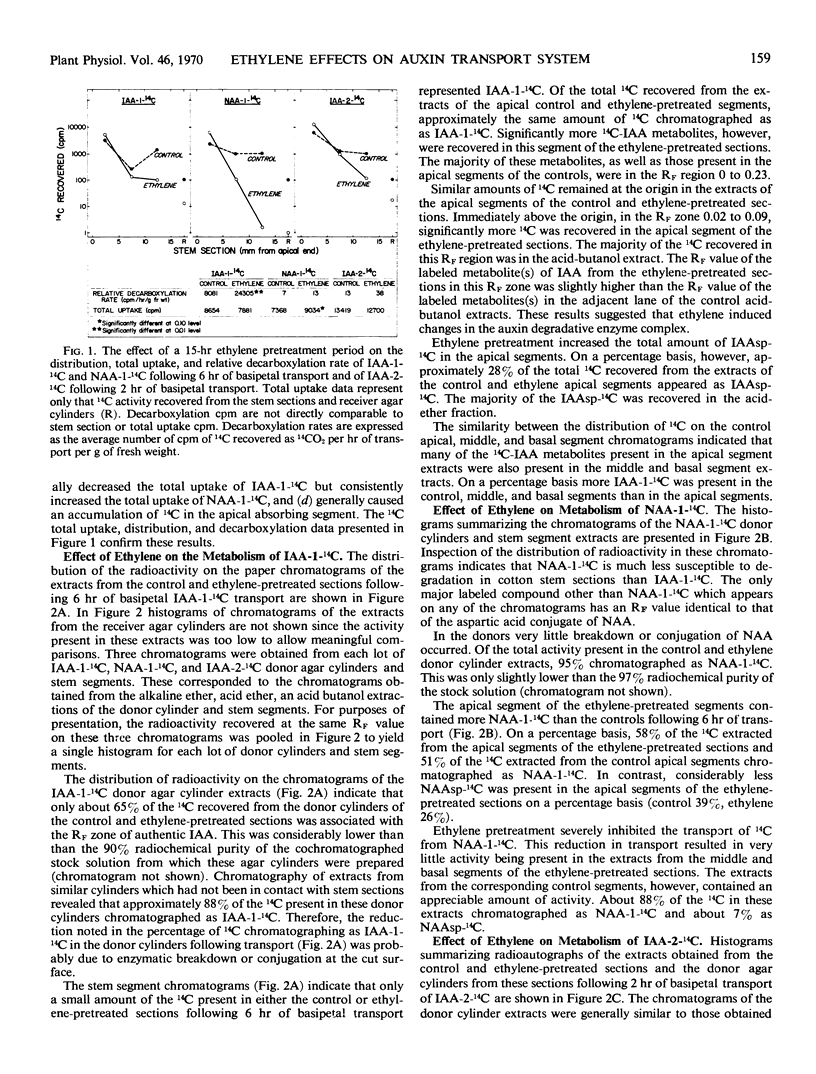
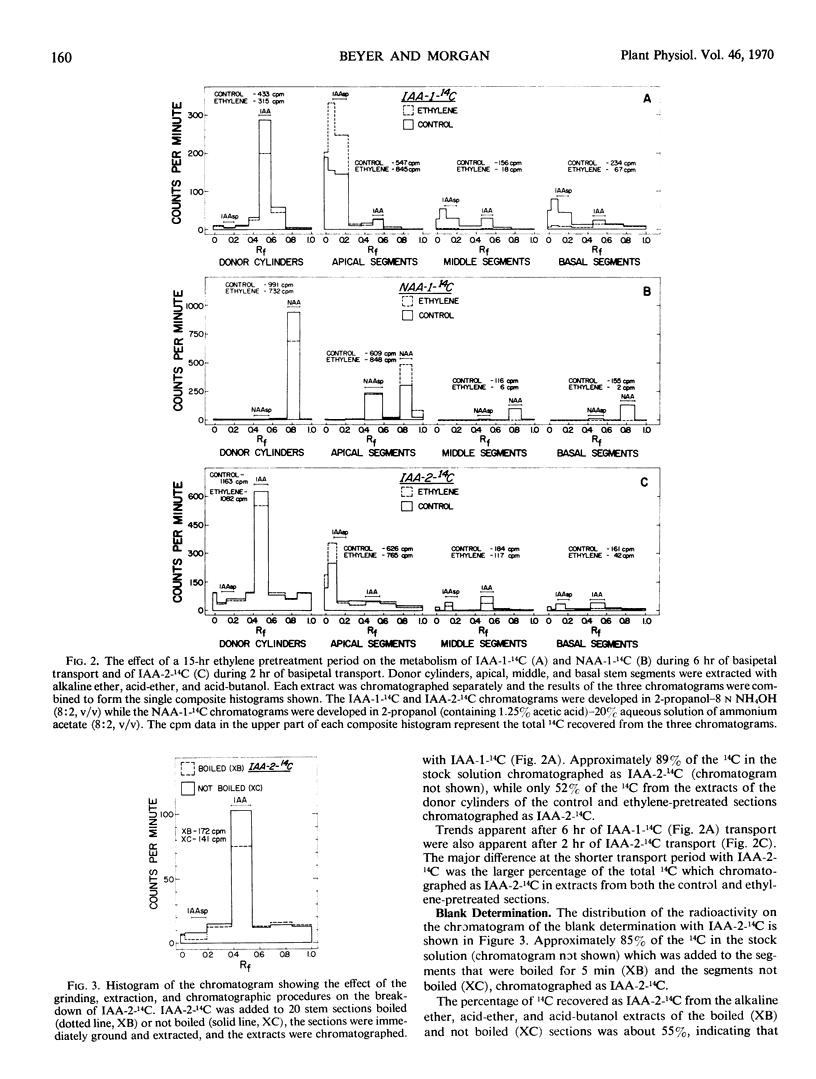
14C-IAA was rapidly converted to indoleacetylaspartic acid and many other metabolites in both control and ethylene-pretreated stem sections. Following transport, similar amounts of 14C-IAA were recovered in the apical absorbing portion of the control and ethylene-pretreated sections. Significantly more 14C-IAA metabolites, however, were recovered in this region of the ethylene-pretreated sections.
Conversely, 14C-NAA was metabolized more slowly than 14C-IAA under identical experimental conditions, with the only major metabolite being naphthaleneacetylaspartic acid. Following transport the apical absorbing portion of ethylene-pretreated stem sections contained significantly more 14C-NAA than the controls. These results suggested that the disruption of auxin transport by ethylene cannot be explained in terms of a more rapid metabolism of auxin in the treated sections. The increased 14C-IAA metabolites in the absorbing portion of ethylene-pretreated sections appear to be the result, rather than the cause, of the ethylene-mediated disruption of IAA transport.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVon Abrams G. J., Pratt H. K. Effect of ethylene on the permeability of excised cantaloupe fruit tissue. Plant Physiol. 1967 Feb;42(2):299–301. doi: 10.1104/pp.42.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreae W. A., Good N. E. The Formation of Indoleacetylaspartic Acid in Pea Seedlings. Plant Physiol. 1955 Jul;30(4):380–382. doi: 10.1104/pp.30.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendaña F. E., Galston A. W., Kaur-Sawhney R., Penny P. J. Recovery of labeled ribonucleic acid following administration of labeled auxin to green pea stem sections. Plant Physiol. 1965 Nov;40(6):977–983. doi: 10.1104/pp.40.6.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M., Morgan P. W. Ethylene modification of an auxin pulse in cotton stem sections. Plant Physiol. 1969 Dec;44(12):1690–1694. doi: 10.1104/pp.44.12.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Inhibition of polar auxin transport by ethylene. Plant Physiol. 1967 Sep;42(9):1224–1228. doi: 10.1104/pp.42.9.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Andreae W. A., Ysselstein M. W. Studies on 3-Indoleacetic Acid Metabolism. II. Some Products of the Metabolism of Exogenous Indoleacetic Acid in Plant Tissues. Plant Physiol. 1956 May;31(3):231–235. doi: 10.1104/pp.31.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCREADY C. C. A direct-plating method for the precise assay of carbon-14 in small liquid samples. Nature. 1958 May 17;181(4620):1406–1406. doi: 10.1038/1811406a0. [DOI] [PubMed] [Google Scholar]
- Morgan P. W., Gausman H. W. Effects of ethylene on auxin transport. Plant Physiol. 1966 Jan;41(1):45–52. doi: 10.1104/pp.41.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson B. J., Forman M., Addicott F. T. Auxin transport and conjugation in cotton explants. Plant Physiol. 1968 Aug;43(8):1321–1323. doi: 10.1104/pp.43.8.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwertner H. A., Morgan P. W. Role of IAA-Oxidase in Abscission Control in Cotton. Plant Physiol. 1966 Nov;41(9):1513–1519. doi: 10.1104/pp.41.9.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S. M., Galston A. W. Experimental Coupling of Indoleacetic Acid to Pea Root Protein In Vivo and In Vitro. Proc Natl Acad Sci U S A. 1953 Nov;39(11):1111–1118. doi: 10.1073/pnas.39.11.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter A., Thimann K. V. Bound indoleacetic Acid in Avena coleoptiles. Plant Physiol. 1966 Feb;41(2):335–342. doi: 10.1104/pp.41.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]


