Abstract
The recent emergence of a new CD4+ T cell subset, Th17, has transformed our understanding of the pathogenetic basis of an increasing number of chronic immune-mediated diseases. Particularly in tissues that interface with the microbial environment — such as the intestinal and respiratory tracts, and skin — where most of the Th17 cells present in the body reside, dysregulated immunity to self, or the extended “self,” the diverse microbiota that normally colonize these tissues, can result in chronic inflammatory disease. In this review, we focus on recent advances in the biology of the Th17 pathway and genome-wide association studies (GWAS) implicating this immune pathway in human disease that are providing new insights into disease mechanisms in these and other tissues.
Keywords: Th17 cells, Th22 cells, autoimmunity, inflammatory bowel disease, Crohn’s disease, ulcerative colitis, asthma, COPD, psoriasis, atopic dermatitis
CD4 T CELLS AND DISEASES OF IMMUNE DYSREGULATION
Many chronic inflammatory diseases are characterized by inappropriate or dysregulated CD4+ T cell responses. Given their central role in regulating innate and adaptive immunity, CD4+ T cells are key to both immune protection and immune pathology. Our understanding of the immunopathology of many chronic inflammatory diseases has been transformed by advances in basic studies of CD4+ T cell biology, most notably the proposition over two decades ago that CD4+ T cells undergo subspecialization for coordinating “help” for distinct types of immunity based on their expression of alternative patterns of cytokines: the T helper(h)1–Th2 hypothesis (1; 2). The discoveries that emerged from examination and testing of the Th1–Th2 model, both in animal models and in human disease, laid the foundation for many tenets of modern immunobiology and have begun to revolutionize the means by which a growing number of immunologic diseases are treated: that is, the introduction into clinical practice of small molecule or biologic therapies, including neutralizing or depleting antibodies, that target specific immune pathways that are linked to aberrant CD4+ T cell immunity.
The notion instigated by the original Th1–Th2 hypothesis that CD4+ T cells emerge from their primary thymic education poised for a secondary education that diverts them down divergent programs of differentiation contingent upon cytokine cues from innate immune cells, has provided a strong mechanistic foundation upon which new advances and discoveries have been integrated. Among the most important of these was the discovery of a third major subset of effector CD4+ T cells, Th17 cells (3). Like their Th1 and Th2 antecedents, Th17 cells are characterized by an alternative pattern of cytokine expression that underpins a distinct type of immunity and has had a substantial impact on our understanding of immune regulation and immune-mediated disease. In this review, we will highlight recent advances that have followed from discovery of the Th17 pathway and their implications for chronic immune-mediated diseases in tissues that Th17 cells have evolved to defend: mucosal barrier tissues, including the gastrointestinal and respiratory tracts, and the skin.
The Th17 Pathway: A New CD4 Effector T Cell Subset with Specialized Functions in Host Protection and New Implications for Chronic Inflammatory Diseases
The original Th1–Th2 hypothesis was based on the description of two general types of cloned mouse T cell lines that came to be characterized by their differential production of the cytokines IFNγ (Th1 cells) or IL-4, IL-5 and IL-13 (Th2 cells) (2). Following their discovery, it became apparent that Th1 cells had evolved to enhance eradication of intracellular pathogens (e.g., intracellular bacteria, viruses and some protozoa), whereas Th2 cells evolved to enhance elimination of parasitic infections (e.g., helminths), primarily from mucosal sites of infection. Thus, the Th1 cytokine, IFNγ, is a potent activator of innate immune cells, particularly macrophages, for enhanced intracellular eradication of pathogens that have evolved mechanisms to evade the microbicidal functions of phagocytes. IFNγ also promotes class-switch recombination of B cells to immunoglobulin (Ig)G isotypes that opsonisze microbes for enhanced uptake by phagocytes. Th2 cytokines coordinate B cell class switching for production IgE antibodies that arm basophils and mast cells for granule release (IL-4 and IL-13), enhanced eosinophil mobilization (IL-5), and increased mucous production and hypermotility (IL-13) – all of which promote the expulsion of worms.
However, this original bifurcation of effector T cell subsets could not fully explain the specialized functions of CD4+ T cells in other types of infections, most notably those mediated by extracellular bacteria and fungi, efficient eradication of which rests on the orchestration of neutrophilic inflammation and class-switched opsonizing IgG antibodies. It was not until the discovery of a third major effector CD4+ T cell, Th17, almost two decades following discovery of Th1 and Th2, that this “hole” in the functional repertoire of effector T cell subsets was filled (3). Th17 cells are so-named for their production of the IL-17 family cytokines, IL-17A and IL-17F, that target innate immune cells and epithelial cells, among others, to produce G-CSF and IL-8 (CXCL8), which induce increased neutrophil production and recruitment, respectively. Th17 cells also produce GM-CSF, another cytokine that promotes enhanced bone marrow production of granulocytes and monocytes, as well as IL-22, a member of the IL-10 cytokine family that acts on epithelial cells of barrier tissues, such as the gut, lung and skin, to enhance anti-microbial defense and epithelial barrier integrity. In view of Th17’s unique links to neutrophils it would appear that each of the three major effector CD4+ T cell lineages (Th1, Th2 and Th17) has evolved to augment and coordinate the functions of, and integrate adaptive immunity with, a different branch of the granulocyte-monocyte pathway for optimal eradication of different classes of pathogens: monocyte/macrophages by Th1; eosinophils, basophils and mast cells by Th2; and neutrophils by Th17 (Figure 1).
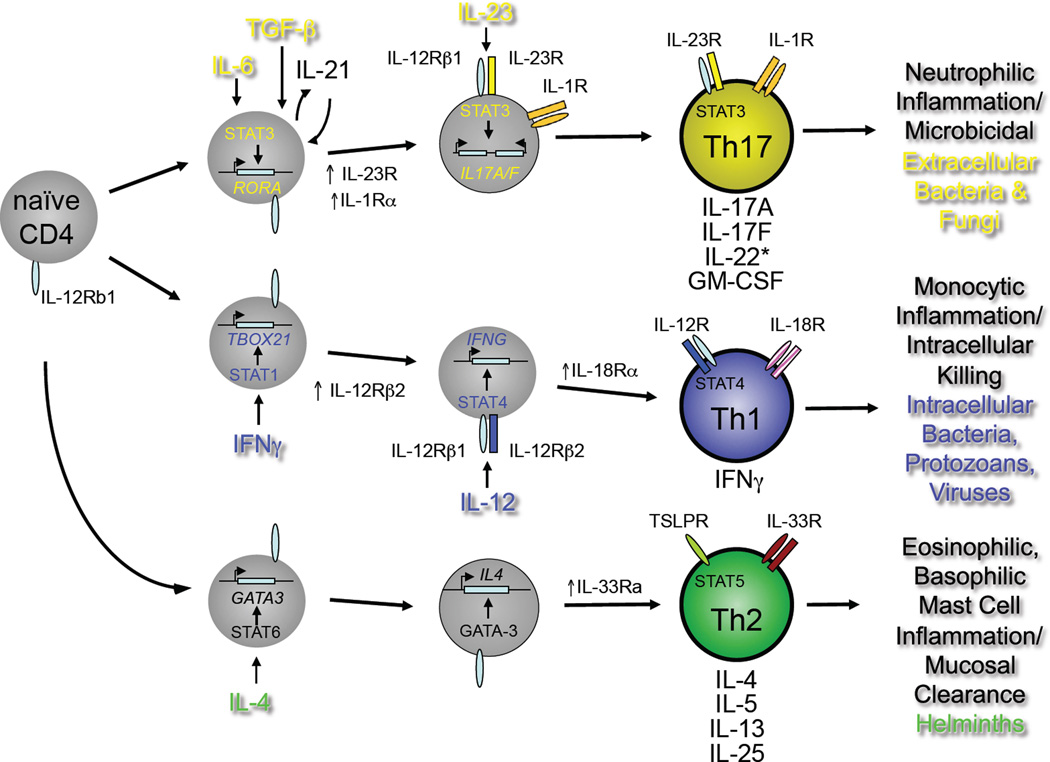
Figure 1.
Developmental programs of Th17, Th1 and Th2 pathways. Naïve CD4+T cells of defined antigen specificity differentiate into Th17, Th1 or Th2 effector cells contingent on the prevailing cytokine milieu (reviewed in ref. (3). For the Th17 pathway, IL-6, TGFβ and IL-1β are critical early factors in specifying Th17 cell development through induction of the transcription factor, RORγt, encoded by the RORC gene. The inducible component of the IL-23 receptor complex, IL-23R, is upregulated downstream of RORγt expression and confers IL-23 responsiveness on maturing Th17 cells. Mature Th17 cells produce IL-17A, IL-17F, GM-CSF and varying amount of IL-22, depending on the concentrations of active TGFβ present during differentiation (see Figure 2, below). As for mature Th1 and Th2 cells, mature Th17 cells can be activated to produce cytokines by either of two pathways: antigen-dependent or antigen-independent. In the latter, stimulation by IL-23 in concert with IL-1β activates independently of a requirement for antigen recognition. Th1 and Th2 cells can also be activated independently of antigen recognition: both rely on ligands for distinct STAT family member-dependent receptors (e.g., IL-12/STAT4 for Th1; TSLP/STAT5 for Th2) acting in concert with ligands for different IL-1 receptor family members (IL-18 for Th1; IL-33 for Th2). The different type of pathogens that preferentially elicit each differentiation program and the innate immune cells with which the mature effectors of each pathway interact are indicated.
Given their defined antigenic specificity and long-lived memory – assets for host protection against pathogens – effector CD4+ T cells pose a potential liability when inappropriately directed against self-antigens or inadequately regulated in response to environmental antigens. As with Th1 and Th2, the evolutionary pressures applied by the types of microbes that Th17 cells evolved to protect against were not without risk to the vertebrate host. For reasons that will be considered below, the development of chronic immune disease mediated by dysregulated Th17 responses appears to be particularly great. In fact, it was through discovery that the cytokine IL-23, an important factor for the development of Th17 cells, was essential for several key models of autoimmune disease previosuly attributed to dysregulated Th1 immunity that led to the discovery of Th17 cells as a distinct effector T cell subset (4). Since then, the Th17 pathway has been linked to a growing number of chronic inflammatory diseases in humans through genome-wide association studies (GWAS) and Th17-specific biomarkers. Notable among these are inflammatory diseases centered on tissues with broad epithelial interfaces with the external environment, specifically mucosal tissues and the skin – sites where cells of the Th17 pathway are normally present in greatest abundance.
The Th17 Pathway: New Branches in the Family Tree
Subsequent to the discovery of Th17 cells, studies that examined the developmental programming of this subset revealed a remarkable propensity for functional diversity, or plasticity, that appears to be a characteristic of this pathway (5; 6). In contrast to Th1 and Th2 cells, which demonstrate more stability following their development from naïve precursors, Th17 cells retain the capacity for divergent cytokine expression profiles and function following their commitment to the Th17 pathway (Figure 2). This appears to be linked to retention of more stem cell–like properties of Th17 cells, which allows them to persist long-term while retaining the capacity to generate functionally divergent progeny when reactivated by antigen (7; 8).
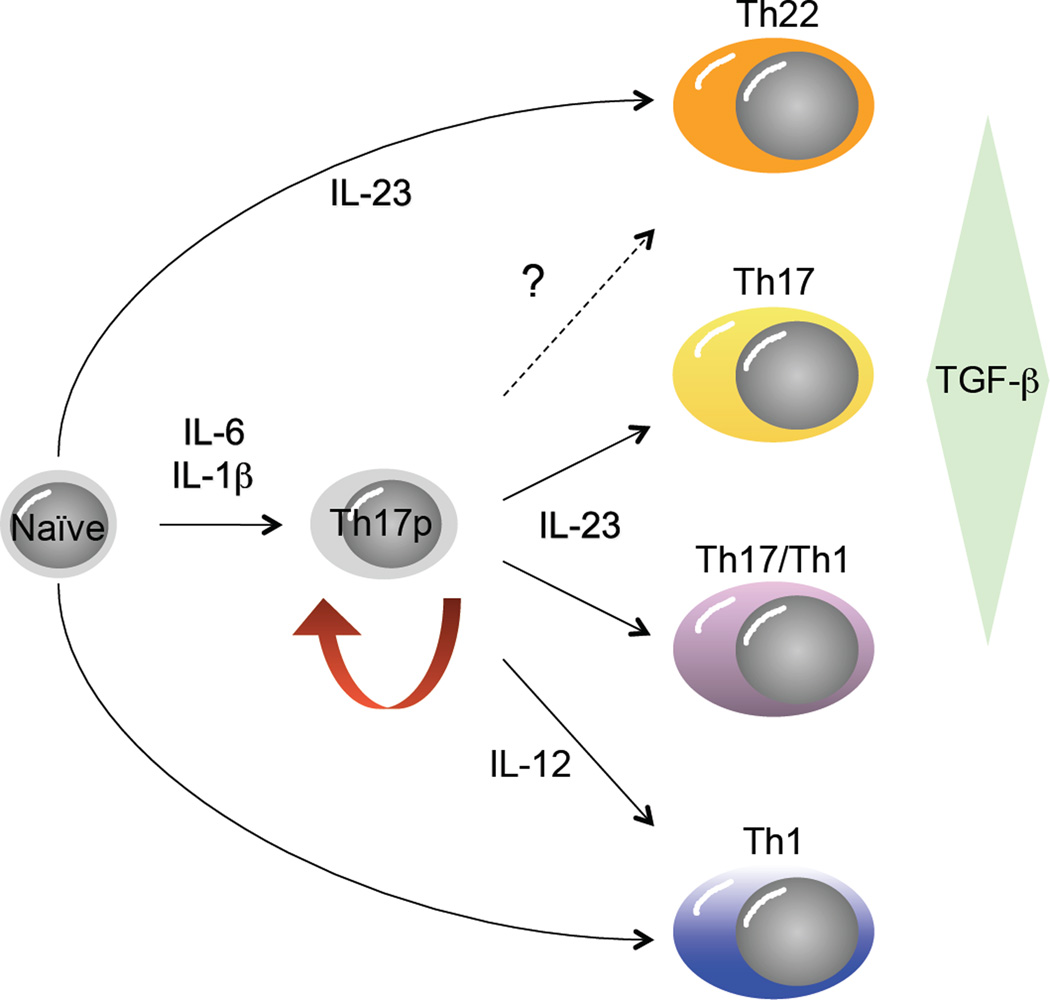
Figure 2.
Developmental plasticity in the Th17 pathway. Originally conceived as a pathway with a single precursor-effector relationship similar to the Th1 and Th2 pathways, recent studies have uncovered substantial developmental flexibility, or plasticity, in the Th17 developmental program. It is now apparent from studies in mice that Th17 precursor cells (Th17p) derived by differentiation in the presence of IL-6, IL-1β and TGFβ can give rise to progeny with many characteristics of classical Th1 cells (socalled, “Th17/Th1” cells), contingent upon the balance of active TGFβ and IL-23 concentrations late in their programming, whereas true Th1 cells can also develop under the influence of IL-12 signaling in Th17p cells. It is also apparent that mature effectors that produce high levels of IL-22, with little of no IL-17A or IL-17F (so-called “Th22” cells), can develop from naïve precursors in the presence of IL-6, IL-1β and IL-23, absent TGFβ. At present it is unclear whether Th22 cells can develop from Th17p cells (dashed arrow).
Most notably, Th17 cells can give rise to IFNγ-producing effectors that bear resemblance to classical Th1 cells, so-called Th17/Th1 cells, by repressing the gene locus that encodes IL-17A and IL-17F and enhancing expression from the locus encoding IFNγ. This has been demonstrated for both human and mouse Th17 cells (9; 10). Importantly, in each of the Th17-associated chronic inflammatory diseases characterized to date, whether in mouse or human, both Th17 and Th1-like cells are found in the involved tissues, as are cells that co-express IL-17 and IFNγ, raising the possibility that the IL-17- and IFNγ-producing cells arise from a common effector precursor pool. While the relative contributions to Th17 and Th17/Th1 cells to chronic inflammatory disease remain to be elucidated, IFNγ producing cells are required for disease pathogenesis in at least some mouse models. Although these Th17/Th1 cells share many features of classical Th1 cells, gene expression analyses indicate important differences that are likely to have implications for immune disease mediated by these cells (10), suggesting that generic categorization of IFNγ-producing CD4+ T cells as Th1 cells is an oversimplification.
In addition to IL-17A, IL-17F, and even IFNγ, the Th17 pathway can give rise to cells that express IL-22, which targets the epithelium of the mucosae and skin for enhanced barrier function (11) (Figure 2; and see below). However, while IL-22 can clearly be produced by Th17 cells, its expression is in developing mouse Th17 cells is antagonized by TGFβ, which enhances IL-17 production by these cells, suggesting a reciprocal relationship between expression of these cytokines by cells of the Th17 pathway (12). Further, in humans, a unique subset of IL-22-expressing CD4+ T cells, referred to as Th22 cells, has been described which is distinguished by its expression of skin-homing receptors (13; 14), as will be discussed below. The functions of Th22 cells in normal immune homeostasis are not well understood at present, nor are the developmental origins of these cells relative to traditional Th17 cells. Also, there has been considerable controversy regarding differences in the developmental programs of Th17 cells in mouse versus human (15). Whereas Th17 cells are clearly present in the memory T cell pool of both species and all Th17 cells to date share expression of the key transcription factor, RORγt (see Figure 1), the relative contributions of TGF-β and pro-inflammatory cytokines, especially IL-1β, to Th17 cell development appear to differ between species. Finally, it should be noted that expression of these cytokines is not unique to CD4+ T cells. CD8 T cells that express IL-17 and IL-22 have been described, particularly in inflammatory diseases of skin, and innate lymphoid cells (ILCs) that share many features of Th17 cells are increasingly appreciated for their contribution to immune responses in mucosal tissues (11).
Th17 Specialization For Immune Regulation At Barrier Sites: Implications For Human Disease
The gastrointestinal (GI) tract, respiratory tract and skin represent, in that order, the three largest geographical contacts with the external environment. The epithelia of these tissues are at the interface with different “external” environments and are the major portals for entry of microbes, whether commensals or pathogens. As such, each of these tissues has evolved highly specialized barrier defense mechanisms to resist threats from the outside environment while performing their major respective functions of nutrient uptake, gas exchange and sensing of/protection from the external world. Although unique in structure due to their specialized functions, the epithelia of each of these tissues also share some common strategies for defense, including those coordinated by cells of the Th17 pathway, which balance needs for host protection against chronic inflammation.
A common strategy employed by these tissues is one of a layered defense that integrates a relatively impenetrable epithelium with innate and adaptive immune cells specialized for protection at each site (Figure 3). The gastrointestinal and respiratory tracts are mucosal tissues, hence the epithelia continuously produce and are invested by a layer of mucous that serves as a first line of defense against microbes. Mucous in the GI tract is continuously propelled along the longitudinal axis by peristalsis, whereas in the respiratory tract it is propelled towards the oropharynx by the continuous, directional beating of ciliated epithelial cells that line the conducting airways. There it is swallowed. In this way, inhaled pathogens can be conveyed to the GI tract where they can be eradicated in the highly acidic environment of the stomach, or failing that, by other GI defense mechanisms. While the mucous layer provides a physical barrier to impede movement of microbes from the lumen to the epithelium, it also serves as a reservoir for retention of microbicidal products of the epithelium, including a large variety of antimicrobial peptides (AMPs) specialized for killing different classes of microbes, as well as secreted antibodies produced by tissue-resident B cells, primarily secretory IgA. Effectively, the mucous layer that bathes these epithelia is a “killing field” that relatively few pathogens have evolved strategies to effectively negotiate.
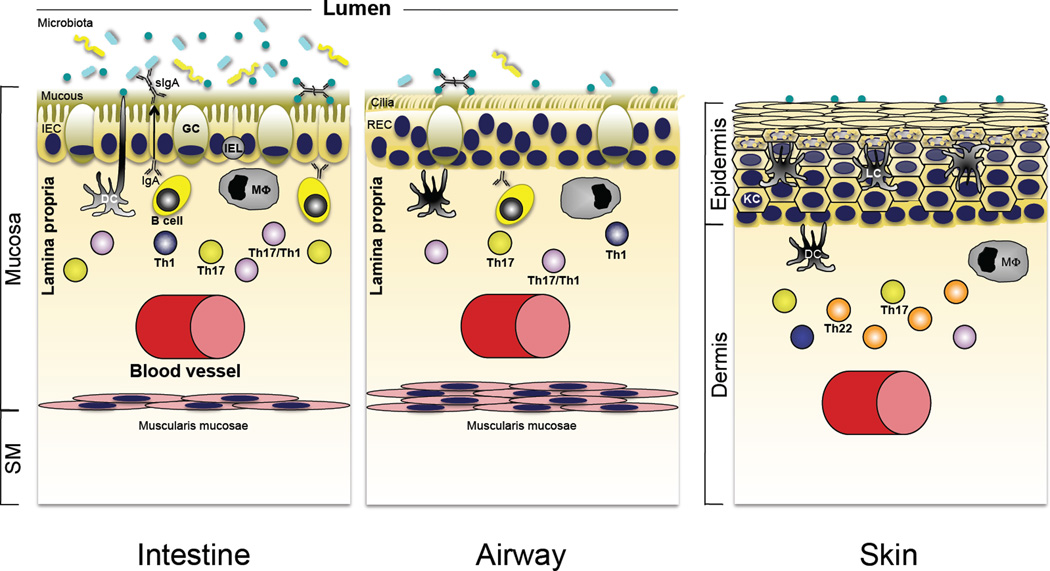
Figure 3.
Role of Th17 cytokines in immune homeostasis in the intestine, lung and skin. Although the interface tissues of the intestine, airways of the lung, and skin are charged with different functional roles, each is a major portal for pathogenic microbes and exists at homeostasis with a resident microbiota that is acquired shortly after birth. Accordingly, there are shared defensive strategies for partitioning microbes to the “outside” environment, and in each tissue robust innate immune mechanisms are reinforced by a propensity for development of Th17 pathway cells that reside in close apposition to the epithelial barriers of each site (see text for details).
The skin does not produce mucous, but instead relies on a multilayered squamous epithelium, the outer layer of which is composed of densely packed, highly keratinized dead cells that forms a protective stratum that acts as a barrier to penetration of the underlying viable epithelial cells, or keratinocytes. As secretory IgA is a specialized adaptation of mucosal tissues, it is absent in skin. However, keratinocytes are also potent producers of AMPs that can eradicate microbes that penetrate the outer barrier of skin.
Thus, the epithelia of mucosal tissues and skin are not simply passive barriers to translocation of microbes. Epithelial cells in these tissues are arrayed with a remarkable diversity of germline-encoded receptors that detect pathogen-associated molecular patterns (PAMPS) through which they can be activated to produce antimicrobial factors that can be secreted apically into the lumen, as well as pro-inflammatory cytokines and chemokines that are directed basolaterally to activate and recruit local innate and adaptive immune cells. Essentially all of the pattern recognition receptors that have been defined to date — whether, toll-like receptors (TLRs), NOD-like receptors (NLRs), etc. — are expressed by epithelial cells of these tissues, some of which are strategically displayed to sense microbes only when the epithelial barriers has been breached. For example, TLR5, the receptor for flagellin, is expressed on the basolateral aspects of the epithelium of the intestines, where it can detect bacteria expressing flagella, which are composed of repeated flagellin monomers (16). Detection of flagellin on the “host” side of the epithelial barrier results in epithelial cell activation downstream of TLR5 signaling to initiate an inflammatory and immune response aimed at eradicating the invading bacterium. The importance of flagellin as both an immune-activating PAMP and antigen targeted by adaptive immune cells is reflected in its high representation among antigens to which serum antibodies from patients with inflammatory bowels disease (IBD) bind (17).
In mucosal tissues and skin, there is an abundant complement of resident innate and adaptive immune cells, reflecting the ongoing recognition of components of a diverse microbiota normally resident in these tissues. As will be discussed in the context of the specific tissues below, by far the greatest number of Th17 cells in the normal T cell repertoire are concentrated in these barrier tissues, especially in the intestines, where they develop in response to colonization by the flora shortly after birth. While a fraction of the GI-resident T cells localize to the eptithelium, where they are referred to as intraepithelial lymphocytes (IELs), the bulk of Th17 cells are found in the lamina propria subjacent to the basement membrane that separates this compartment from the epithelium. Antibody-producing B cells are also abundant in this tissue compartment, particularly IgA plasmacytes, where they continuously produce IgA that is actively transported across the epithelium into the lumen. As demonstrated by studies of microbiota-reconstituted germ-free and defined flora gnotobiotic mice, there appear to be certain components of the commensal flora that favor development of Th17 cells (18), although much additional work is needed to clarify this interaction.
At homeostasis, the dialog between the microbiota, epithelilium and innate and adaptive immune cells favors regulatory dominance that prevents inflammatory or immune-mediated disease. Indeed, the innate defenses are sufficiently robust that much of the host response to the microbiota progresses without involvement of CD4+ T cell dependent responses (19). Further, much of the IgA response that contributes to partitioning of commensal organisms away from the epithelium is generated without requirement for T cell help (20). Finally, at steady state, antigen presentation by dendritic cells of in the intestines, at least, favors the development of regulatory T cells (Tregs) that act to suppress effector T cells responses in this tissue to prevent inflammation (21). Nevertheless, dysregulated responses to the microbiota or other environmental antigens to which these tissues are exposed can cause chronic inflammatory disease and autoimmunity, and Th17 cells are central to many of these diseases.
THE TH17 PATHWAY IN INTESTINAL HOMEOSTASIS AND DISEASE
Th17 cells in the GI tract
The intestine is an immunologic organ, and necessarily so, as it has the largest single epithelial interface in the body and is populated by the greatest number and diversity of resident microbes. Metagenomic analysis of the normal human intestinal microbiome of 124 individuals provides an estimate of over 1000 bacterial species and some 3.3 million bacterial genes (22). Thus, it is not surprising that the intestine contains most of the B cells and T cells in the body, which are mainly dedicated to maintaining the hostmicrobiota mutualism. Prominent among the T cells present in the normal intestinal lamina propria are Th17 cells, which likely serve to maintain intestinal homeostasis (23). The Th17 cytokines, IL-17A, IL-17F, and IL-22, have major effects on the intestinal epithelium enhancing barrier function by stimulation of mucin and antimicrobial peptide production, increasing tight junction function, and increasing IgA transport into the lumen by upregulating pIgR expression, among other effects (11; 24).
In addition to Th17 cells, a number of other cells in the intestine produce the Th17 cytokines IL-22 and IL-17, including innate lymphoid cells (ILCs), natural killer (NK)T cells, and a subset of γδ T cells. These share some common features. All are RORγt+, CCR6+, IL-23R+, and produce IL-22 and/or IL-17 upon stimulation with IL-23 (25; 26). Some also express the aryl hydrocarbon receptor (AHR) and are activated by AHR ligands (27). Cytokine secretion by these cells is rapid and they appear to be the initial source of IL-22 during intestinal bacterial infection. One of these cells, a CD4linckit+ CD127+CCR6+, is a dominant source of IL-22 early during Citrobacter rodentium infection in mice (26). This early IL-22 production and the resulting epithelial cell production of the antimicrobial peptide REGIIIγ is essential for host protection against C. rodentium infection (28).
Th17 cytokines are protective against a number of pathogens at both barrier and systemic sites (29). These cells also presumably act in a continuous feedback loop with the microbiota, releasing IL-22 and/or IL-17 upon sensing microbiota metabolites or constituents, thus stimulating the epithelial cell secretion of antimicrobial peptides, which inhibit or kill bacteria in the vicinity of the epithelial cell surface. The important role these cells play in the digesive tract is demonstrated by the RORγt-deficient mouse (30) in which both innate and CD4+ Th17 cell subsets are absent. RORγt-deficient mice demonstrate a dramatic expansion of gut lymphoid follicles, increased numbers of gut CD4+ Th1 cells and IgG+ B cells, and have an extreme sensitivity to colon injury with dextran sulfate sodium (DSS). The relative role of innate IL-22-producing vs. CD4+ Th17 cells in protection from infection and maintenance of intestinal homeostasis is not yet understood.
CD4+ Th17 cells are not found in the germ-free mouse intestine, indicating that this subset is generated in response to the microbiota (31). A particularly potent bacterial inducer of Th17 cells in the intestine is Candidatus arthromatus, more popularly known as segmented filamentous bacteria (SFB), an organism that inhabits the ileum and cecum in low abundance (18). SFB stimulates many other mucosal cells as well (32), but the mechanism for its potency is unknown. Other microbiota bacteria can also stimulate intestinal Th17 cells, including the eight bacteria comprising the altered Schaedler’s flora (ASF). Such stimulation is dependent on the status of the host immune response at the time of the exposure (33). The induction of Th17 cells in the lamina propria by SFB has been shown to provide protection against Citrobacter infection by stimulating REGIIIγ production (18). Such protection comes at a cost in that SFB in the intestine also provides susceptibility to experimental autoimmunity in the K/BxN arthritis (34) and EAE models (35), which are both mediated by Th17 cells. Remarkably, therefore, sensing of certain pro-inflammatory constituents of the intestinal microbiota can promote autoimmunity in distal tissues. At present, however, it is unknown how or whether microbes such as SFB directly or indirectly induce Th17 cells reactive to autoantigens.
Gut injury and repair
IL-17 and IL-22 signaling are protective during colonic epithelial injury due to feeding of dextran sulfate sodium (DSS), that is, DSS-induced colitis is worse in the absence of IL-17 (36; 37) or IL-22 signaling (38). Consistent with these data, IL-23R-deficient RAG2-deficient mice given DSS exhibited exacerbated disease, increased mucosal damage, reduced phosphorylated STAT3 in the epithelium, and delayed recovery following DSS exposure. This phenotype was rescued with exogenous IL-22-Fc, which restored epithelial pSTAT3 (39). In this study, the source of endogenous IL-22 was Thy1.1+ innate lymphoid cells (ILCs). In contrast, IL-23R-deficient and IL-23p19-deficient mice, with adaptive immune cells present, had less weight loss and reduced inflammatory infiltrate following DSS injury (39), possibly due to increased numbers of intestinal Treg cells, which have been reported to occur in the absence of IL-23 (40). The production of IL-17 and/or IL-22 by innate non-Th17 cells appears to be responsible for protection from acute injury from DSS, but Th17 cells may also contribute. The weight of the data favors the notion that endogenous intestinal Th17 cytokines are serving primarily a homeostatic, protective function in the mucosa (23). Th17 cells can be potent mediators of colitis as well, as will be discussed next, and how these two discordant roles can be reconciled is not entirely clear.
Th17 cells in experimental colitis
The finding that IL-23, but not IL-12, was required for the spontaneous development of colitis in IL-10–deficient mice (41) was the first indication that Th17 cells might play an important role in inflammatory bowel disease (IBD). Subsequently, many other models of colitis have been shown to involve the Th17 subset. For example, in the CD45RBhi transfer model of colitis, in which the transfer of CD4 T cells isolated from the T cell repertoire of normal mice causes colitis in T cell deficient mice, transferred RORγt-deficient T cells failed to develop into Th17 cells or induce colitis in recipients. Furthermore, both IL-17A and IL-17F had to be deficient or neutralized to block disease after transfer of wild type CD4+CD45RBhi T cells (42). Transfer of CD4+CD45RBhi T cells into RAG-deficient IL-23p19-deficient mice does not cause colitis (43), nor does transfer of CD4+CD45RBhi T cells that lack IL-23R expression (44). In yet another transfer model in which microbiota-reactive T cells are administered to immunodeficient recipients, a large expansion of mucosal CD4+ Th17 cells was found and small numbers of Th17 cells were able to transfer colitis (45). In this model, antibody-mediated neutralization of IL-23 not only prevented colitis, it also reversed established colitis. Colitis induced by administration of the chemical irritant, 2,4,6-trinitrobenzenesulfonic acid (TNBS), also failed to develop in IL-17RA-deficient mice, which are defective in signaling by both IL-17A and IL-17F (46).
Although a pathogenetic role for the Th17 pathway is established in models of colitis, transfer of CD4+CD45RBhi T cells deficient in T-bet, the master transcription factor for Th1 responses, does not cause colitis, indicating that Th1-like cells play an important role in this model. In a recent report, transfer of CD4 T cells from IL-17A-deficient mice caused increased colitis that was associated with high levels of IFNγ in the lesions. This was postulated to be due to a lack of IL-17A inhibition of T-bet in Th1 cells (29). As discussed above, interpretation of these results is confounded by findings from several studies that Th17 cells have a propensity to give rise to Th1-like cells that express IFNγ and extinguish expression of IL-17, contingent upon induction of T-bet (10). Th17 cells stimulate IL-12 and IL-23 production in colonic dendritic cells, which would serve to enhance the diversion of Th17 precursors to Th1-like cells (47). Th17 and Th1 subsets in the colon may compete with one another as well (48). One view is that Th17 cells are not directly pathogenic but are modulating Th1 cell activity and the level of IFNγ in lesions via IL-17 (29; 49). An alternate view is that Th17 cells are important for disease progression in experimental chronic colitis. This issue remains controversial and will need to be resolved by future studies.
Th17 pathway in Crohn’s disease
Crohn’s disease (CD) is, along with ulcerative colitis, one of the two major forms of IBD in humans. It is characterized by chronic, relapsing inflammation that can involve any level of the GI tract, although it typically involves the small and large intestine. The involved segments of involvement are often sharply demarcated and can be discontinuous, with lesions separated by relatively normal segments of intestine (socalled “skip lesions”). Histopathologically, fully developed lesions included noncaseating granulomatous inflammation that is often transmural, which can lead to formation of fistulae between other segments of intestine or non-intestinal tissues. The exact mechanism(s) by which Th17 cells are pathogenic in CD is unknown, as are the relative roles of Th17 vs. Th1 subsets, although a wealth of data from mouse models, genetic associations and analysis of human tissues strongly implicate this pathway (Figure 4). Increased expression of IL-17 and IL-23 has been found in CD lesions (9; 50–55). In humans the Th17 subset appears to reside in a sub-population of CD4 T cells that are CCR6+, IL-23R+, and CD161+ (56). This subset is increased in the lesions of IBD (57). Data from genome-wide association studies has implicated IL-23R as being linked to IBD (58). The first major IL-23R SNP to be discovered, which is protective, encodes an alternately spliced secreted variant of IL-23R that is an inhibitor of IL-23 signaling (59). Additional IBD-linked genetic susceptibility variants of IL23R have now been identified, as have other genes of the Th17 pathway, including Jak2, Tyk2, STAT3, ICOSLG, and TNFSF15, among others (58; 60) (Table I). Patients with Crohn’s disease have increased IL-22 in the active lesions (51), as well as increased serum levels of IL- 22 that correlate with disease activity (61).
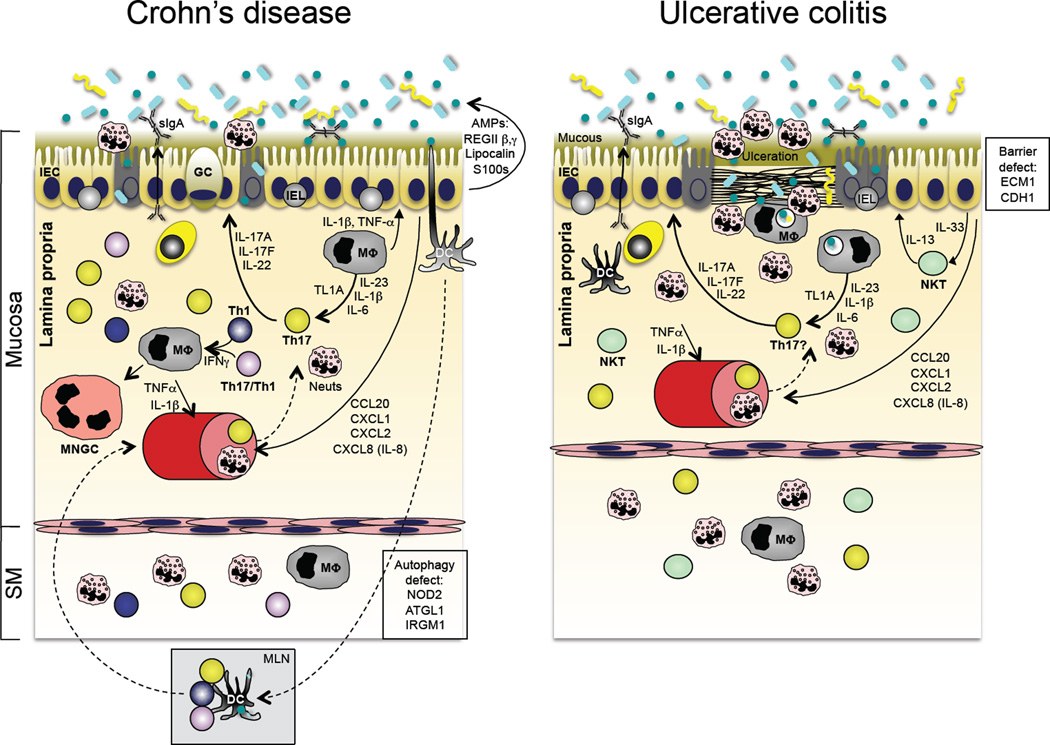
Figure 4.
Immunopathogenesis of inflammatory bowel disease (IBD). The two major forms of IBD, Crohn’s disease (CD; left panel) and ulcerative colitis (UC; right panel), have distinct patterns of tissue involvement and immunopathogenic bases. However, recent GWAS data have identified shared Th17 pathway attributes in both (see Table I), and both share abnormal responses to the enteric microbiota. Although disease pathogenesis is complex and there appear to be different immune abnormalities in individual patients, CD is associated with defects in autophagy (an evolutionarily conserved mechanism to deliver intracellular microbes or cytoplasmic organelles to the lysosomal compartment), bacterial sensing and excessive Th17 pathway activation. UC, on the other hand, has been associated with excessive IL-13, thought to be produced by invariant NKT cells, as well as defects in epithelial barrier integrity and excessive Th17 pathway activation.
Table 1.
Immune gene variants linked to inflammatory diseases of the GI tract, respiratory tract, and skin
| Gene/locus | Encoded protein | Disease associations |
Immune Function/Pathway |
|---|---|---|---|
| IL23R | IL-23R (CD | CD, UC, psoriasis | ILC activation; Th17 differentiation |
| IL12B | IL-12p40 | CD, UC, psoriasis | Common subunit of IL-12/IL-23; Th1 & Th17 |
| IL23A | IL-12p19 | Psoriasis | Th17 differentiation |
| IL27 | IL-27 | CD | Development of IL-10-producing T cells; Th1 |
| IL10 | IL-10 | CD, UC | Anti-inflammatory immune modulator |
| IL10RA | IL-10R1 (CD210a) | CD | IL-10-specific receptor component |
| IL10RB | IL-10R2 (CD210b) | CD | Shared receptor for IL-10, IL-22 and IL-26 |
| IL2/IL21 | IL-2/IL-21 (?) | UC | T cell development; Treg vs Th17 balance |
| TSLP | TSLP | asthma | Innate cell derived inducer of Th2 |
| IL2RA | IL-2Rα (CD25) | CD | High affinity IL-2R component; Tregs |
| IL2RB | IL-2Rβ (CD122) | asthma | Shared subunit of IL-2 and IL-15 receptors |
| IFNG/IL26 | IFNγ/IL-26 (?) | UC | IFNγ or IL-26 expression |
| TNFSF11 | RANKL | CD | DC survival/function |
| TNFSF15 | TL1A | CD, UC | Augments IFNγ and IL-17 production |
| ICOSLG | ICOS ligand | CD, UC | Costimulatory molecule; T cell development |
| IL1R1 | IL-1Rα (CD121a) | asthma | Receptor component for IL-1α/β signaling; Th17 |
| IL1R2 | IL-1R2 (CD121b) | CD, UC | Decoy receptor for IL-1α/β |
| IL18R1 | IL-18R (CD218a) | asthma | Receptor component for IL-18 signaling; Th1 |
| IL18RAP | IL-18Rap (CD218b) | CD | Co-receptor for IL-18 signaling; Th1 |
| Il1RL1 | ST2/IL-33R | Asthma, AD | Receptor for IL-33; Th2 |
| IL33 | IL-33 | UC, asthma, AD | Innate cell derived inducer of Th2 |
| CCR6 | CCR6R (CD196) | CD | Chemokine receptor for CCL20; Th17 |
| CCL2/CCL7 | MCP-1/MCP-3 | CD | Chemokine ligands for CCR2; monocytes |
| IL8RA/IL8RB | CXCR2 (CD182) | UC | Chemokine receptor for CCL8/IL-8; neutrophils |
| STAT3 | STAT3 | CD, UC | Signal transduction/transcription factor; Th17 |
| JAK2 | JAK2 | CD, UC | STAT signaling kinase; multiple pathways |
| TYK2 | TYK2 | CD, UC | STAT signaling kinase; multiple pathways |
| SMAD3 | SMAD3 | CD, UC, asthma | TGFβ receptor signaling; multiple pathways |
| PTPN2 | T-cell PTP | CD, UC | T cell signaling |
| PTPN22 | Lymphoid-specific PTP | CD, UC, psoriasis | T cell signaling |
| REL | C-Rel | CD, UC | NF-κB component; inflammatory signaling |
| PRDM1 | BLIMP1 | CD, UC | Antagonizes Bcl-6; promotes T effector develop. |
| ERAP1 | ER aminopeptidase 1 | Psoriasis | MHC class I antigen processing |
| ERAP2 | ER aminopeptidase 1 | CD | MHC class I antigen processing |
| CARD9 | CARD9 | CD, UC | Adaptor protein for NF-κB signaling |
| NOD2 | NOD2/CARD15 | CD | Bacterial sensing; autophagy |
| IRGM | IRGM | CD | Autophagy |
| ATG16L1 | ATG16L | CD | Autophagy |
| ORMDL3 | ORMDL3 | CD, UC, asthma | Linked to increased IL-17; mech. ? |
| MUC1 | Mucin 1 | CD | Epithelial mucin/barrier function |
| ECM1 | ECM1 | UC | Extracellular matrix; epithelial barrier integrity |
| CDH1 | E-cadherin (CD324) | UC | Epithelial barrier integrity, intestines |
| LCE3B/3C/3D | Late cornified env.3B/C/D | Psoriasis | Epidermal differentiation, skin |
| FLG | Fillagrin | AD | Epithelial barrier integrity-skinn |
Abbreviations: CD, Crohn’s disease; UC, ulcerative colitis; AD, Atopic dermatitis
IL-23 can support both Th17 and Th1 responses in humans (62). Monoclonal antibodies to the common subunit of IL-23 and IL-12, IL-12/IL-23p40, have been effective in clinical trials of Crohn’s disease, although it is unknown whether blockade of IL-23, IL-12 or both is responsible for treatment efficacy (63; 64). Clinical trials specifically targeting IL-23 are currently underway and should be telling. Interestingly, a monoclonal anti-IL-17A was tried in patients with CD but was found to be ineffective (49). Given the supporting data for a role for Th17 in Crohn’s disease, the reasons for this failure are unclear and will require further study.
Ulcerative colitis and the Th17 pathway
Ulcerative colitis (UC) is distinguished from Crohn’s disease by involvement limited to the colon, with contiguous extension of disease proximally from the rectum. Also in contrast with CD, inflammation typically not transmural, rather it is limited to the mucosa and submucosa, and includes prominent ulceration without granulomas. As in Crohn’s disease, pathogenetic mechanisms that result in UC are incompletely understood. A major working hypothesis is that pathogenesis it is mediated by dysregulated production of IL-13 (49) (Figure 4). Support for this concept comes from the oxazolone colitis model in mice that is mediated by IL-13 produced by CD1d-restricted, invariant NKT cells (49). Indeed, IL-13 production has been found in lamina propria CD4+ T cells isolated from the colons of ulcerative colitis patients (65). IL-13 has been shown to be toxic for intestinal epithelial cells in vitro (66). Because IFNβ can down-regulate IL-13, a small trial of IFNβ therapy in patients with ulcerative colitis was undertaken. Whereas patients not responding to IFNβ therapy were found to have elevated mucosal IL-17 and IL-6, patients who did respond to IFNβ therapy demonstrated decreased IL-13 expression in the colon mucosa suggesting that, at least in this subset of patients, IL-13 might be linked to pathogenesis.
In addition to data implicating IL-13 in UC pathogenesis, GWAS have linked IL23R and Th17 pathway and related immune-mediated disease genes to ulcerative colitis, including several also linked to CD: Jak2, Tyk2, STAT3, ICOSLG, IL10, and TNFSF15 (60) (Table 1). Consistent with these genetic studies, increased IL-17 and IL-22 have been found in UC lesions, although less so than in CD lesions (50; 52). This might imply that both Th17 and IL-13-dominated responses are occurring to varying proportions in different individuals with ulcerative colitis, or that disease in different patients has a distinct pathogenetic basis. This will require further investigation, as will interactions among IL-13, IL-23, and IL-17. Notably, GWAS have also identified variants of genes encoding constituents of the colonic epithelial barrier as susceptibility traits, suggesting a role for impaired barrier function as a contributor to UC pathogenesis in at least some patients (60; 67) (Table 1).
IBD and cancer
Chronic inflammatory bowel disease, particularly ulcerative colitis, is known to predispose to the development of colon cancer. The extent of involvement of the colon and the duration of disease are major risk factors in individuals with ulcerative colitis. Such patients are frequently screened for dysplasia as a harbinger of cancer development. A number of mouse strains with experimental colitis also develop colon cancer, including IL-10-deficient and Gαi2-deficient mice. There is great interest in the potential role of the microbiota in the development of colon cancer in general, as well as in the setting of IBD. A recent study in mice highlights this potential interaction. Chronic Th17-mediated colitis can be induced in C57Bl/6 mice by colonization with an enterotoxigenic B. fragilis, a strain isolated from the intestinal microbiota of humans. The enterotoxin cleaves epithelial cell E-cadherin, an adhesion molecule important to the epithelial barrier (68; 69). This increases STAT3 in enterocytes, a transcription factor important in epithelial restitution and enterocyte survival. However, overexpression of STAT3 in enterocytes can lead to epithelial cell cancer (70; 71). Consistent with the prolonged increase in STAT3 expression in enterocytes due to the enterotoxigenic B. fragilis, colitic Min mice developed colonic tumors (72). Blocking the inflammation vastly reduced the incidence of cancers in this system. Enterotoxigenic B. fragilis is present in the feces of up to 20% of normal humans, raising the question of whether this organism or some other components of the microbiota may be contributing to the high risk for cancer in patients with IBD.
THE TH17 PATHWAY IN LUNG DISEASE
CD4+ T cells have long been known to play important roles in many lung diseases. Their critical role in pulmonary mucosal immunity in humans was exemplified by the human immunodeficiency virus (HIV) epidemic. The acquired immunodeficiency syndrome (AIDS) that results from CD4 T cell depletion in HIV infection causes increased susceptibility to a number of opportunistic pulmonary infections, including Pneumocystis jiroveci and bacteremic S. pneumoniae. Defective control of chronic infections by CD4 T cells was also exposed by the AIDS epidemic, as shown by the prevalence of reactivation tuberculosis and defective control of herpesviruses, including HHV8, which causes pulmonary Kaposi’s sarcoma.
Before the discovery of Th17 cells, it was thought that the heightened susceptibility to infections such as Pneumocystis in AIDS must be due to defective Th1 or Th2 responses. However, mice deficient in Th1 and Th2 immunity could still clear Pneumocystis infection, whereas mice deficient for CD4 T cells were susceptible (73–76). It is now clear that a number of pulmonary infections previously thought to be controlled by Th1 cells are in fact dependent on Th17 cells. As is the case for other barrier sites discussed in this review, receptors for each of the key effector cytokines produced by Th17 cells, including IL-17A, IL-17F, IL-21, GM-CSF and IL-22, are expressed in lung tissue. Accordingly, each of these cytokines can mediate both protective and pathological responses in the lung.
The receptor for IL-17A and IL-17F, which is composed of the IL-17RA and IL-17RC subunits, is expressed on the basolateral surface of human lung epithelium (77) (78), and these ligands induce the expression of CXCL1. CXCL2, CXCL8, and G-CSF in primary polarized human lung epithelial cells (77) (78). In addition, IL-17A leads to the expression of antimicrobial peptide, human β-defensin 2 (HBD-2) (79). IL-17A also increases apical bicarbonate expression (80), which may optimize the antimicrobial environment in the apical surface liquid where HBD-2 mediates its antimicrobial activity, as HBD-2 has optimal killing in the presence of bicarbonate anion (81) (Figure 1). IL- 17RA is also expressed on lung fibroblasts (82), pulmonary endothelial cells, and airway smooth muscle cells (83) and signaling through this receptor can induce chemokines as well as IL-6. Recently it has been shown that IL-17 can also induce airway smooth muscle contraction that could explain in part the ability of IL-17 to mediate airways hyperresponsiveness to methacholine {Sheppard Nat Med, in press}.
Human bronchial epithelial cells also express IL-22R1 and IL-10R2, components of the hetrodimeric receptor necessary for IL-22 signaling, and IL-22 activates STAT3 signaling in these cells (84). The IL-22 receptor complex is enriched in basal cells that also co-express aquaporin 3 (85), and IL-22 increases the clonogenic potential of these cells in vitro (84). IL-22 also accelerates the recovery of trans-epithelial resistance in injured human bronchial epithelial cells, indicating its role in epithelial repair following injury (84). In support of this concept, it has been shown that recombinant IL-22 can reduce extravasation of serum proteins in to the lung in a model of ventilator induced lung injury (86).
Role of Th17 Cytokines in Infection
Mice deficient in IL-17RA, the common receptor component thought to be shared by all members of the IL-17 receptor family (87), control Th1-dependent infections such as Mycobacteria tuberculosis (84) but succumb to several extracelluar pathogens including Klebsiella pneumoniae (88) IL17RA-deficient mice fail to induce G-CSF and have a defective granulopoietic response to pulmonary K. pneumoniae infection. Moreover these mice show reduced emigration of neutrophils from the vascular space into the alveolar space, which is associated with bacterial dissemination (88). Blockade of IL-22 is also associated with enhanced lethality in this model (84) and associated with very high rates of bacterial dissemination from the lung. In addition to potentially controlling barrier function of the epithelium, IL-22 can also increase the expression of a number of anti-microbial and anti-apoptotic genes such as lipocalin-2, which contributes to mucosal immunity in the lung (84). In this model much of the early IL-17 and IL-22 derives from γδ T-cells (89) as has also been reported for E. coli as well (90). The γδ T-cell production of IL-17 and IL-22 requires IL-23 that can be produced by both alveolar macrophages as well as lung dendritic cells (84). Other potential sources of IL-17 and IL-22 include NKT cells as well as innate lymphoid cells (ILCs). Although neutrophils and macrophages have also been reported to produce IL-17 transcripts or protein by intracellular cytokine staining (91; 92), the recent development of various IL-17 reporter mice suggests that these are minor sources of IL-17 in the lung (unpublished observations).
Although there is a consensus that Th17 effector cytokines are major players in mediating host defense against extracelluar pathogens, several intracellular pathogens have been shown to require IL-17 including Mycoplasma pneumoniae (93) and Franciscella tularensis. The latter pathogen is a poor inducer of IL-12p40 and requires IL-17 production to induce IL-12p70 from lung macrophages and dendritic cells to prime normal Th1 immunity (94). Thus, in this setting, IL-17 controls the production of IFNγ that is the ultimate effector that controls the intracellular growth of this pathogen. Moreover, augmenting Th17 responses thru vaccination can augment the local expression of CXCR3 ligands necessary for enhanced Th1 recruitment and ultimately vaccine induced immunity against intracellular pathogens such as M. tuberculosis. Further, vaccine induced immunity against diverse groups of microbes such as S. pneumoniae (95), North American endemic mycoses (96), and K. pneumoniae (89) require Th17 cells and/or IL-17. In the latter setting, Th17 cells undergo expansion and contraction in hilar lymph nodes and lung parenchyma and show MHC class II restricted proliferative responses to outer membrane proteins in the cell wall of the bacteria (89). As these proteins are conserved across this genus of bacteria, this Th17 response can provide heterologous immunity to several different capsular serotypes of the bacteria. Thus, using these types of conserved protein sequences as T-cell dependent vaccines may provide broader immunity than using polysaccharide antigens that target humoral immunity.
ROLE OF TH17/TH22 CYTOKINES IN INFLAMMATORY LUNG DISEASES
Although there is much evidence that Th17 effector cytokines can play protective roles against infectious agents in the lung, there is growing evidence that this pathway can also result in lung pathology. This may result from dysregulation of a normal immune response, an autoimmune response or through the development of structural lung disease that could potentially interfere with normal mucosal immunity. Regarding the latter, many chronic lung diseases, including chronic asthma and chronic obstructive pulmonary disease (COPD), are associated with an abnormal lung microbiome that could exacerbate Th17 inflammation.
Autoimmune and Fibrotic Lung Diseases
Because of its large vascular supply and high exposure to inhaled environmental agents, the lung is an organ that is often involved by chronic immune inflammation, whether as a component of systemic or lung-specific inflammatory disease. Pulmonary vasculitis and capillaritis can occur as complications of systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) (97) (98). Interstitial fibrosis is a complication of a number of systemic autoimmune diseases, including RA and scleroderma (systemic sclerosis). RA can also be associated with pleural effusion, pulmonary nodulosis, and alveolar hemorrhage. Whether these pulmonary complications are associated with Th17 effector cytokine signaling remains to be determined. It has been shown that the IL-17:IL-17R signaling is required for the formation of ectopic lymphoid follicles in the lung (99) and thus there is at least preclinical evidence that the IL-17 signaling pathway may be involved in some pulmonary complications of RA. Of note it has been reported that RA patients treated with certain anti-TNF therapies can be associated with granulomatous lung disease, thought to be of non-infectious etiology (100).
Interstitial fibrosis can result from a large variety of pulmonary insults, including chronic hypersensitivity pneumonitis (CHP), sarcoidosis, and certain drugs (e.g., bleomycin, used in cancer chemotherapy), or can occur in an idiopathic form (idiopathic pulmonary fibrosis, or IPF). In a mouse model of CHP induced by repeated exposure to the Gram-positive bacterium, Saccharopolyspora rectivirgula, a cause of so-called “farmer’s lung,” increased Th17 cells were found in the lung and the development of peribronchiolar inflammation and fibrosis were attenuated in the absence of IL-17RA signaling (101). Among IL-17R ligands, this response appears to be mediated by IL-17A as similar results were observed in IL-17A-deficient mice (102). IL-17 appears to be a double-edged sword in this disease as well, as it is also essential for clearance of some bacteria that mediate CHP, such as Bacillus subtilis (103). This may also be relevant in diseases such as cystic fibrosis, in which infection persists despite elevated IL-17 in sputum and increased numbers of Th17 cells in lung (77; 104; 105). Because a major effect of IL-17 the regulation of ion transport in the airways (80), this Th17 response may be ineffective at eradicating infection and resulting in end-organ damage.
Recently, IL-17A has also been implicated in interstitial pulmonary fibrosis caused by bleomycin or the particulate, silica (106). Moreover both IL-17A and IL-1β were elevated in the bronchoalveolar lavage fluid of patients with idioathic pulmonary fibrosis compared to controls. An IL-17 gene signature has also been recently reported in lung fibroblasts from IPF patients and patients with idiopathic pulmonary hypertension (107). Sarcoidosis is a granulomatous lung disease that can also lead to the development of interstitial fibrosis and is characterized by elevated CD4 T-cells in lung lavage fluid. Although classically thought of as a Th1 disease, IL-17 producing cells have also been found to be elevated in peripheral blood as well as in lung lavage fluid of these patients (108). Moreover both single IL-17A+ and double IL-17/ IFNγ+ cells have been reported in sarcoid granulomatous tissue (109). Given the potential dual nature of Th17 and Th1 immunity in sarcoidosis, a clinical trial has been initiated to test an anti-IL-12p40 monoclonal antibody for possible efficacy in this disease (NCT00955279).
Although not autoimmune per se, there is growing evidence supporting a role for the Th17 pathway in transplantation-associated inflammatory disease of the lung. A common complication of hematopoietic stem cell (HSC), or bone marrow, transplantation is graft versus host disease. Although GVHD was originally thought to be Th1-mediated, recent studies have demonstrated that the transplantation or transfer of allogeneic cells deficient for IFNγ or T-bet result in exacerbated disease in some mouse models of GVHD (110; 111). Further, adoptive transfer of allogeneic Th17 cells induced lethal GVHD with prominent involvement of the lung and skin (110). Interestingly although cutaneous manifestations of GVHD could be blocked with anti-IL-17A, anti-TNFα was more effective at blocking weight loss and overall GVHD organ pathology (110). This differential pathology may be due to homing receptors and the role of Th2 cells in mediating some aspects of the lung pathology (112).
Obliterative bronchiolitis (or bronchiolitis obliterans), a fibrosing destruction of the small airways, is the most serious, life-threatening complication of lung transplantation and is thought to be due to T-cell mediated allograft injury. Recently, two independent groups have shown that neutralization of IL-23 or IL-17 can prevent allograft fibrosis, suggesting Th17 involvement. Anti-IL-23 prevented large airway fibrosis in a tracheal allograft model (113), and anti-IL-17 or an IL-17R:Fc chimera was shown to prevent obliterative bronchiolitis in a minor MHC mismatch lung allograft transplant model (114).
Asthma
Asthma is a syndrome of recurrent wheezing and shortness of breath characterized by airways inflammation, increased mucous production and reversible airflow obstruction that is typically caused by immune hypersensitivity to environmental antigens (115; 116). Although asthma has largely been thought to be a disease mediated by Th2 cells, up to 50% of patients are non-atopic. Moreover some patients with severe asthma and patients in status asthmaticus are found to have neutrophilic airways inflammation as opposed to Th2-associated eosinophilic inflammation (117) (Figure 5). As IL-17 can mediate neutrophilic inflammation in the lung, there has been intense interest in the possibility that a Th17-based immune pathology might explain some aspects of the heterogeneity found in this disease. IL-17A has been shown to be elevated in the sputum of patients with severe asthma and the levels correlated with neutrophil chemokines such as IL-8 as well as neutrophil numbers (118). Increased production of IL-17F has also been reported in stimulated PBMCs and BAL fluid from asthmatics compared to controls (119). In this regard, mice with a genetic deletion in IL-17F have reduced antigen-induced neutrophilia in the lungs (37).
Figure 5.
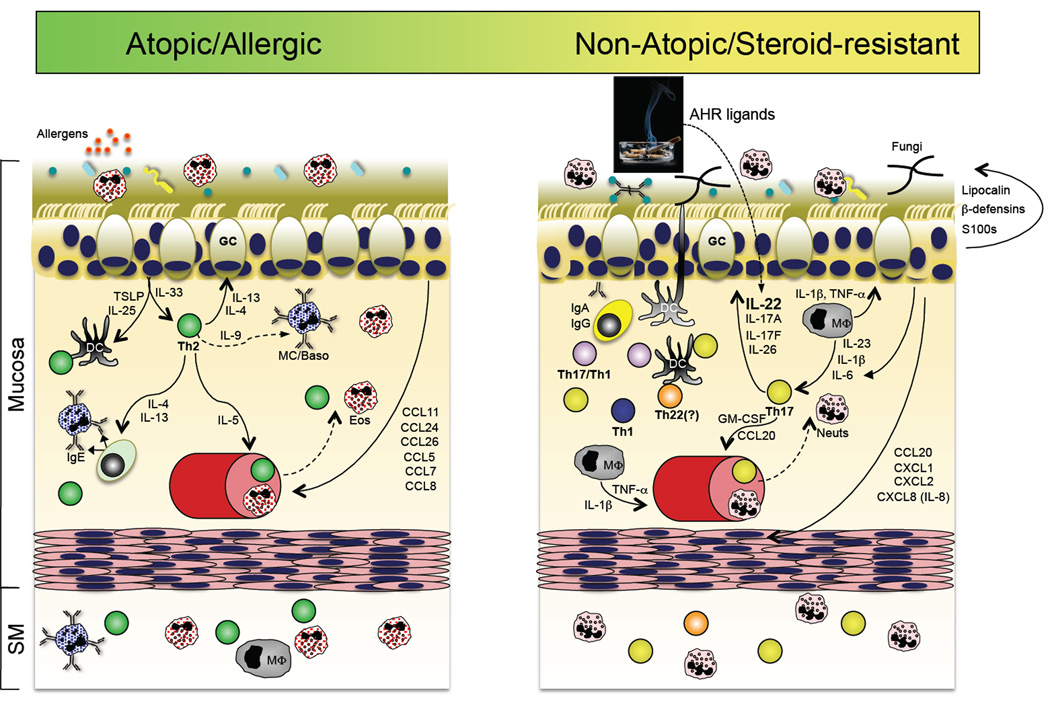
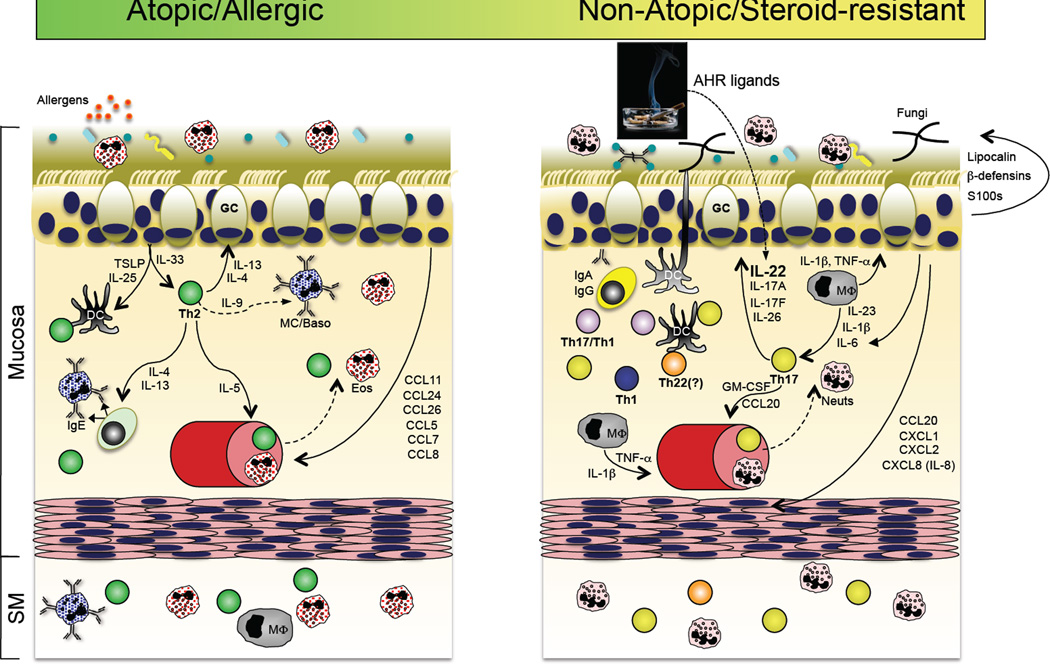
A role for the Th17 pathway in a subset of asthma patients? Accumulating evidence suggests that in addition to the well-accepted Th2 pathway of asthma pathogenesis (left panel), a subset of patients with steroid-refractory disease might have a prominent contribution by Th17 pathway inflammation. In the former, sensitization to respiratory allergens primes a dysregulated Th2 response, perhaps via dysregulated TSLP and IL-33 production by the repiratory epithelium, resulting in the production of IgE-producing antibodies that arm tissue resident or infiltrating mast cells and basophils for hypersensitivity responses to allergen re-exposure. In addition to actions on B cells, the Th2 cytokines IL-4 and IL-13 act on respiratory epithelial cells to stimulate increased mucous production. IL-5 produced by Th2 cells stimulates the increased production and recruitment of eosinophils, which are a principal component of bronchoalveolar lavage (BAL) fluid in patients with active disease. In a subset of patients with refractory disease, Th17 pathway features are found, including elevated neutrophils and IL-17 in BAL. It is therefore possible that many of the Th17 pathway responses stimulated in response to respiratory bacterial or fungal infection (right panel) could be dysregulated in this subset of asthma patients, thereby contributing to disease pathogenesis (reviewed in ref. (217)).
Genetic studies on the role of IL-17F have been conflicting. Although many SNPS were identified (including several non-synonymous ones) in one study, none were associated with physician-diagnosed asthma (120). In a cohort from Japan, homozygosity for a H161R variant in IL17F was inversely associated with asthma (121). However, to date these data have not been replicated. It was also hypothesized that this variant may encode a loss of function however expression studies have shown that this variant of IL-17F can induce chemokine expression in lung epithelial cells (122).
Mice deficient in IL-17RA show reduced airways inflammation in allergen-induced asthma (123), however IL-17RA is also a receptor for other IL-17 cytokine family members such as IL-25 that can regulate Th2 responses and thus this approach does not address whether IL-17 or TH17 cells per se are required for this phenotype. In contrast, neutralization of IL-17 in a Th2 biased model induced by immunization with alum actually exacerbated airways inflammation and eosinophilia (123). Using a Koch’s postulate approach to determine if Th17 could mediate asthma in the mouse, McKinley adoptively transferred in vitro polarized ovalbumin-specific Th17 cells into mice followed by airway challenge with ovalbumin. Adoptive transfer of Th2 and Th17 cells mediated increases in airways hyperresponsiveness to methacholine (124). However the airway inflammation was predominantly neutrophilic in the Th17 transfer model as opposed to eosinophilic with the transfer of Th2 cells. Interestingly the production of effector cytokines in vitro by Th2 cells as well as airways inflammation and bronchial hyperresponsiveness were steroid sensitive. In contrast, the production of effector cytokines in vitro by Th17 cells, airways inflammation, and bronchial hyperresponsiveness were steroid resistant (124).
It has also been recently shown that mouse strain differences in response to house dust mite antigen that subsequently dictate the severity of bronchospasm to methacholine is in part determined by the degree of complement activation and IL-17 (125). Furthermore ozone, which can also exacerbate pre-existing asthma, mediates bronchial hyperresponsiveness through IL-17 production by invariant NKT cells (126). The precise target cells of IL-17 signaling in these models remains to be determined. Based on these pre-clinical data there are ongoing clinical trials targeting the IL-17R pathway in asthma. Further genetic evidence of a potential role of the Th17 pathway is emerging as well. Recently there has been a significant association with a SNP in IL-1R1 as well as in RORA (127). These have also been replicated in a recent metaanalysis (128).
Chronic Obstructive Pulmonary Disease (COPD)
COPD encompasses the respiratory disorders chronic bronchitis and emphysema, with individual patients manifesting variable contributions of these two disorders. Disease is caused by long-term exposure to toxic inhalants, with cigarette smoking by far the leading contributor. In contrast to asthma, wherein airway obstruction is episodic, airway inflammation and obstruction (chronic bronchitis component) and destruction of alveoli (emphysematous component) are progressive in COPD, and COPD is the third leading cause of death in the US. Variable susceptibility and familial clustering of disease in patients with similar smoking histories indicates a genetic component.
While GWA studies have begun to implicate a number of immune function genes in COPD, to date most of these studies have not been adequately powered to definitively identify SNPs that confer increased susceptibility or resistance. However, due to the fact that many patients with COPD have colonization with non-typeable Hemophilus influenza and neutrophilic airway inflammation and mucous hypersecretion, it has been hypothesized that IL-17 could play a role in COPD (129; 130). In this regard, polymorphisms in IL-27, which can negatively regulate IL-17 responses, have been associated with a reduced risk of COPD in a Chinese cohort (131), and severe COPD is characterized by ectopic lymphoid follicles (132; 133), which in the mouse requires IL- 17RA signaling (99). Compared to wild-type mice, IL-17RA-deficient mice failed to develop emphysema after six months of cigarette smoke exposure, and mice that overexpress IL-17 develop more severe emphysema upon exposure to cigarette smoke (134). Moreover, it was recently shown that the number of IL-22 and IL-23 expressing cells is increased in the lung epithelium of patients with stable COPD, and the number of IL-17A and IL-22 positive cells was also increased (135). Interestingly, smoking alone was associated with an increase in the number of IL-22 positive cells, and it has been shown that cigarette smoke extract can augment IL-22 expression in Th17 cells in part through the aryl-hydrocarbon receptor (AHR) (134). Finally, the progression of disease in patients that have quit smoking has suggested a possible autoimmune component. Although not yet well characterized, a subset of COPD patients develop anti-elastin antibodies (136) as well as increase in TH17 responses (137). Collectively, these studies suggest that the Th17 pathway may represent a therapeutic target in COPD.
Lung Cancer
Given the close association of COPD and the development of non-small cell lung cancer (NSCLC) the role of the Th17 pathway in lung cancer has been recently explored. In one study, increases in IL-17 positive cells were identified in 25 of 52 lung cancer specimens and the presence of these cells was associated with poorer survival (138). IL-17 has been shown to increase angiogenesis in pre-clinical models of NSCLC (139). CCL20, a chemokine regulated by IL-17 that serves to recruit CCR6 positive cells, including Th17 cells, may also promote NSCLC progression (140). IL-22 has been shown to have anti-apoptotic activity in NSLC and may also promote tumor progression and/or survival (141). Taken together, there is mounting evidence of a potential Th17 pathway that is shared between COPD and lung cancer, which raises the issue of targeting Th17 cells or their effector cytokines for treatment or chemoprophylaxis of NSCLC (Figure 2).
THE TH17 PATHWAY IN SKIN DISEASE
In contrast to the intestinal and respiratory tracts, the skin is a non-mucosal barrier that develops from ectoderm, rather than endoderm. And while it is much smaller in total surface area than these mucosal tissues, it is the largest organ of the body. Although the skin contributes to the generation of essential metabolites, including active vitamin D, and plays a major role in regulating body temperature, its main function is protective. The tissue constituents of the skin have evolved to protect against a variety of physical, radiant, chemical and microbial threats. The skin consists of the outer epidermis and inner dermis, which are in many ways analogous in function to the respective epithelium and lamina propria of mucosal tissues of the intestinal and respiratory tracts. However, unlike the single-cell epithelium of the intestines or pseudostratified epithelium of the respiratory tract, which are invested by a layer of mucous that provides a physical barrier to many environmental threats, the epithelium of the skin lacks mucous. Rather, continuously proliferating keratinocytes within the basal epidermis differentiate as they mature towards the skin surface to create an outer, densely keratinized and cross-linked matrix of anucleated dead squamous cells, the stratum corneum, which prevents fluid loss and acts as a relatively impenetrable barrier. The underlying dermal tissue consists of fibroblasts within a connective matrix that supports hair follicles, glands, and nerve endings and a rich supply of lymphatic and blood vessels. This vascularization enables the extravasation of circulating immune cells to mostly the dermis but also the epidermis (142–144). In particular, the skin is home to a large number of resident T cells that respond to antigens presented by Langerhans cells (LC) in the epidermis and distinct types of dendritic cells in the dermis (145). As a result of its relative accessibility, much of our knowledge about Th17, and particularly Th22, cells in human solid tissue is derived from study of the skin. Most notably, the discovery and study of Th17 cells and associated cytokines has revolutionized our mechanistic understanding of psoriatic immunopathology (146; 147).
As at mucosal barriers, the constant exposure of the skin to the external environment, including a resident commensal microbiota, requires a constant dialog between the epithelium and immune cells to maintain homeostasis. Keratinocytes, the epithelial constituents of the epidermis, have considerable immunological and inflammatory functions, acting together with monocyte-derived intraepidermal Langerhans cells as first-line sentinels that respond to environmental/microbial agents that penetrate the superficial epidermis (144) (Figure 1). Keratinocytes express pattern-recognition and NOD-like receptors (PRR and NLR) for both pathogen-associated molecular pattern (PAMP) and damage-associated molecular pattern (DAMP) ligands that are detected or produced in the context of infectious or non-infectious threats, respectively (148–150). The type of assault dictates the quality of these ligands (151), which in turn, stimulate production of cytokines and chemokines that initiate and coordinate the activation and recruitment innate immune cells and promote the development of distinct subsets of CD4+ T cells that coordinate both the innate and adaptive immune response (152).
Among the cytokines produced by keratinocytes in response to inflammatory stimuli, those normally provoked by extracellular bacteria and fungi that breach the outer epithelial barrier promote the development of pathogen-specific CD4 T cells of the Th17 pathway, including both classical Th17 cells and Th22 cells. Keratinocytes activated through the appropriate PRRs produce abundant IL-1β, IL-6 and other cytokines (153– 155) that amplify production of IL-23 by intraepidermal Langerhans cells and dermalresident dendritic cells and promote their migration to regional lymph nodes where they prime CD4 T cell development (156–160). Th17 and Th22 cells that develop in response to antigens presented by activated dendritic cells from skin express homing receptors that promote their trafficking to sites of inflammation in the skin. Specifically, skin-homing Th17 and Th22 cells are CCR6+CCR4+CCR10+CLA+, which directs their E selectin-dependent extravasation mediated by cutaneous lymphocyte antigen (CLA) and migration along gradients of the chemokines CCL20, CCL22, and CCL27 (161–165). In addition, the binding of environmental small molecules by the AHR transcription factor expressed by Th17 and Th22 cells induces IL-22 expression and is a further synergistic signaling pathway towards the generation of this subset’s specific inflammatory responses in the immediate vicinity of the insult (166). In fact, chronic and systemic human exposure to dioxin, a toxic AHR ligand product and by-product of manufacturing processes, appears to be associated with a detectable skewing of circulating T cells to the Th22 lineage (167).
As in the gut and lungs, Th17 (and Th22) cells that home to skin produce cytokines that signal back to the tissue-resident epithelial cells, keratinocytes, to influence their barrier-protective properties. The receptors for IL-17A and IL-17F (IL-17RA/C), IL-22 and TNFα are expressed by keratinocytes, and these ligands each have unique and synergistic actions on keratinocytes to induce production of a variety of cytokines (e.g., IL-32, IL-36’s,), chemokines (e.g. CXCL1, CCL20) and antimicrobial peptides (i.e., β-defensin 2, S100A7/psoriacin and other S100 family peptides) (168–173). While antimicrobial peptides (AMPs) can be directly bactericidal, these molecules can also further amplify local inflammatory responses through their actions on immune cells (174; 175). In addition, Th17 and Th22-derived cytokines are able to contribute directly (i.e., IL-22) or indirectly (i.e., IL-17A and IL-22’s induction of IL-20 and IL-24 from keratinocytes) to enhance repair of the injured epidermis by inducing the proliferation of keratinocytes (176; 177). Thus, the crosstalk between skin-resident keratinocytes and dendritic cells and the Th17 and Th22 effector cells whose development and recruitment they promote facilitates both immune and non-immune functions that drive cutaneous inflammation. With appropriate feedback regulation of, and subsequent recovery from, these inflammatory responses –typically commensurate with the clearance of foreign antigens, the skin is returned to physiologic homeostasis.
Psoriasis
Psoriasis is a chronic inflammatory disease that exemplifies a dysregulated immune response in the skin (146; 147). Psoriatic lesions are characterized by rapid hyperproliferation and impaired differentiation of keratinocytes that results in considerable thickening (acanthosis) and scaling (hyperkeratosis/parakeratosis) of the epidermis. The epidermal abnormalities are associated with an influx or expansion of immune cells, with increased of CD4+T cells in the involved dermis and infiltration of CD8+ T cells into the involved epidermis, consistent with an autoimmune etiology. Antecedent to discovery of the Th17 pathway, psoriasis was proposed to be a Th1/Tc1 autoimmune disease: a dysregulated T cell response involving IFNγ-producing CD4+ (Th1) and CD8+ (Tc1) cells. This was based on the identification of these cells in direct isolates and cloned lines derived from lesional biopsies, and detection of increased transcripts for IFNγ in psoriatic lesions (178; 179).
The efficacy of neutralizing antibodies that target human IL-12 in treating psoriasis was initially interpreted as confirmatory of the Th1 basis for psoriatic autoimmunity (180; 181). Ustekinumab, an anti-IL-12p40 monoclonal antibody, was originally chosen for its high affinity and potency for blocking the activity of IL-12. It was conceived as an anti- Th1 therapy for the treatment of psoriasis and has been proven very effective for both induction and maintenance therapy (182; 183). However, the subsequent discovery that the IL-12p40 subunit is shared between IL-12 and IL-23 has raised the possibility that part, or all, of the clinical efficacy of this biologic might be due to its targeting of the IL-23/IL-17 axis, and the Th17 pathway. Indeed, a preponderance of mechanistic and clinical data collected since discovery of the Th17 pathway suggests that psoriasis is primarily driven by the IL-23/IL-17/Th17 axis rather than the classical IL-12/IFNγ/Th1 axis (146). Thus, the instrumental, yet ultimately serendipitous and fortuitous, early design and clinical development of a high affinity anti-IL-12(IL-23)p40 neutralizing antibody, prior to the discovery of the IL-23(p19p40) cytokine, has created a transformative therapeutic for the treatment of psoriasis.
The significance of the IL-23 signaling pathway to the promotion of psoriasis pathology has been strongly reinforced by GWAS (Table 1). Psoriasis susceptibility SNPs have been identified in the IL23R, IL23A and IL12B gene loci (184; 185). Furthermore, clinical data from a phase I safety study suggests that targeting IL-17A is effective at resolving psoriatic lesions, giving additional credence to the role of Th17 cells in this disease (186). Thus, the onset of psoriasis is now proposed to be due to local, dysregulated, IL-23-dependent expansion of CD4+(Th17) and CD8+ (Tc17) αβ T cells (146; 187; 188) (Figure 6). Cytokines secreted from these cells, particularly IL- 17A, IL-22, and TNFα, induce keratinocytes to express of a variety of genes – cytokines, chemokines and AMPS – that are proposed to sustain the inflammatory cascade in psoriatic lesions. In particular, IL-17, TNFα, and IL-22 act synergistically to induce several AMPs that are thought to underlie the sterile state of psoriatic skin. A mechanism by which an AMP also contributes to sustaining an inflammatory cascade is exemplified by LL37. This AMP is able to bind to self nucleic acid such that auto-reactive activation of DCs can be induced by TLRs (189). While non-self antigens are presumed to instigate early psoriatic disease, self antigens, exemplified by nucleic acids, could sustain the ability of DCs to activate T cells independently of on-going contribution by foreign antigens.
Figure 6.
Mechanisms by which Th17 and Th22 pathways contribute to psoriasis and atopic dermatitis. Psoriasis (left panel) and atopic dermatitis (right panel) are chronic inflammatory diseases of skin with distinct immunopathogeneses. In psoriasis, hyperproliferation and impaired differentiation of keratinocytes in the epidermis is caused by an inflammatory cascade primarily mediated by the signaling of cytokines produced by cells of the Th17 pathway within the dermis. IL-17A signaling into keratinocytes, in synergy with IL-22 and TNFα and potentially IFNγ, induces the production of a variety of pro-inflammatory proteins (AMPS, chemokines and cytokines), which results in a heightened immune state and the further recruitment and activation of immune cells that maintain chronic inflammation. IL-17A and IL-22 also induce the production of IL-20 and IL-24 by keratinocytes, which, with IL-22, induce keratinocyte hyperproliferation and hyperplasia, or acanthosis, that is a hallmark of the psoriatic pathology. Atopic dermatitis (AD) is distinguished by both defects in the terminal differentiation of the epidermis and the pattern of sustained inflammation (right panel). In AD, the barrier function of the cornified layer of the epidermis can be defective, resulting in increased sensitization to environmental antigens that primes a dysregulated Th2 response. Th2 cytokines promote the generation of antigen-specific IgE and the recruitment/expansion of mast cells, basophils and eosinophils. Similar to, yet distinct from, psoriasis, a variety of antimicrobial peptides (AMPs), cytokines and chemokines are up-regulated in AD, which contribute to maintenance of the underlying inflammation. It has been recently demonstrated that Th22 (and Tc22 cells; not shown) are prevalent in AD lesions, which contribute IL-22 that induces the epidermal hyperplasia that is characteristic of AD lesions. The relative contributions of Th2 and Th22 cells to pathogenesis has yet to be well explored, and there might well be a spectrum of disease in individual patients.
In addition to inflammation, IL-17A, IL-22, and TNFα synergize to maintain the acanthosis that is a hallmark of this disease. These Th17-associated cytokines promote the expression of IL-20 and IL-24, IL-10 family cytokines that, like IL-22, are capable of inducing the hyperproliferation of keratinocytes (177; 190; 191). In summary, while data documenting the presence of increased IFNγ-producing T cells in psoriatic lesions is irrefutable, whether these cells arise from the Th17 pathway as a consequence of its considerable developmental plasticity (Th17/Th1 cells) or from a classical Th1 pathway remains to be determined, as does their contribution to disease pathogenesis. Collectively, the data from the bench and the clinic indicate that sustained Th17 cell responses are a major contributor to chronic pathology of psoriasis (146; 192).
Three anti-TNF monoclonal antibodies – etanercept, infliximab, and adalimumab – are approved for the treatment of psoriasis in the US, the development of the first two preceding that of ustekinumab (193). The established efficacy of anti-TNF therapies begs the question as to their mechanism of action. Gene expression profiling indicated that the down-modulation of ‘immediate response TNF genes’ to baseline levels occurred in the lesions of etanercept-treated patients, whether or not there was a response to therapy (194). Instead, suppression of IL-17 pathway genes occurred in responders, but was not observed in non-responders (194; 195). Nevertheless, even resolved skin lesions from patients who responded to etanercept retained residual inflammation as detected by elevated mRNA levels, despite nearly complete histopathologic resolution (196). A clinical study has shown that ustekinumab is superior to etanercept after 12 weeks of treatment, although it has not been reported whether this heightened efficacy reflects complete resolution of inflammatory mRNA transcripts (197). While the durability of clinical remissions achieved by blockade of IL-23 signaling remains to be fully assessed, it would appear that both TNFα and IL-12/IL-23p40 inhibitors will require long-term administration to restrain disease, suggesting that the IL-23/Th17 pathway is not irreversibly suppressed by these biologic agents.
Atopic dermatitis
Atopic dermatitis (AD), also referred to as atopic eczema, is another immune-mediated disease of the skin (187; 198; 199). As its name indicates, AD has an allergic component, with identifiable environmental antigens in some patients. The skin in patients with AD skin is dry and intensely pruritic (itchy), leading to scratching and, thereby, mechanical insults that exacerbate disease. The lesions contain an influx and/or expansion of dermal and epidermal immune cells, including dendritic cells, CD4+T cells, mast cells and eosinophils. Although the weight of evidence does not support a role for Th17 cells in the immunopathogenesis of AD — like asthma, it has been traditionally considered a Th2-mediated disease — new data has revealed a contribution by Th22 cells, hence its inclusion here.
A leading hypothesis in AD pathogenesis is that genetic predisposition to defective barrier function of the epidermis leads to increased exposure of the skin immune defenses to sensitizing antigens that drive abnormal, overlapping Th2 and Th22 immunity (Figure 6). There is substantial evidence supporting a genetic component in AD, although the specific genes involved are only beginning to be elucidated. Recent GWAS data older genetic linkage data that implicated loss-of-function mutations in the gene that encodes fillaggrin (FLG), which leads to altered terminal differentiation of keratinocytes that results in defective generation of the outer, cornified layer of the epidermis (stratum corneum) in the ~30% of AD patients who are affected (200; 201). The gene that encodes fillagrin resides in a cluster of genes involved in epidermal differentiation on chromosome 1 in humans. Fillagrin is expressed by keratinocytes in the granular layer of the epidermis (stratum granulosum), where it binds and condenses the keratin cytokeleton to effect cell compaction required for the generation of the squames that compose the outer epidermal barrier (200). When defective, the skin may become more permeable to environmental antigens and is less resistant to moisture loss, setting the stage for hypersensitization to allergens. As in ulcerative colitis, and perhaps even asthma, it would appear that an inherited predisposition to epithelial barrier defects might compromise the normal partitioning of environmental antigens and stimuli that activate innate, and ultimately, adaptive immunity.
More recently, GWAS data have identified associations of other genes with AD susceptibility, most notably genes that link the production of epidermal cytokines to the Th2 response, and Th2 cytokines themselves (Table 1). Specifically, AD-associated SNPs have been identified in gene loci that encode IL-33 and its specific receptor component, ST2 or IL-1RL1, as well as IL-13 and IL-4 (201; 202). IL-33 is an IL-1 family cytokine (IL-1F11) produced by epithelial cells of barrier tissues, including keratinocytes. Like IL-1 and IL-18, IL-33 is produced as a precursor form that, interestingly, can translocate to the nucleus where it can act to repress chromatin through interactions with histones (203). It is also released from dying cells that express it, acting as an alarmin following it extracellular cleavage to an active cytokine form by neutrophil-derived proteolytic enzymes (204). The cleaved form of IL-33 signals through a heterodimeric receptor composed of a specific component, ST2 (or IL-1RL1), and a common subunit, IL-1RAP, which is shared by the receptors for IL-1α, IL-1β and the IL-36 subfamily (205). The IL-33 receptor is highly expressed on cells of the Th2 pathway, including mast cells, eosinophils and Th2 cells themselves, which it costimulates to produce the Th2 cytokines, IL-4 and IL-13. Thus, newer GWAS data support a contribution of dysregulated Th2 cytokine responses to the inflammatory pathology of AD that is triggered by the activation of immune cells downstream of injury to keratinocytes exposed to mechanical, environmental, and/or infectious insults (187; 198; 199; 206). In support of a Th2 pathway link to AD pathogenesis, the majority of AD patients have high levels of IgE in the circulation with the isolation of Th2 cells from, and the detection of transcript for associated cytokines IL-13, IL-4, and IL-5 in, AD lesions. IL-23 and IL-17 transcript levels are relatively low in RNA from skin biopsies, suggesting that Th17 cells do not have a major role in this disease (207), and perhaps reflecting the potent inhibition of Th17 development by Th2 cytokines (3).
Genetic defects in the terminal differentiation of the epidermis concomitant with a Th2-mediated suppression of Th17 function might explain the chronic colonization of lesional as well as non-lesional skin of AD patients by the extracellular bacterial pathogen, Staphylcoccus aureus (199). This is in contrast to psoriasis, in which lesional skin is a sterile, attributable to the strong induction of AMP gene expression by keratinocytes as well as the chemokine-dependent recruitment of neutrophils to the hyperplastic epidermis (146; 187; 198). IL-17A is proposed to be a dominant inducer of several AMPs. The immune deficiency to S. aureus in the context of AD pathology might therefore be due, at least in part, to an insufficient production of IL-17A and, thereby, specific AMPs. In fact, a comparison of AMP transcripts or peptides in biopsies from psoriasis to AD patients indicates lower levels in the latter (208; 209), despite a prominent contribution of IL-22-prodiucing T cells in then skin of AD patients (see below).
Although IL-17 producing cells are lacking in AD lesions, T cells that express IL-22 are present and appear to contribute to the AD pathology (210). Two groups independently reported a subset of CD4+memory T cells from human peripheral blood that express IL-22 without co-expression of IL-17A or IL-4, and variable co-expression of IFNγ, so-called Th22 cells (13; 14). Th22 cells from blood of healthy donors co-expressed CCR6, CCR4, CCR10, and CLA, indicative of a skin-homing phenotype, and produced TNFα and IL-13 along with IL-22. Activation of the AHR signaling pathway favored development of Th22 lineage and induced increased production of IL-22 (14). Notably, a subsequent report identified IL-22-producing αβ T cells circulating in normal human blood that were restricted by the non-classical MHC I-like molecule, CD1a, which is highly expressed by Langerhans cells (LCs) and presents lipid antigens to αβ T cells (159; 211; 212). These T cells express the same skin-homing profile as previously reported Th22 cells, and, remarkably, a major fraction of CD1a-reactive IL-22 producers were CD4+CD8− T cells that could be activated by skin-derived LCs (159). A subset of T cells isolated from skin of healthy donors was also CD1a autoreactive and produced predominantly IL-22 (159). Further, LCs preferentially induced the allogeneic expansion of Th22 cells from human peripheral blood (213). Collectively, these observations suggest that Langerhans cells have the capacity to present lipid self antigens via CD1a to induce the development of skin-resident Th22 cells. Both Th22 and IL-22-producing CD8+ T cells (Tc22 cells) are detected at high frequency in AD lesions (170; 210), with Tc22 frequencies, in particular, strongly correlating with the severity of a patient’s disease.
It is tempting to speculate based on the above observations that Th22 and Tc22 cells contribute to AD pathogenesis (187). Compared to skin from healthy donors, nonlesional skin from AD patients has a molecular and histological profile indicative of impaired terminal differentiation of the epidermis and more immune cell infiltrates (214). In particular, and as a result of mechanical damage to the intrinsically defective AD epidermis, environmental non-self, lipid antigens presented by CD1a on the surface of the LC dendritic network within the epidermis could contribute to the development and activation of skin-resident Th22 and Tc22 cells. IL-22 and TNFα, produced by an expansion of the Th22 and Tc22 subsets and in the context of a Th2 cytokine milieu, would activate keratinocytes to produce inflammatory proteins, including cytokines, chemokines, TLRs and complement subunits (170). While AD is described as a Th2 subset-associated disease, the mechanism by which epidermal hyperplasia is mediated has not been elucidated. The presence of Th22 and Tc22 cells in this pathology could contribute the IL-22-dependant mechanism by which the acanthosis of AD is induced. Thus, the convergence of the Th2 and Th22 signaling mechanisms may define a cytokine milieu that is unique to and exacerbates the AD pathology,and will need to be further explored.
Inflammation and immunity can be instigated and maintained in geographically distinct local environments in the skin. A recent report of rare patients that present with both psoriatic and AD lesions, and distinct Th17/Th1- or Th2-dominant local T cell infiltrates, respectively, emphasizes this conclusion (215). Whereas Th17 and Th2 responses are typically counter-regulatory, perhaps explaining the rarity of patients with both psoriasis and AD, increasingly it is appreciated that T cells with convergent patterns of cytokine expression exist and might contribute to distinct patterns of tissue-specific immunopathology. Furthermore, inflammation and immune mechanisms have evolved that are tissue specific. As has been described above for mucosal tissues, IL-17A and IL-22-producing innate lymphoid cells (ILCs) appear to be critical to early host defense in this context. Yet, studies have not detected ILCs in the skin. While this may be an experimental issue, it is tempting to speculate that the large number of resident memory T cells that appear to be long-term, non-recirculating residents of the skin – twice as many T cells than are circulating – might well make ILCs unnecessary in this barrier organ (145; 216). The yang of this ‘abundant skin T cell’ yin is that these primed, albeit resting, T cell sentinels can be sensitively reactivated with the potential for Th17/Th22 signaling mechanisms to contribute to the psoriatic and AD skin-specific pathologies.
CONCLUDING REMARKS
Discoveries that laid the foundation for on-going advances in the delineation of the Th17 pathway in immune homeostasis and host protection have provided new opportunities for understanding chronic immune-mediated disease. Whereas these discoveries have largely emanated from mouse models, the tide is rapidly turning. The advent of new technologies for interrogating the genome of individuals with greater affordability will continue to identify important nodes for immune regulation and transform the approach to human immune-mediated disease initiated by GWAS studies. Because consortia coordinated for GWAS studies of IBD were organized early and have led the way, many more genes/loci have been identified for CD and UC to date, but this will no doubt change in the next few years.
It is noteworthy that the original disease models that led to discovery of Th17 were not diseases of “barrier” tissues, specifically the EAE model of MS and collagen-induced model of rheumatois arthritis (3; 4). However, new discoveries increasingly support the notion that tissue-specific autoimmunity that bears a Th17 signature may well be linked to interactions between the host and its microbiota — particularly the microbiota of the lower gut, which dwarfs that of other barrier sites in its diversity and abundance. Thus, specific components of the microbiota have been implicated in diseases as divergent as multiple sclerosis and type II diabetes (34; 35). Indeed, as the application of genomic tools to define the microbiome in individuals accelerates, it is anticipated that a wealth of new links between the host-microbiome dialog and human disease will emerge, as will new opportunities for more precisely targeting immune regulatory pathways with which to treat disease mediated by dysregulation of the Th17 pathway.
ACKNOWLEDGMENTS
The authors are grateful to members of their research groups, past and present, as well as colleagues and collaborators who have contributed insights related to this review. We offer our apologies to colleagues whose work could not be adequately cited or discussed due to space limitations. This work was supported by the NIH (C.T.W., COE. and J.K.K.) and the Crohn’s and Colitis Foundation of America, (C.T.W. and C.O.E).
Contributor Information
Casey T. Weaver, Email: cweaver@uab.edu.
Charles O. Elson, Email: coelson@uab.edu.
Lynette A. Fouser, Email: lfouser@mac.com.
Jay K. Kolls, Email: Jay.Kolls@chp.edu.
LITERATURE CITED
- 1.Coffman RL. Origins of the TH1-TH2 model: a personal perspective. Nat Immunol. 2006;7:539–541. doi: 10.1038/ni0606-539. [DOI] [PubMed] [Google Scholar]
- 2.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annual Review of Immunology. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 3.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annual Review of Immunology. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 4.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annual Review of Immunology. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 5.Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Current Opinion in Immunology. 2009;21:274–280. doi: 10.1016/j.coi.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Murphy KM, Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat Immunol. 2010;11:674–680. doi: 10.1038/ni.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kryczek I, Zhao E, Liu Y, Wang Y, Vatan L, et al. Human TH17 cells are long-lived effector memory cells. Science translational medicine. 2011;3:104ra0. doi: 10.1126/scitranslmed.3002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, et al. Th17 Cells Are Long Lived and Retain a Stem Cell-like Molecular Signature. Immunity. 2011 doi: 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, et al. Phenotypic and functional features of human Th17 cells. The Journal of experimental medicine. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 12.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annual Review of Immunology. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 13.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 14.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 15.Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. Human Th17 cells: are they different from murine Th17 cells? European journal of immunology. 2009;39:637–640. doi: 10.1002/eji.200839050. [DOI] [PubMed] [Google Scholar]
- 16.Vijay-Kumar M, Aitken JD, Gewirtz AT. Toll like receptor-5: protecting the gut from enteric microbes. Seminars in Immunopathology. 2008;30:11–21. doi: 10.1007/s00281-007-0100-5. [DOI] [PubMed] [Google Scholar]
- 17.Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, et al. Bacterial flagellin is a dominant antigen in Crohn disease. The Journal of clinical investigation. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivanov I, Atarashi K, Manel N, Brodie E, Shima T, et al. Induction of Intestinal Th17 Cells by Segmented Filamentous Bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maynard CL, Weaver CT. Intestinal effector T cells in health and disease. Immunity. 2009;31:389–400. doi: 10.1016/j.immuni.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macpherson AJ, Geuking MB, McCoy KD. Homeland Security: IgA immunity at the frontiers of the body. Trends in Immunology. 2012 doi: 10.1016/j.it.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Mucida D, Park Y, Cheroutre H. From the diet to the nucleus: vitamin A and TGF-beta join efforts at the mucosal interface of the intestine. Semin Immunol. 2009;21:14–21. doi: 10.1016/j.smim.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohnmacht C, Marques R, Presley L, Sawa S, Lochner M, Eberl G. Intestinal microbiota, evolution of the immune system and the bad reputation of pro-inflammatory immunity. Cellular microbiology. 2011;13:653–659. doi: 10.1111/j.1462-5822.2011.01577.x. [DOI] [PubMed] [Google Scholar]
- 24.Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Seminars in immunopathology. 2010;32:17–31. doi: 10.1007/s00281-009-0188-x. [DOI] [PubMed] [Google Scholar]
- 25.Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu J, Heller JJ, Guo X, Chen Z-mE, Fish K, et al. The Aryl Hydrocarbon Receptor Regulates Gut Immunity through Modulation of Innate Lymphoid Cells. Immunity. 2011:1–13. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nature medicine. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 29.O'Connor W, Zenewicz LA, Flavell RA. The dual nature of T(H)17 cells: shifting the focus to function. Nat Immunol. 2010;11:471–476. doi: 10.1038/ni.1882. [DOI] [PubMed] [Google Scholar]
- 30.Lochner M, Bérard M, Sawa S, Hauer S, Gaboriau-Routhiau V, et al. Restricted microbiota and absence of cognate TCR antigen leads to an unbalanced generation of Th17 cells. Journal of immunology (Baltimore, Md : 1950) 2011;186:1531–1537. doi: 10.4049/jimmunol.1001723. [DOI] [PubMed] [Google Scholar]
- 31.Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, et al. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science (New York, NY) 2010;330:665–669. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- 32.Gaboriau-Routhiau V, Rakotobe S, Lécuyer E, Mulder I, Lan A, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 33.Geuking MB, Cahenzli J, Lawson MAE, Ng DCK, Slack E, et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34:794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 34.Wu H-J, Ivanov II, Darce J, Hattori K, Shima T, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Microbes and Health Sackler Colloquium: Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2010 [Google Scholar]
- 36.Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clinical Immunology. 2004;110:55–62. doi: 10.1016/j.clim.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Yang XO, Chang SH, Park H, Nurieva R, Shah B, et al. Regulation of inflammatory responses by IL-17F. The Journal of experimental medicine. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cox JH, Kljavin NM, Ota N, Leonard J, Roose-Girma M, et al. Opposing consequences of IL-23 signaling mediated by innate and adaptive cells in chemically induced colitis in mice. Mucosal Immunology. 2012;5:99–109. doi: 10.1038/mi.2011.54. [DOI] [PubMed] [Google Scholar]
- 40.Izcue A, Hue S, Buonocore S, Arancibia-Cárcamo CV, Ahern PP, et al. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008;28:559–570. doi: 10.1016/j.immuni.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. The Journal of clinical investigation. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leppkes M, Becker C, Ivanov II, Hirth S, Wirtz S, et al. RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 2009;136:257–267. doi: 10.1053/j.gastro.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 43.Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–318. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 44.Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, et al. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity. 2010;33:279–288. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, et al. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–2370. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflammatory Bowel Diseases. 2006;12:382–388. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 47.Feng T, Qin H, Wang L, Benveniste EN, Elson CO, Cong Y. Th17 cells induce colitis and promote Th1 cell responses through IL-17 induction of innate IL-12 and IL-23 production. Journal of immunology (Baltimore, Md : 1950) 2011;186:6313–6318. doi: 10.4049/jimmunol.1001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mikami Y, Kanai T, Sujino T, Ono Y, Hayashi A, et al. Competition between colitogenic Th1 and Th17 cells contributes to the amelioration of colitis. European journal of immunology. 2010;40:2409–2422. doi: 10.1002/eji.201040379. [DOI] [PubMed] [Google Scholar]
- 49.Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–1767. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, et al. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005;129:969–984. doi: 10.1053/j.gastro.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 51.Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, et al. IL-22 is increased in active Crohn's disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. American journal of physiology Gastrointestinal and liver physiology. 2006;290:G827–G838. doi: 10.1152/ajpgi.00513.2005. [DOI] [PubMed] [Google Scholar]
- 52.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kobayashi T, Okamoto S, Hisamatsu T, Kamada N, Chinen H, et al. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn's disease. Gut. 2008;57:1682–1689. doi: 10.1136/gut.2007.135053. [DOI] [PubMed] [Google Scholar]
- 54.Nielsen OH, Kirman I, Rüdiger N, Hendel J, Vainer B. Upregulation of interleukin-12 and-17 in active inflammatory bowel disease. Scandinavian journal of gastroenterology. 2003;38:180–185. doi: 10.1080/00365520310000672. [DOI] [PubMed] [Google Scholar]
- 55.Sakuraba A, Sato T, Kamada N, Kitazume M, Sugita A, Hibi T. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn's disease. Gastroenterology. 2009;137:1736–1745. doi: 10.1053/j.gastro.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 56.Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. Journal of Experimental Medicine. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. The Journal of experimental medicine. 2009;206:525–534. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Budarf ML, Labbé C, David G, Rioux JD. GWA studies: rewriting the story of IBD. Trends in genetics : TIG. 2009;25:137–146. doi: 10.1016/j.tig.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 59.Kan S-h, Mancini G, Gallagher G. Identification and characterization of multiple splice forms of the human interleukin-23 receptor alpha chain in mitogen-activated leukocytes. Genes and immunity. 2008;9:631–639. doi: 10.1038/gene.2008.64. [DOI] [PubMed] [Google Scholar]
- 60.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmechel S, Konrad A, Diegelmann J, Glas J, Wetzke M, et al. Linking genetic susceptibility to Crohn's disease with Th17 cell function: IL-22 serum levels are increased in Crohn's disease and correlate with disease activity and IL23R genotype status. Inflammatory Bowel Diseases. 2008;14:204–212. doi: 10.1002/ibd.20315. [DOI] [PubMed] [Google Scholar]
- 62.Kamada N, Hisamatsu T, Okamoto S, Chinen H, Kobayashi T, et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. The Journal of clinical investigation. 2008;118:2269–2280. doi: 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mannon PJ, Fuss IJ, Mayer L, Elson CO, Sandborn WJ, et al. Anti-interleukin-12 antibody for active Crohn's disease. The New England journal of medicine. 2004;351:2069–2079. doi: 10.1056/NEJMoa033402. [DOI] [PubMed] [Google Scholar]
- 64.Sandborn WJ, Feagan BG, Fedorak RN, Scherl E, Fleisher MR, et al. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn's disease. Gastroenterology. 2008;135:1130–1141. doi: 10.1053/j.gastro.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 65.Fuss IJ, Heller F, Boirivant M, Leon F, Yoshida M, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. The Journal of clinical investigation. 2004;113:1490–1497. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heller F, Florian P, Bojarski C, Richter J, Christ M, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 67.Lees CW, Barrett JC, Parkes M, Satsangi J. New IBD genetics: common pathways with other diseases. Gut. 2011;60:1739–1753. doi: 10.1136/gut.2009.199679. [DOI] [PubMed] [Google Scholar]
- 68.Rabizadeh S, Rhee K-J, Wu S, Huso D, Gan CM, et al. Enterotoxigenic bacteroides fragilis: a potential instigator of colitis. Inflammatory Bowel Diseases. 2007;13:1475–1483. doi: 10.1002/ibd.20265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rhee K-J, Wu S, Wu X, Huso DL, Karim B, et al. Induction of persistent colitis by a human commensal, enterotoxigenic Bacteroides fragilis, in wild-type C57BL/6 mice. Infection and immunity. 2009;77:1708–1718. doi: 10.1128/IAI.00814-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saleh M, Trinchieri G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nature reviews. Immunology. 2011;11:9–20. doi: 10.1038/nri2891. [DOI] [PubMed] [Google Scholar]
- 72.Wu S, Rhee K, Albesiano E, Rabizadeh S, Wu X, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nature medicine. 2009;15:1016–1023. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garvy BA, Ezekowitz RA, Harmsen AG. Role of gamma interferon in the host immune and inflammatory responses to Pneumocystis carinii infection. Infect Immun. 1997;65:373–379. doi: 10.1128/iai.65.2.373-379.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garvy BA, Wiley JA, Gigliotti F, Harmsen AG. Protection against Pneumocystis carinii pneumonia by antibodies generated from either T helper 1 or T helper 2 responses. Infect Immun. 1997;65:5052–5056. doi: 10.1128/iai.65.12.5052-5056.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shellito J, Suzara VV, Blumenfeld W, Beck JM, Steger HJ, Ermak TH. A new model of Pneumocystis carinii infection in mice selectively depleted of helper T lymphocytes. J Clin Invest. 1990;85:1686–1693. doi: 10.1172/JCI114621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009;2:403–411. doi: 10.1038/mi.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol. 2005;175:404–412. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuestner RE, Taft DW, Haran A, Brandt CS, Brender T, et al. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J Immunol. 2007;179:5462–5473. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kao CY, Chen Y, Thai P, Wachi S, Huang F, et al. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J Immunol. 2004;173:3482–3491. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 80.Kreindler JL, Bertrand CA, Lee RJ, Karasic T, Aujla S, et al. Interleukin-17A induces bicarbonate secretion in normal human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;296:L257–L266. doi: 10.1152/ajplung.00344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dorschner RA, Lopez-Garcia B, Peschel A, Kraus D, Morikawa K, et al. The mammalian ionic environment dictates microbial susceptibility to antimicrobial defense peptides. FASEB J. 2006;20:35–42. doi: 10.1096/fj.05-4406com. [DOI] [PubMed] [Google Scholar]
- 82.van den Berg A, Kuiper M, Snoek M, Timens W, Postma DS, et al. Interleukin-17 induces hyperresponsive interleukin-8 and interleukin-6 production to tumor necrosis factor-alpha in structural lung cells. Am J Respir Cell Mol Biol. 2005;33:97–104. doi: 10.1165/rcmb.2005-0022OC. [DOI] [PubMed] [Google Scholar]
- 83.Vanaudenaerde BM, Wuyts WA, Dupont LJ, Van Raemdonck DE, Demedts MM, Verleden GM. Interleukin-17 stimulates release of interleukin-8 by human airway smooth muscle cells in vitro: a potential role for interleukin-17 and airway smooth muscle cells in bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2003;22:1280–1283. doi: 10.1016/s1053-2498(02)01234-2. [DOI] [PubMed] [Google Scholar]
- 84.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nature medicine. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kreda SM, Gynn MC, Fenstermacher DA, Boucher RC, Gabriel SE. Expression and localization of epithelial aquaporins in the adult human lung. Am J Respir Cell Mol Biol. 2001;24:224–234. doi: 10.1165/ajrcmb.24.3.4367. [DOI] [PubMed] [Google Scholar]
- 86.Hoegl S, Bachmann M, Scheiermann P, Goren I, Hofstetter C, et al. Protective properties of inhaled IL-22 in a model of ventilator-induced lung injury. Am J Respir Cell Mol Biol. 2011;44:369–376. doi: 10.1165/rcmb.2009-0440OC. [DOI] [PubMed] [Google Scholar]
- 87.Ely LK, Fischer S, Garcia KC. Structural basis of receptor sharing by interleukin 17 cytokines. Nat Immunol. 2009;10:1245–1251. doi: 10.1038/ni.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. The Journal of experimental medicine. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen K, McAleer JP, Lin Y, Paterson DL, Zheng M, et al. Th17 cells mediate clade-specific, serotype-independent mucosal immunity. Immunity. 2011;35:997–1009. doi: 10.1016/j.immuni.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 91.Li L, Huang L, Vergis AL, Ye H, Bajwa A, et al. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120:331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Werner JL, Gessner MA, Lilly LM, Nelson MP, Metz AE, et al. Neutrophils produce interleukin 17A (IL-17A) in a dectin-1- and IL-23-dependent manner during invasive fungal infection. Infection and Immunity. 2011;79:3966–3977. doi: 10.1128/IAI.05493-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu Q, Martin RJ, Rino JG, Breed R, Torres RM, Chu HW. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 2007;9:78–86. doi: 10.1016/j.micinf.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, et al. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009;31:799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moffitt KL, Gierahn TM, Lu YJ, Gouveia P, Alderson M, et al. T(H)17-based vaccine design for prevention of Streptococcus pneumoniae colonization. Cell Host Microbe. 2011;9:158–165. doi: 10.1016/j.chom.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wuthrich M, Gern B, Hung CY, Ersland K, Rocco N, et al. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J Clin Invest. 2011;121:554–568. doi: 10.1172/JCI43984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Capobianco J, Grimberg A, Thompson BM, Antunes VB, Jasinowodolinski D, Meirelles GS. Thoracic manifestations of collagen vascular diseases. Radiographics. 2012;32:33–50. doi: 10.1148/rg.321105058. [DOI] [PubMed] [Google Scholar]
- 98.Panopoulos ST, Sfikakis PP. Biological treatments and connective tissue disease associated interstitial lung disease. Curr Opin Pulm Med. 2011;17:362–367. doi: 10.1097/MCP.0b013e3283483ea5. [DOI] [PubMed] [Google Scholar]
- 99.Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, Hwang JY, Kusser K, et al. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol. 2011;12:639–646. doi: 10.1038/ni.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Toussirot E, Berthelot JM, Pertuiset E, Bouvard B, Gaudin P, et al. Pulmonary nodulosis and aseptic granulomatous lung disease occurring in patients with rheumatoid arthritis receiving tumor necrosis factor-alpha-blocking agent: a case series. J Rheumatol. 2009;36:2421–2427. doi: 10.3899/jrheum.090030. [DOI] [PubMed] [Google Scholar]
- 101.Simonian PL, Roark CL, Wehrmann F, Lanham AK, Diaz del Valle F, et al. Th17-polarized immune response in a murine model of hypersensitivity pneumonitis and lung fibrosis. J Immunol. 2009;182:657–665. [PMC free article] [PubMed] [Google Scholar]
- 102.Joshi AD, Fong DJ, Oak SR, Trujillo G, Flaherty KR, et al. Interleukin-17-mediated immunopathogenesis in experimental hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2009;179:705–716. doi: 10.1164/rccm.200811-1700OC. [DOI] [PubMed] [Google Scholar]
- 103.Simonian PL, Roark CL, Wehrmann F, Lanham AM, Born WK, et al. IL-17A-expressing T cells are essential for bacterial clearance in a murine model of hypersensitivity pneumonitis. J Immunol. 2009;182:6540–6549. doi: 10.4049/jimmunol.0900013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brodlie M, McKean MC, Johnson GE, Anderson AE, Hilkens CM, et al. Raised interleukin-17 is immunolocalised to neutrophils in cystic fibrosis lung disease. Eur Respir J. 2011;37:1378–1385. doi: 10.1183/09031936.00067110. [DOI] [PubMed] [Google Scholar]
- 105.Tan HL, Regamey N, Brown S, Bush A, Lloyd CM, Davies JC. The Th17 pathway in cystic fibrosis lung disease. Am J Respir Crit Care Med. 2011;184:252–258. doi: 10.1164/rccm.201102-0236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, et al. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. The Journal of experimental medicine. 2010;207:535–552. doi: 10.1084/jem.20092121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hsu E, Shi H, Jordan RM, Lyons-Weiler J, Pilewski JM, Feghali-Bostwick CA. Lung tissues in patients with systemic sclerosis have gene expression patterns unique to pulmonary fibrosis and pulmonary hypertension. Arthritis Rheum. 2011;63:783–794. doi: 10.1002/art.30159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Facco M, Cabrelle A, Teramo A, Olivieri V, Gnoato M, et al. Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax. 2011;66:144–150. doi: 10.1136/thx.2010.140319. [DOI] [PubMed] [Google Scholar]
- 109.Ten Berge B, Paats MS, Bergen IM, van den Blink B, Hoogsteden HC, et al. Increased IL-17A expression in granulomas and in circulating memory T cells in sarcoidosis. Rheumatology (Oxford) 2012;51:37–46. doi: 10.1093/rheumatology/ker316. [DOI] [PubMed] [Google Scholar]
- 110.Carlson MJ, West ML, Coghill JM, Panoskaltsis-Mortari A, Blazar BR, Serody JS. In vitro-differentiated TH17 cells mediate lethal acute graft-versus-host disease with severe cutaneous and pulmonary pathologic manifestations. Blood. 2009;113:1365–1374. doi: 10.1182/blood-2008-06-162420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gowdy KM, Nugent JL, Martinu T, Potts E, Snyder LD, et al. Protective Role of T-bet and Th1 Cytokines in Pulmonary Graft-versus-Host Disease and Peribronchiolar Fibrosis. Am J Respir Cell Mol Biol. 2012;46:249–256. doi: 10.1165/rcmb.2011-0131OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 112.Yi T, Chen Y, Wang L, Du G, Huang D, et al. Reciprocal differentiation and tissue-specific pathogenesis of Th1, Th2, and Th17 cells in graft-versus-host disease. Blood. 2009;114:3101–3112. doi: 10.1182/blood-2009-05-219402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cao H, Lan Q, Shi Q, Zhou X, Liu G, et al. Anti-IL-23 antibody blockade of IL-23/IL-17 pathway attenuates airway obliteration in rat orthotopic tracheal transplantation. Int Immunopharmacol. 2011;11:569–575. doi: 10.1016/j.intimp.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 114.Fan L, Benson HL, Vittal R, Mickler EA, Presson R, et al. Neutralizing IL-17 prevents obliterative bronchiolitis in murine orthotopic lung transplantation. Am J Transplant. 2011;11:911–922. doi: 10.1111/j.1600-6143.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tubby C, Harrison T, Todd I, Fairclough L. Immunological basis of reversible and fixed airways disease. Clin Sci (Lond) 2011;121:285–296. doi: 10.1042/CS20110062. [DOI] [PubMed] [Google Scholar]
- 116.Holgate ST. A look at the pathogenesis of asthma: the need for a change in direction. Discov Med. 2010;9:439–447. [PubMed] [Google Scholar]
- 117.Zhang JY, Wenzel SE. Tissue and BAL based biomarkers in asthma. Immunol Allergy Clin North Am. 2007;27:623–632. doi: 10.1016/j.iac.2007.09.003. vi. [DOI] [PubMed] [Google Scholar]
- 118.Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, et al. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006;7:135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kawaguchi M, Onuchic LF, Li XD, Essayan DM, Schroeder J, et al. Identification of a novel cytokine, ML-1, and its expression in subjects with asthma. J Immunol. 2001;167:4430–4435. doi: 10.4049/jimmunol.167.8.4430. [DOI] [PubMed] [Google Scholar]
- 120.Ramsey CD, Lazarus R, Camargo CA, Jr, Weiss ST, Celedon JC. Polymorphisms in the interleukin 17F gene (IL17F) and asthma. Genes Immun. 2005;6:236–241. doi: 10.1038/sj.gene.6364170. [DOI] [PubMed] [Google Scholar]
- 121.Kawaguchi M, Takahashi D, Hizawa N, Suzuki S, Matsukura S, et al. IL-17F sequence variant (His161Arg) is associated with protection against asthma and antagonizes wild-type IL-17F activity. J Allergy Clin Immunol. 2006;117:795–801. doi: 10.1016/j.jaci.2005.12.1346. [DOI] [PubMed] [Google Scholar]
- 122.Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, et al. Interleukin-17 is a negative regulator of established allergic asthma. The Journal of experimental medicine. 2006;203:2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, et al. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol. 2010;11:928–935. doi: 10.1038/ni.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pichavant M, Goya S, Meyer EH, Johnston RA, Kim HY, et al. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. The Journal of experimental medicine. 2008;205:385–393. doi: 10.1084/jem.20071507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, et al. A large-scale, consortium-based genomewide association study of asthma. The New England journal of medicine. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Torgerson DG, Ampleford EJ, Chiu GY, Gauderman WJ, Gignoux CR, et al. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nature genetics. 2011;43:887–892. doi: 10.1038/ng.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Linden A, Hoshino H, Laan M. Airway neutrophils and interleukin-17. Eur Respir J. 2000;15:973–977. doi: 10.1034/j.1399-3003.2000.15e28.x. [DOI] [PubMed] [Google Scholar]
- 130.Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem. 2003;278:17036–17043. doi: 10.1074/jbc.M210429200. [DOI] [PubMed] [Google Scholar]
- 131.Huang N, Liu L, Wang XZ, Liu D, Yin SY, Yang XD. Association of interleukin (IL)-12 and IL-27 gene polymorphisms with chronic obstructive pulmonary disease in a Chinese population. DNA Cell Biol. 2008;27:527–531. doi: 10.1089/dna.2007.0715. [DOI] [PubMed] [Google Scholar]
- 132.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. The New England journal of medicine. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 133.McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. The New England journal of medicine. 2011;365:1567–1575. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen K, Pociask DA, McAleer JP, Chan YR, Alcorn JF, et al. IL-17RA is required for CCL2 expression, macrophage recruitment, and emphysema in response to cigarette smoke. PloS one. 2011;6:e20333. doi: 10.1371/journal.pone.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Di Stefano A, Caramori G, Gnemmi I, Contoli M, Vicari C, et al. T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clinical and experimental immunology. 2009;157:316–324. doi: 10.1111/j.1365-2249.2009.03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nature medicine. 2007;13:567–569. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- 137.Shan M, Yuan X, Song LZ, Roberts L, Zarinkamar N, et al. Cigarette smoke induction of osteopontin (SPP1) mediates T(H)17 inflammation in human and experimental emphysema. Science translational medicine. 2012;4:117ra9. doi: 10.1126/scitranslmed.3003041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen X, Wan J, Liu J, Xie W, Diao X, et al. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer. 2010;69:348–354. doi: 10.1016/j.lungcan.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 139.Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, et al. IL-17 enhances the net angiogenic activity and in vivo growth of human non- small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175:6177–6189. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 140.Kirshberg S, Izhar U, Amir G, Demma J, Vernea F, et al. Involvement of CCR6/CCL20/IL-17 axis in NSCLC disease progression. PloS one. 2011;6:e24856. doi: 10.1371/journal.pone.0024856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang W, Chen Y, Wei H, Zheng C, Sun R, et al. Antiapoptotic activity of autocrine interleukin-22 and therapeutic effects of interleukin-22-small interfering RNA on human lung cancer xenografts. Clin Cancer Res. 2008;14:6432–6439. doi: 10.1158/1078-0432.CCR-07-4401. [DOI] [PubMed] [Google Scholar]
- 142.Hoffjan S, Stemmler S. On the role of the epidermal differentiation complex in ichthyosis vulgaris, atopic dermatitis and psoriasis. The British journal of dermatology. 2007;157:441–449. doi: 10.1111/j.1365-2133.2007.07999.x. [DOI] [PubMed] [Google Scholar]
- 143.Marieb EN, Mallatt J, Wilhelm PB. Human anatomy. San Francisco: Pearson/Benjamin Cummings; 2005. p. xxvii.p. 818. [Google Scholar]
- 144.Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nature reviews. Immunology. 2009;9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 146.Nestle FO, Kaplan DH, Barker J. Psoriasis. The New England journal of medicine. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 147.Perera GK, Di Meglio P, Nestle FO. Psoriasis. Annual review of pathology. 2012;7:385–422. doi: 10.1146/annurev-pathol-011811-132448. [DOI] [PubMed] [Google Scholar]
- 148.Baker BS, Ovigne JM, Powles AV, Corcoran S, Fry L. Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasis. The British journal of dermatology. 2003;148:670–679. doi: 10.1046/j.1365-2133.2003.05287.x. [DOI] [PubMed] [Google Scholar]
- 149.Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 150.Song PI, Park YM, Abraham T, Harten B, Zivony A, et al. Human keratinocytes express functional CD14 and toll-like receptor. The Journal of investigative dermatology. 2002;119:424–432. doi: 10.1046/j.1523-1747.2002.01847.x. [DOI] [PubMed] [Google Scholar]
- 151.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 152.Lebre MC, van der Aar AM, van Baarsen L, van Capel TM, Schuitemaker JH, et al. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. The Journal of investigative dermatology. 2007;127:331–341. doi: 10.1038/sj.jid.5700530. [DOI] [PubMed] [Google Scholar]
- 153.Lee Y, Kim H, Kim S, Shin MH, Kim YK, et al. Myeloid differentiation factor 88 regulates basal and UV-induced expressions of IL-6 and MMP-1 in human epidermal keratinocytes. The Journal of investigative dermatology. 2009;129:460–467. doi: 10.1038/jid.2008.261. [DOI] [PubMed] [Google Scholar]
- 154.Sugita K, Kabashima K, Atarashi K, Shimauchi T, Kobayashi M, Tokura Y. Innate immunity mediated by epidermal keratinocytes promotes acquired immunity involving Langerhans cells and T cells in the skin. Clinical and experimental immunology. 2007;147:176–183. doi: 10.1111/j.1365-2249.2006.03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zepter K, Haffner A, Soohoo LF, De Luca D, Tang HP, et al. Induction of biologically active IL-1 beta-converting enzyme and mature IL-1 beta in human keratinocytes by inflammatory and immunologic stimuli. J Immunol. 1997;159:6203–6208. [PubMed] [Google Scholar]
- 156.Chamilos G, Ganguly D, Lande R, Gregorio J, Meller S, et al. Generation of IL-23 producing dendritic cells (DCs) by airborne fungi regulates fungal pathogenicity via the induction of T(H)-17 responses. PloS one. 2010;5:e12955. doi: 10.1371/journal.pone.0012955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dennehy KM, Willment JA, Williams DL, Brown GD. Reciprocal regulation of IL-23 and IL-12 following co-activation of Dectin-1 and TLR signaling pathways. Eur J Immunol. 2009;39:1379–1386. doi: 10.1002/eji.200838543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Roses RE, Xu S, Xu M, Koldovsky U, Koski G, Czerniecki BJ. Differential production of IL-23 and IL-12 by myeloid-derived dendritic cells in response to TLR agonists. J Immunol. 2008;181:5120–5127. doi: 10.4049/jimmunol.181.7.5120. [DOI] [PubMed] [Google Scholar]
- 159.de Jong A, Pena-Cruz V, Cheng T-Y, Clark RA, Van Rhijn I, Moody DB. CD1a-autoreactive T cells are a normal component of the human T cell repertoire. Nat Immunol. 2010;11:1102–1109. doi: 10.1038/ni.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Igyarto BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011;35:260–272. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Berg EL, Yoshino T, Rott LS, Robinson MK, Warnock RA, et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. The Journal of experimental medicine. 1991;174:1461–1466. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Homey B, Alenius H, Muller A, Soto H, Bowman EP, et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nature medicine. 2002;8:157–165. doi: 10.1038/nm0202-157. [DOI] [PubMed] [Google Scholar]
- 163.Horikawa T, Nakayama T, Hikita I, Yamada H, Fujisawa R, et al. IFN-gamma-inducible expression of thymus and activation-regulated chemokine/CCL17 and macrophage-derived chemokine/CCL22 in epidermal keratinocytes and their roles in atopic dermatitis. International immunology. 2002;14:767–773. doi: 10.1093/intimm/dxf044. [DOI] [PubMed] [Google Scholar]
- 164.Katou F, Ohtani H, Nakayama T, Ono K, Matsushima K, et al. Macrophage-derived chemokine (MDC/CCL22) and CCR4 are involved in the formation of T lymphocyte-dendritic cell clusters in human inflamed skin and secondary lymphoid tissue. The American journal of pathology. 2001;158:1263–1270. doi: 10.1016/S0002-9440(10)64077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kennedy-Crispin M, Billick E, Mitsui H, Gulati N, Fujita H, et al. Human keratinocytes' response to injury upregulates CCL20 and other genes linking innate and adaptive immunity. The Journal of investigative dermatology. 2012;132:105–113. doi: 10.1038/jid.2011.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Stockinger B, Hirota K, Duarte J, Veldhoen M. External influences on the immune system via activation of the aryl hydrocarbon receptor. Semin Immunol. 2011;23:99–105. doi: 10.1016/j.smim.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 167.Brembilla NC, Ramirez JM, Chicheportiche R, Sorg O, Saurat JH, Chizzolini C. In vivo dioxin favors interleukin-22 production by human CD4+ T cells in an aryl hydrocarbon receptor (AhR)-dependent manner. PloS one. 2011;6:e18741. doi: 10.1371/journal.pone.0018741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Carrier Y, Ma HL, Ramon HE, Napierata L, Small C, et al. Inter-regulation of Th17 cytokines and the IL-36 cytokines in vitro and in vivo: implications in psoriasis pathogenesis. The Journal of investigative dermatology. 2011;131:2428–2437. doi: 10.1038/jid.2011.234. [DOI] [PubMed] [Google Scholar]
- 169.Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, Nograles KE, Tian S, et al. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. The Journal of investigative dermatology. 2011;131:677–687. doi: 10.1038/jid.2010.340. [DOI] [PubMed] [Google Scholar]
- 170.Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hegyi Z, Zwicker S, Bureik D, Peric M, Koglin S, et al. Vitamin D Analog Calcipotriol Suppresses the Th17 Cytokine-Induced Proinflammatory S100"Alarmins" Psoriasin (S100A7) and Koebnerisin (S100A15) in Psoriasis. The Journal of investigative dermatology. 2012 doi: 10.1038/jid.2011.486. [DOI] [PubMed] [Google Scholar]
- 172.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. Journal of Experimental Medicine. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suarez-Farinas M, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. British Journal of Dermatology. 2008;159:1092–1102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bernard JJ, Gallo RL. Protecting the boundary: the sentinel role of host defense peptides in the skin. Cellular and molecular life sciences : CMLS. 2011;68:2189–2199. doi: 10.1007/s00018-011-0712-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ghannam S, Dejou C, Pedretti N, Giot J-P, Dorgham K, et al. CCL20 and - defensin-2 induce arrest of human Th17 cells on inflamed endothelium in vitro under flow conditions. Journal of Immunology. 2011;186:1411–1420. doi: 10.4049/jimmunol.1000597. [DOI] [PubMed] [Google Scholar]
- 176.Eyerich S, Wagener J, Wenzel V, Scarponi C, Pennino D, et al. IL-22 and TNF-alpha represent a key cytokine combination for epidermal integrity during infection with Candida albicans. Eur J Immunol. 2011 doi: 10.1002/eji.201041197. [DOI] [PubMed] [Google Scholar]
- 177.Wolk K, Witte E, Warszawska K, Schulze-Tanzil G, Witte K, et al. The Th17 cytokine IL-22 induces IL-20 production in keratinocytes: a novel immunological cascade with potential relevance in psoriasis. Eur J Immunol. 2009;39:3570–3581. doi: 10.1002/eji.200939687. [DOI] [PubMed] [Google Scholar]
- 178.Austin LM, Ozawa M, Kikuchi T, Walters IB, Krueger JG. The majority of epidermal T cells in Psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. The Journal of investigative dermatology. 1999;113:752–759. doi: 10.1046/j.1523-1747.1999.00749.x. [DOI] [PubMed] [Google Scholar]
- 179.Schlaak JF, Buslau M, Jochum W, Hermann E, Girndt M, et al. T cells involved in psoriasis vulgaris belong to the Th1 subset. The Journal of investigative dermatology. 1994;102:145–149. doi: 10.1111/1523-1747.ep12371752. [DOI] [PubMed] [Google Scholar]
- 180.Kauffman CL, Aria N, Toichi E, McCormick TS, Cooper KD, et al. A phase I study evaluating the safety, pharmacokinetics, and clinical response of a human IL-12 p40 antibody in subjects with plaque psoriasis. The Journal of investigative dermatology. 2004;123:1037–1044. doi: 10.1111/j.0022-202X.2004.23448.x. [DOI] [PubMed] [Google Scholar]
- 181.Krueger GG, Langley RG, Leonardi C, Yeilding N, Guzzo C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. The New England journal of medicine. 2007;356:580–592. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- 182.Benson JM, Sachs CW, Treacy G, Zhou H, Pendley CE, et al. Therapeutic targeting of the IL-12/23 pathways: generation and characterization of ustekinumab. Nature biotechnology. 2011;29:615–624. doi: 10.1038/nbt.1903. [DOI] [PubMed] [Google Scholar]
- 183.Kimball AB, Gordon KB, Fakharzadeh S, Yeilding N, Szapary PO, et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis: results from the PHOENIX 1 trial through up to 3 years. The British journal of dermatology. 2012 doi: 10.1111/j.1365-2133.2012.10901.x. [DOI] [PubMed] [Google Scholar]
- 184.Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. American Journal of Human Genetics. 2007;80:273–290. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nature genetics. 2009;41:199–204. doi: 10.1038/ng.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Science translational medicine. 2010;2:52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- 187.Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis--part II: immune cell subsets and therapeutic concepts. J Allergy Clin Immunol. 2011;127:1420–1432. doi: 10.1016/j.jaci.2011.01.054. [DOI] [PubMed] [Google Scholar]
- 188.Helms C, Saccone NL, Cao L, Daw JA, Cao K, et al. Localization of PSORS1 to a haplotype block harboring HLA-C and distinct from corneodesmosin and HCR. Human genetics. 2005;118:466–476. doi: 10.1007/s00439-005-0048-2. [DOI] [PubMed] [Google Scholar]
- 189.Lande R, Gilliet M. Plasmacytoid dendritic cells: key players in the initiation and regulation of immune responses. Ann N Y Acad Sci. 2010;1183:89–103. doi: 10.1111/j.1749-6632.2009.05152.x. [DOI] [PubMed] [Google Scholar]
- 190.Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. Journal of Immunology. 2005;174:3695–3702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- 191.Sa SM, Valdez PA, Wu J, Jung K, Zhong F, et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. Journal of Immunology. 2007;178:2229–2240. doi: 10.4049/jimmunol.178.4.2229. [erratum appears in J Immunol. 2007 Jun 1;178(11):7487]. [DOI] [PubMed] [Google Scholar]
- 192.Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. Journal of Investigative Dermatology. 2008;128:1207–1211. doi: 10.1038/sj.jid.5701213. [see comment]. [DOI] [PubMed] [Google Scholar]
- 193.Alwawi EA, Krulig E, Gordon KB. Long-term efficacy of biologics in the treatment of psoriasis: what do we really know? Dermatologic therapy. 2009;22:431–440. doi: 10.1111/j.1529-8019.2009.01259.x. [DOI] [PubMed] [Google Scholar]
- 194.Zaba LC, Suarez-Farinas M, Fuentes-Duculan J, Nograles KE, Guttman-Yassky E, et al. Effective treatment of psoriasis with etanercept is linked to suppression of IL-17 signaling, not immediate response TNF genes. J Allergy Clin Immunol. 2009;124:1022–1010. e1–e395. doi: 10.1016/j.jaci.2009.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zaba LC, Cardinale I, Gilleaudeau P, Sullivan-Whalen M, Suarez Farinas M, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. Journal of Experimental Medicine. 2007;204:3183–3194. doi: 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Suarez-Farinas M, Fuentes-Duculan J, Lowes MA, Krueger JG. Resolved psoriasis lesions retain expression of a subset of disease-related genes. Journal of Investigative Dermatology. 2011;131:391–400. doi: 10.1038/jid.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Griffiths CE, Strober BE, van de Kerkhof P, Ho V, Fidelus-Gort R, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. The New England journal of medicine. 2010;362:118–128. doi: 10.1056/NEJMoa0810652. [DOI] [PubMed] [Google Scholar]
- 198.Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis--part I: clinical and pathologic concepts. J Allergy Clin Immunol. 2011;127:1110–1118. doi: 10.1016/j.jaci.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 199.Oyoshi MK, He R, Kumar L, Yoon J, Geha RS. Cellular and molecular mechanisms in atopic dermatitis. Advances in immunology. 2009;102:135–226. doi: 10.1016/S0065-2776(09)01203-6. [DOI] [PubMed] [Google Scholar]
- 200.Sandilands A, Sutherland C, Irvine AD, McLean WHI. Filaggrin in the frontline: role in skin barrier function and disease. Journal of Cell Science. 2009;122:1285–1294. doi: 10.1242/jcs.033969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Paternoster L, Standl M, Chen C-M, Ramasamy A, Bønnelykke K, et al. Meta-analysis of genome-wide association studies identifies three new risk loci for atopic dermatitis. Nature genetics. 2012;44:187–192. doi: 10.1038/ng.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ober C, Yao T-C. The genetics of asthma and allergic disease: a 21st century perspective. Immunological reviews. 2011;242:10–30. doi: 10.1111/j.1600-065X.2011.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nature Reviews Immunology. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 204.Lefrançais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, et al. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proceedings of the National Academy of Sciences. 2012;109:1673–1678. doi: 10.1073/pnas.1115884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 206.Savinko T, Matikainen S, Saarialho-Kere U, Lehto M, Wang G, et al. IL-33 and ST2 in Atopic Dermatitis: Expression Profiles and Modulation by Triggering Factors. The Journal of investigative dermatology. 2012 doi: 10.1038/jid.2011.446. [DOI] [PubMed] [Google Scholar]
- 207.Guttman-Yassky E, Lowes MA, Fuentes-Duculan J, Zaba LC, Cardinale I, et al. Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. Journal of Immunology. 2008;181:7420–7427. doi: 10.4049/jimmunol.181.10.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nakatsuji T, Gallo RL. Antimicrobial peptides: old molecules with new ideas. The Journal of investigative dermatology. 2012;132:887–895. doi: 10.1038/jid.2011.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. The New England journal of medicine. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 210.Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, et al. IL-22-producing "T22" T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol. 2009;123:1244–1252. doi: 10.1016/j.jaci.2009.03.041. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hunger RE, Sieling PA, Ochoa MT, Sugaya M, Burdick AE, et al. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J Clin Invest. 2004;113:701–708. doi: 10.1172/JCI19655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mizumoto N, Takashima A. CD1a and langerin: acting as more than Langerhans cell markers. J Clin Invest. 2004;113:658–660. doi: 10.1172/JCI21140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fujita H, Nograles KE, Kikuchi T, Gonzalez J, Carucci JA, Krueger JG. Human Langerhans cells induce distinct IL-22-producing CD4+ T cells lacking IL-17 production. Proc Natl Acad Sci U S A. 2009;106:21795–21800. doi: 10.1073/pnas.0911472106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Suarez-Farinas M, Tintle SJ, Shemer A, Chiricozzi A, Nograles K, et al. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol. 2011;127:954–964. e1–e4. doi: 10.1016/j.jaci.2010.12.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Eyerich S, Onken AT, Weidinger S, Franke A, Nasorri F, et al. Mutual antagonism of T cells causing psoriasis and atopic eczema. The New England journal of medicine. 2011;365:231–238. doi: 10.1056/NEJMoa1104200. [DOI] [PubMed] [Google Scholar]
- 216.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Alcorn JF, Crowe CR, Kolls JK. TH17 cells in asthma and COPD. Annual review of physiology. 2010;72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]