Abstract
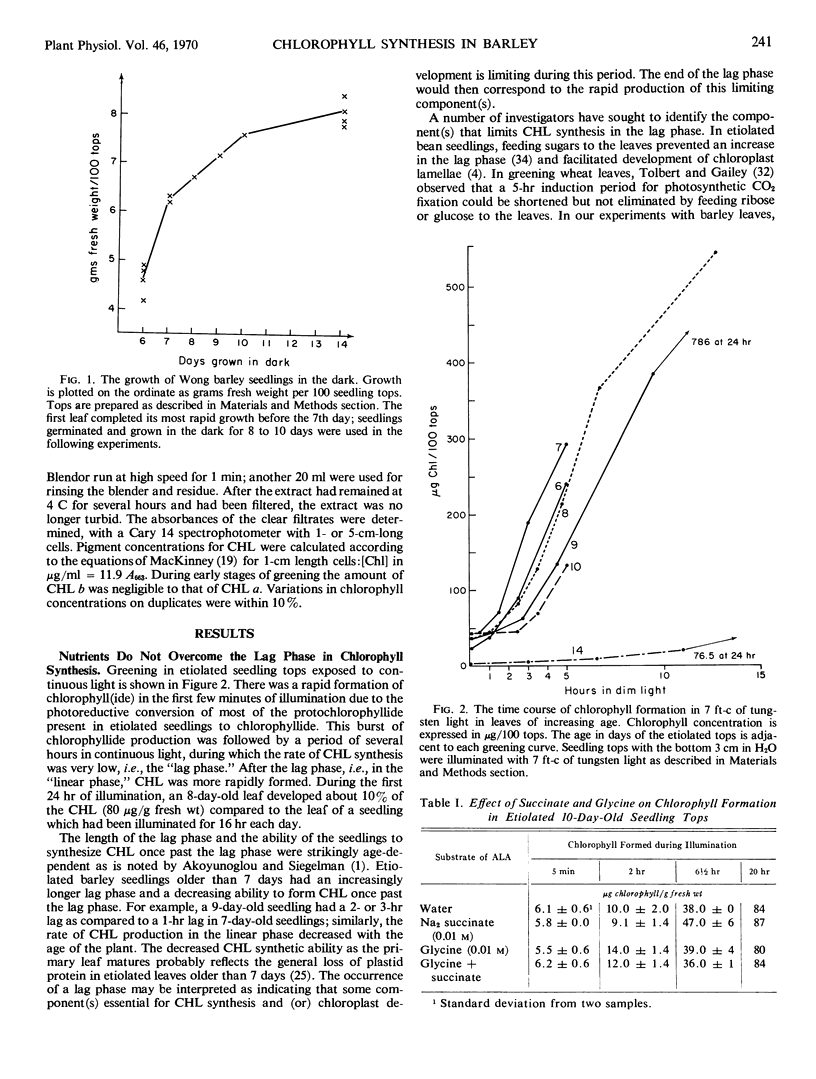
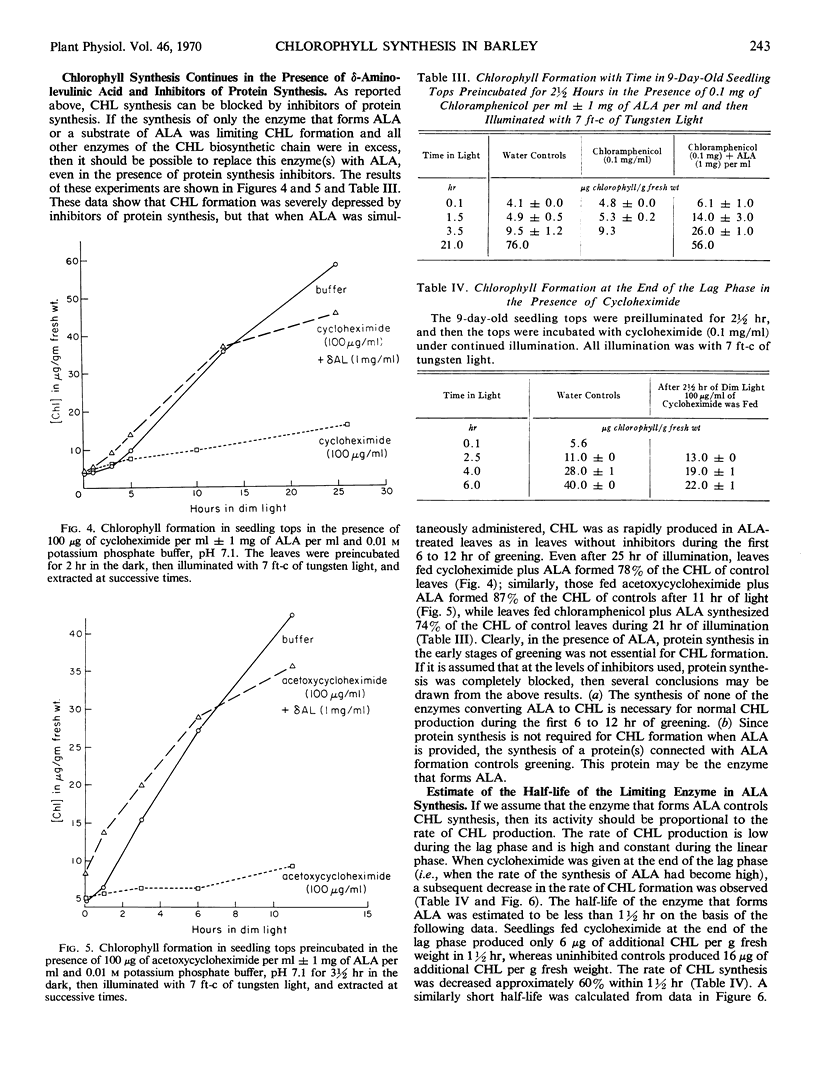
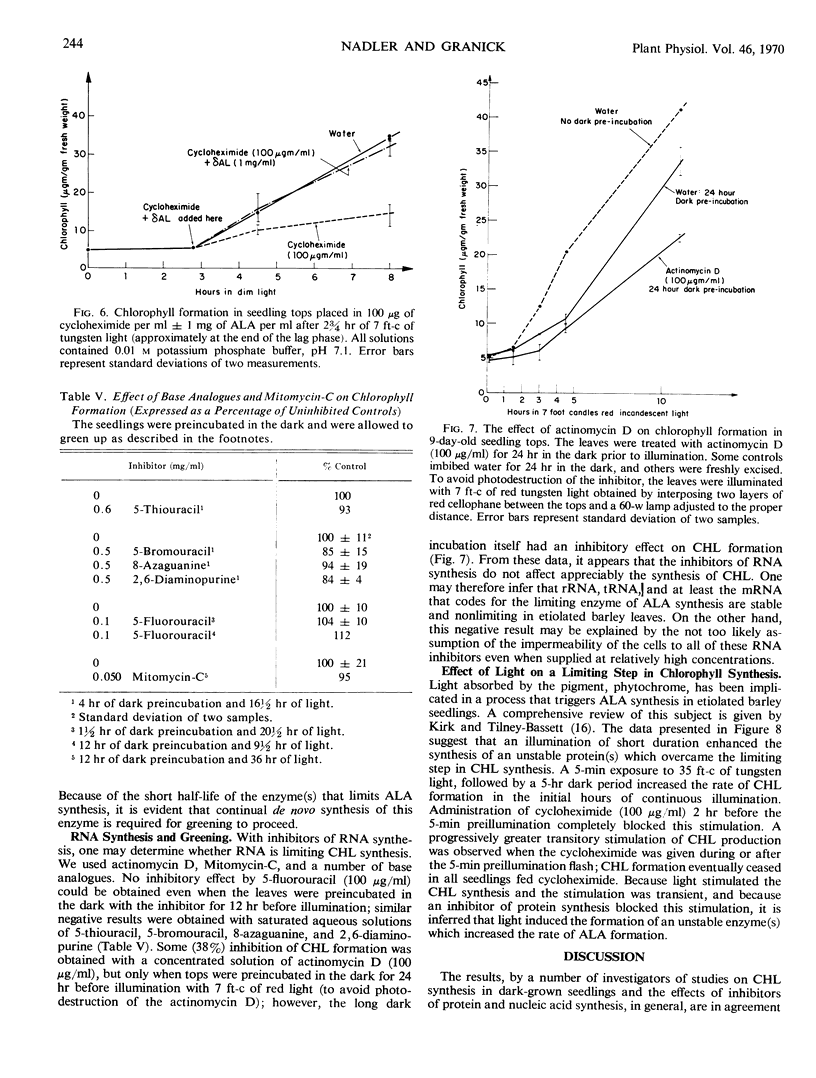
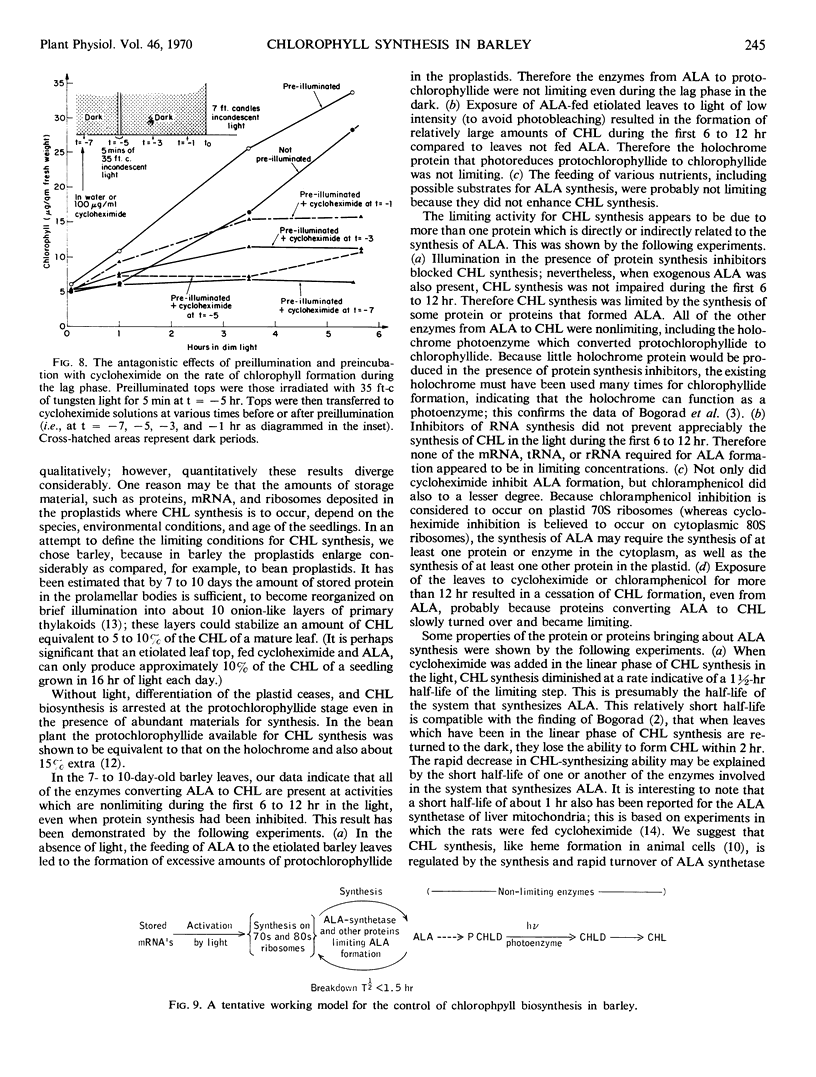
In 7- to 10-day-old leaves of etiolated barley (Hordeum vulgare), all of the enzymes that convert δ-aminolevulinic acid to chlorophyll are nonlimiting during the first 6 to 12 hours of illumination, even in the presence of inhibitors of protein synthesis. The limiting activity for chlorophyll synthesis appears to be a protein (or proteins) related to the synthesis of δ-aminolevulinic acid, presumably δ-aminolevulinic acid synthetase. Protein synthesis in both the cytosol and plastids may be required to produce nonlimiting amounts of δ-aminolevulinic acid. The half-life of a limiting protein controlling the synthesis of δ-aminolevulinic acid appears to be about 1½ hours, when determined with inhibitors of protein synthesis. Acceleration of chlorophyll synthesis by light is not inhibited by inhibitors of nucleic acid synthesis, but is inhibited by inhibitors of protein synthesis. A model for control of chlorophyll synthesis is proposed, based on a light-induced activation at the translational level of the synthesis of proteins forming δ-aminolevulinic acid, as well as the short half-life of these proteins. Evidence is presented confirming the idea that the holochrome on which protochlorophyllide is photoreduced to chlorophyllide functions enzymatically.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akoyunoglou G. A., Siegelman H. W. Protochlorophyllide resynthesis in dark-grown bean leaves. Plant Physiol. 1968 Jan;43(1):66–68. doi: 10.1104/pp.43.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EILAM Y., KLEIN S. The effect of light intensity and sucrose feeding on the fine structure in chloroplasts and on the chlorophyll content of etiolated leaves. J Cell Biol. 1962 Aug;14:169–182. doi: 10.1083/jcb.14.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassman M., Bogorad L. Control of chlorophyll production in rapidly greening bean leaves. Plant Physiol. 1967 Jun;42(6):774–780. doi: 10.1104/pp.42.6.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassman M., Bogorad L. Studies on the regeneration of protochlorophyllide after brief illumination of etiolated bean leaves. Plant Physiol. 1967 Jun;42(6):781–784. doi: 10.1104/pp.42.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassman M., Pluscec J., Bogorad L. delta-Aminolevulinic Acid Transaminase in Chlorella vulgaris. Plant Physiol. 1968 Sep;43(9):1411–1414. doi: 10.1104/pp.43.9.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granick S., Gassman M. Rapid regeneration of protochlorophyllide(650). Plant Physiol. 1970 Feb;45(2):201–205. doi: 10.1104/pp.45.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granick S. The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem. 1966 Mar 25;241(6):1359–1375. [PubMed] [Google Scholar]
- Hayashi N., Yoda B., Kikuchi G. Mechanism of allylisopropylacetamide-induced increase of delta-aminolevulinate synthetase in liver mitochondria. IV. Accumulation of the enzyme in the soluble fraction of rat liver. Arch Biochem Biophys. 1969 Apr;131(1):83–91. doi: 10.1016/0003-9861(69)90107-6. [DOI] [PubMed] [Google Scholar]
- Kirk J. T., Allen R. L. Dependence of chloroplast pigment synthesis on protein synthesis: effect of actidione. Biochem Biophys Res Commun. 1965 Dec 21;21(6):523–530. doi: 10.1016/0006-291x(65)90516-4. [DOI] [PubMed] [Google Scholar]
- LASCELLES J. Adaptation to form bacteriochlorophyll in Rhodopseudomonas spheroides: changes in activity of enzymes concerned in pyrrole synthesis. Biochem J. 1959 Jul;72:508–518. doi: 10.1042/bj0720508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies M. M. Effect of Chloramphenicol on Formation of Chloroplast Structure and Protein During Greening of Etiolated Leaves of Phaseolus vulgaris. Plant Physiol. 1966 Jun;41(6):992–1003. doi: 10.1104/pp.41.6.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi D. L., Waygood E. R. Biosynthesis of porphyrins in wheat leaves. I. Succinate:coA ligase (ADP). Can J Biochem. 1965 Oct;43(10):1605–1614. doi: 10.1139/o65-177. [DOI] [PubMed] [Google Scholar]
- Ohad I., Siekevitz P., Palade G. E. Biogenesis of chloroplast membranes. II. Plastid differentiation during greening of a dark-grown algal mutant (Chlamydomonas reinhardi). J Cell Biol. 1967 Dec;35(3):553–584. doi: 10.1083/jcb.35.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. M., Wedding R. T. Purification and properties of succinyl-CoA synthetase from Jerusalem artichoke mitochondria. Biochim Biophys Acta. 1966 Jan 11;113(1):167–174. doi: 10.1016/s0926-6593(66)80131-5. [DOI] [PubMed] [Google Scholar]
- RHODES M. J., YEMM E. W. DEVELOPMENT OF CHLOROPLASTS AND THE SYNTHESIS OF PROTEINS IN LEAVES. Nature. 1963 Dec 14;200:1077–1080. doi: 10.1038/2001077a0. [DOI] [PubMed] [Google Scholar]
- Steer B. T., Gibbs M. Changes in Succinyl CoA Synthetase Activity in Etiolated Bean Leaves Caused by Illumination. Plant Physiol. 1969 May;44(5):775–780. doi: 10.1104/pp.44.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Gailey F. B. Carbon Dioxide Fixation by Etiolated Plants after Exposure to White Light. Plant Physiol. 1955 Nov;30(6):491–499. doi: 10.1104/pp.30.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFF J. B., PRICE L. The effect of sugars on chlorophyll biosynthesis in higher plants. J Biol Chem. 1960 Jun;235:1603–1608. [PubMed] [Google Scholar]



