ABSTRACT
Many patients of all ages have multiple conditions, yet clinicians often lack explicit guidance on how to approach clinical decision-making for such people. Most recommendations from clinical practice guidelines (CPGs) focus on the management of single diseases, and may be harmful or impractical for patients with multimorbidity. A major barrier to the development of guidance for people with multimorbidity stems from the fact that the evidence underlying CPGs derives from studies predominantly focused on the management of a single disease. In this paper, the investigators from the Improving Guidelines for Multimorbid Patients Study Group present consensus-based recommendations for guideline developers to make guidelines more useful for the care of people with multimorbidity. In an iterative process informed by review of key literature and experience, we drafted a list of issues and possible approaches for addressing important coexisting conditions in each step of the guideline development process, with a focus on considering relevant interactions between the conditions, their treatments and their outcomes. The recommended approaches address consideration of coexisting conditions at all major steps in CPG development, from nominating and scoping the topic, commissioning the work group, refining key questions, ranking importance of outcomes, conducting systematic reviews, assessing quality of evidence and applicability, summarizing benefits and harms, to formulating recommendations and grading their strength. The list of issues and recommendations was reviewed and refined iteratively by stakeholders. This framework acknowledges the challenges faced by CPG developers who must make complex judgments in the absence of high-quality or direct evidence. These recommendations require validation through implementation, evaluation and refinement.
KEY WORDS: guidelines, multimorbidity, comorbidity, grading evidence
INTRODUCTION
Many patients have more than one health condition, but clinicians often lack explicit guidance on approaching clinical decision-making with people with multiple chronic conditions, or multimorbidity.1–6 Recommendations from clinical practice guidelines (CPGs) for the management of single conditions may be impractical, irrelevant or even harmful in the patient with multimorbidity.2,5,7
Some CPGs have considered issues of comorbidity and multimorbidity, but the degree to which specific recommendations are adaptable to patients with more than one disease remains quite limited.5,7–9 Current CPG development approaches do not optimally orient CPG developers to routinely consider whether the benefits of CPG-recommended care are sensitive to common, important coexisting conditions (comorbidities). Neither do they provide tools for adapting recommendations to the patient with greater complexity or for prioritizing the most important recommendations within a single condition, let alone between conditions. Development of CPGs relevant to people with multimorbidity is an aim of the Department of Health and Human Services.10
A major barrier to developing CPGs relevant to people with multimorbidity is that CPGs are built on an evidence base typically focused on the management of a single disease.2,11,12 Only rarely are data available that are directly applicable to patients with multimorbidity. Extrapolation of evidence from groups with lesser complexity to individuals with greater complexity may increase clinical uncertainty, weaken the strength of the recommendation and/or reduce its applicability.2,12 Such extrapolation is most often left to the clinician.
We define multimorbidity as the presence of two or more conditions in which each condition may influence optimal clinical management of other condition(s) through interactions between the conditions and the related treatments, between the treatments, or through limitations of life expectancy (Table 1).
Table 1.
Important Interactions to Consider Regarding Multimorbidity
| 1. Condition A x Condition B |
| Example: Depression is more common in diabetes.13 Depression may affect self-management, while the burden of long-term self-management may worsen depressive symptoms. |
| 2. Treatment A x Condition B |
| Example: use of non-steroidal anti-inflammatory drugs for osteoarthritis may lead to acute renal failure in individuals with chronic kidney disease (CKD). |
| 3. Treatment A x Treatment B |
| Example: Many potential drug interactions occur in people on multiple medications, such as the interaction between warfarin and antibiotics. |
| 4. Condition A and Life Expectancy |
| Example: The presence of end-stage chronic obstructive pulmonary disease may change the potential benefit of screening for colon cancer.14 , 15 |
Conditions may be recognized diseases such as asthma, or may be conditions like risk for falls. We use both terms in this report, but in general refer to conditions as a more generic term. Treatments may be pharmacological or non-pharmacological.
In this paper, we provide consensus-based recommendations to help CPG developers craft CPGs that are more useful for the care of people with multimorbidity; companion papers discuss the generation, analysis and reporting of primary data, and evidence synthesis and integration.11,16,17
METHODS
Our main focus was on CPGs relevant to treatments and interventions, rather than on evaluation and diagnosis. While systematic reviews are often integral parts of CPG development, this is the focus of an accompanying paper and is only briefly discussed here.16 Important issues considered outside of our scope included end-of-life issues, hospice, cost and resource use, CPG implementation, and performance measurement.18
We organized our work by the generic CPG development steps extracted from the review of leading, collaborative CPG initiatives.19–27 Next, we iteratively drafted and refined a list of issues and existing approaches relevant for addressing multimorbidity in each step of CPG development, based on review of key literature and investigators’ experience. An expert panel (see acknowledgements, also co-authors) with clinical and methods expertise in CPG development, methods of evidence synthesis, epidemiology, and multimorbidity provided feedback on the list of issues identified by CB, KU, BL, and were asked to identify examples of how existing CPGs currently address or do not address multimorbidity. This list of issues was reviewed and critiqued at an in-person retreat by all Improving Guidelines for Multimorbid Patients Investigators Group. With the expert panel, we then developed and refined draft recommendations to address the gaps identified for each step.
A large stakeholder group conference on Improving Guidelines for Multimorbid Patients (Baltimore, MD, October 2010) was attended by clinicians, methodologists, and researchers from various disciplines and stakeholders from government, payors and industry. The list of issues was reviewed and recommendations were vetted in facilitated group discussions using a modified Delphi approach (Participants in the Guideline Breakout Groups, see acknowledgements), where participants critiqued the draft recommendations based on importance, scientific and face validity, and feasibility. The final set of issues and recommendations was created after the incorporation of ideas discussed at the conference and a revised draft of the recommendations was circulated to all investigators and all members of the expert panel for final feedback.
RESULTS
Table 2 shows the steps, issues, and recommendations for developing CPGs that consider individuals with more than one condition, organized by generic CPG development steps. Since CPG development and systematic review proceed iteratively rather than sequentially, several issues and recommendations relate to more than one step (e.g. consideration of values and preferences spans several steps). The third column contains issues related to combinations of relevant conditions or multimorbidity for CPG developers to consider at each step of CPG development. The fourth column contains recommendations on how to resolve or ameliorate the identified issues. Below, we provide additional explanation for the recommendations.
Table 2.
Recommendations for Consideration of Multimorbidity in the Development of Clinical Practice Guidelines
| Item # | Guideline Development Step | Issue(s) for CPG Developers to Consider in CPG Development | Recommendations |
|---|---|---|---|
| 1, 2 | Topic nomination and topic scoping | When selecting a topic for guideline development, what may be important interactions between conditions or treatments to address? | a) Consider how disease-disease, disease-treatment, and treatment-treatment interactions, or limitations of life expectancy may result in specific consequences for clinical management. b) Determine whether the guideline should focus on an index condition with consideration of specific coexisting conditions or whether the guideline should focus on a combination of conditions. c) Review or estimate the scope and quality of evidence for the conditions under consideration. |
| 3 | Commissioning Work Group: Selection of Members | Who should be included in the guideline panel to provide expertise on relevant conditions? | a) Include experts who have substantial experience managing the relevant patient groups, participate in coordination of care, and regularly engage in shared decision-making. b) Incorporate views or values of patients with relevant coexisting conditions, patient advocates and consumer representatives. |
| 4 | Refining the key questions | How should relevant coexisting conditions be considered in the formulation of the guideline’s key questions according to PICO criteria: Population, Intervention, Comparator, and Outcomes? | Consider impact of relevant coexisting conditions in the formulation of all components of key questions: a) Determine how coexisting conditions affect the definitions of populations of interest, inclusion and exclusion criteria. b) Determine how coexisting conditions may affect effectiveness and harms of interventions. c) Determine how coexisting conditions affect the choice and range of relevant outcomes, including harms and treatment burden. If surrogates are considered as substitutes for outcomes, determine if the degree of linkage between surrogate and outcomes varies for populations with specific combinations of conditions. d) Determine the appropriate timeframe for assessing benefits and harms in the context of coexisting conditions, related prognosis, and competing risks. |
| 5 | Ranking importance of outcomes | How do relevant coexisting conditions affect the ranking of importance of outcomes? | a) Consider values and preferences of people with the relevant combination of conditions when determining importance of each outcome. b) Assess the importance of mortality, morbidity, harms and treatment burden in the target population with the coexisting conditions. c) Report explicitly any values and judgments used in ranking of outcomes. |
| 6 | Conducting systematic review of the evidence | How do relevant coexisting conditions affect the conduct of systematic reviews and synthesis of evidence? | a) Determine how searches need to be modified to include or specifically target studies in people with the relevant combination of conditions. b) Determine the criteria for eligible study designs in view of the trade-offs between greater validity from experimental studies and greater applicability from observational studies, and the appropriateness of combining evidence across different study designs. c) Search for relevant subgroup analyses or tests for interactions or modeling of baseline risk and risk of harms. d) Determine appropriateness of combining studies that included or excluded people with the coexisting conditions. e) Explore how the coexisting conditions impact baseline risk and effectiveness due to treatment responsiveness, harms or the opportunity for the treatment effect to manifest itself despite competing risks. |
| 7 | Assessing quality of evidence for each outcome, including directness of evidence or applicability for each outcome | How will relevant coexisting conditions affect the appraisal of the quality of evidence for each outcome, including appraisal of directness or applicability of evidence? | a) Determine how consideration of some coexisting conditions changes appraisal of quality of evidence for each outcome. b) Consider specifically how consideration of some coexisting conditions may modify directness of evidence or change applicability of evidence for a particular outcome. |
| 8 | Assessing quality of evidence overall, including directness or applicability | How will relevant coexisting conditions affect the appraisal of the quality of evidence across all outcomes, including appraisal of directness or applicability of overall evidence? | a) Determine how consideration of some coexisting conditions changes appraisal of quality of evidence overall. b) Consider specifically how consideration of some coexisting conditions may modify directness or change applicability of overall evidence. |
| 9 | Trading off benefits and harms | How do relevant coexisting conditions affect the net balance of benefits and harms including treatment burden? How do coexisting conditions affect the degree to which determining the net benefit may be more or less sensitive to individual preferences? |
a) Assess how benefits and harms and the resulting net balance change as a result of different baseline risk, different direction of effect, or different absolute or relative effect sizes in persons with relevant coexisting conditions. b) Specify the estimated time horizon for the net effect or important trade-offs over time. c) Assess to what degree coexisting conditions make the determination of net benefit more sensitive to individual preferences. d) Trade off benefits and harms and certainty based on what is known about the above (a-c). |
| 10 | Formulating recommendations and grading their strength | How does the formulation of a recommendation and its strength change in light of relevant coexisting conditions? Hoes do relevant coexisting conditions change the priority of recommendations? |
a) Either formulate recommendations for overarching groups with caveats for subgroups with relevant coexisting conditions, or formulate specific recommendations for certain populations. b) Determine how the strength of a recommendation needs to be modified for subgroups or people with relevant coexisting conditions or morbidity burden. Assess how coexisting conditions impact on the strength of a recommendation in view of the estimated time horizon for net benefit. c) Identify any people with coexisting conditions who are not likely to benefit or are likely to be harmed by following a recommendation. |
Steps 1 and 2: Topic Nomination and Topic Scoping
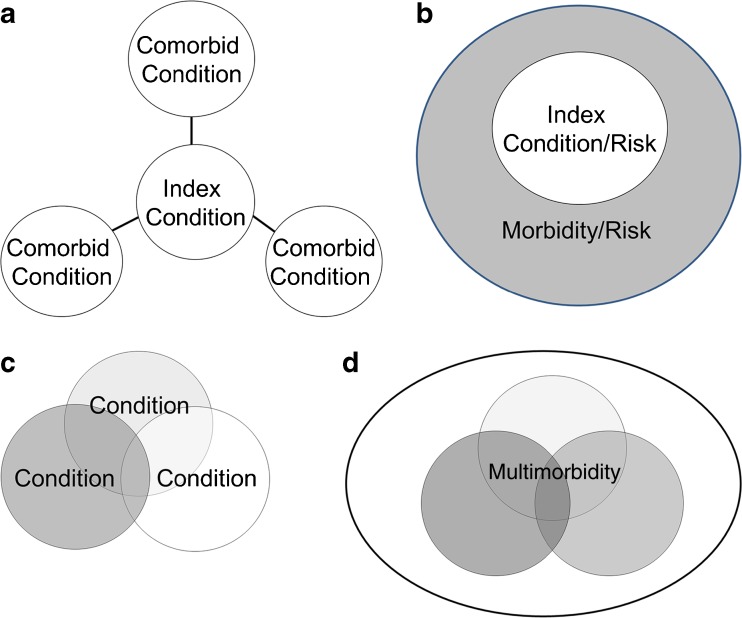
CPG developers are obligated to choose a topic and refine its scope. In the context of multimorbidity, they must also adopt a clear strategy to appropriately refine its scope around multimorbidity. A CPG may focus on a main (or index) condition with consideration of relevant coexisting conditions, or focus on specific combinations of conditions (Fig. 1). An example of a CPG for an index condition is one on hypertension, with a section focused on specific coexisting conditions; for example, hypertension in people with osteoarthritis (Fig. 1a). A CPG could focus on an index condition, but consider comorbidity through the frame of coexistent morbidity burden or risk indices (Fig. 1b). An example of a CPG focusing on a specific combination is a CPG for the care of people with diabetes and chronic kidney disease (CKD), or a CPG on HIV and CKD, and this approach could address specific combinations of three or more conditions as well (Fig. 1c).28,29 A CPG could focus on multimorbidity or its consequences, without naming specific individual conditions (Fig. 1d). CPG developers may formally consider clinical knowledge, epidemiological data, and biological plausibility as well as expert experience during the scoping phase to determine important conditions. The potential list of important conditions should consider not only diseases, but also conditions like risk for falls and cognitive impairment. Input from clinical experts is critical to focus on the coexisting conditions of greatest interest (i.e. relevant), likely conditions that frequently co-occur, carry a risk of poor outcomes, and whose presence or absence may lead to different actions. This process of choosing relevant coexisting conditions allows the formulation of an analytic framework that can focus literature searches and analyses.
Figure 1.
a) A guideline may focus on an index condition, and choose to address some number of comorbid (coexisting) conditions, or comorbidities. b) A guideline may focus on an index condition, or risk factor, and may choose to address how some marker of overall morbidity affects the management of the index condition. For example, the guideline may focus on diabetes, but may discuss how management would change in people with varying degrees of morbidity or mortality risk or other risk. c) A guideline may focus on a specific combination of conditions—where all (two conditions, three conditions, or more) are specified. An example is a guideline on the management of people with HIV and chronic kidney disease. d) A guideline may focus on multimorbidity, or its consequences. Examples may include a guideline focused on care coordination for multimorbid patients, or a guideline focused on polypharmacy or falls. Guidelines may use more than one of the above approaches, within the same guideline. This is meant for illustrative purposes.
Preliminary literature searches are often conducted for scoping. Searching for data on persons with more than one condition may require greater breadth in literature searches and abstract screening. Results on interactions and subgroups are usually not comprehensively identifiable with searches of key words and abstracts, and may require full text screening.
Step 3: Commissioning Workgroup: Selection of Members
A CPG workgroup that aims to consider a combination of conditions rather than a single condition will have to incorporate a greater range of expertise and explicitly take a patient-centered approach. Broadening the scope beyond a single index condition will require a greater range of judgment in evidence synthesis and appraisal. Methodological expertise is essential to support formulation of recommendations.25 Since the synthesis may require summary of potential benefits and harms across a wide array of disparate outcomes, adequate representation of specialists and generalists is key. While challenging to incorporate people with multimorbidity or caregivers in workgroups, their voice should be sought. A review to identify existing literature on patient values for the conditions of interest can be conducted(Step 5). At a minimum, patient input should be provided early through a review of the scope of work, and then again in the public review phase of the draft CPG.25 Finally, since the development of CPGs for this population will often require the use of less direct evidence, judgments by the workgroup will commonly be challenging and require adequate time and process for consensus building. As in all cases, a workgroup will require appropriate management of potential conflicts of interest.25,30
Step 4: Refining the Key Questions
In order to refine the key questions, the CPG workgroup should consider how the specific constellation of conditions or multimorbidity may impact the CPG’s key questions, with corresponding refinement of the “PICO” elements of population, intervention, comparator, and outcomes of interest. In particular, multimorbidity usually expands the number of potentially relevant outcomes. For example, important outcomes for management of hypertension in individuals with CKD include progression of kidney disease as well as cardiovascular events.31,32 Harms have to be explicitly considered in the form of overall treatment burden as unintended consequences, such as contrast-dye-induced acute kidney injury in a patient with CKD and cardiovascular disease (CVD) undergoing angiographic evaluation.33,34 Further, the key questions or protocol should specify the desired duration of follow-up for assessment of benefits and harms, allowing CPG users to interpret the estimates in the context of prognosis.
The use of surrogates as outcomes is discouraged in CPG development.25,35 When surrogates are used as substitutes, comorbidity may affect how well the surrogate will substitute because the degree of linkage between a surrogate and a patient-important outcome may be altered. For example, in individuals with CKD who also have renal osteodystrophy, fracture risk does not reliably correlate with bone mineral density (BMD), decreasing the certainty that an improvement in BMD will translate into benefit from preventing fractures.36
Step 5: Ranking Importance of Outcomes
CPG developers must acknowledge that multimorbidity may alter the relative importance of chosen outcomes.4 Importance of an outcome may be affected by competing risk for other outcomes. For a patient with CKD and diabetes, progression of CKD to kidney failure may be less likely than death from CVD. Symptomatic relief and reducing treatment burden may become more important with older age. Similarly, a person with multimorbidity at high immediate risk for outcomes (such as a heart attack, stroke, or death), may consider improvement in the long-term likelihood for an outcome from another disease (such as fracture from osteoporosis) relatively less important.
Thus, when multiple outcomes are possible, their relative importance may be sensitive to the patient preferences. This underscores the need for information on values and preferences of people with the relevant combination of conditions, to determine the importance of outcomes and the degree of variability. Information may be sought through literature review, interviews in focus groups, or direct input by patients or their representatives in the CPG development process.20,37–39 The American College of Chest Physicians systematically reviewed literature relating to values and preferences of patients considering antithrombotic therapy.40 The absence of informative data would highlight an important research need that can be articulated in the CPG. In the absence of informative data, CPG workgroups must make judgments regarding typical values and preferences and the extent of variability in these, to guide their recommendations.20,41 Those values and preferences should be stated explicitly and transparently and, if possible, quantitatively.
Step 6: Conducting Systematic Review of the Evidence
The issues and recommendations pertinent for the conduct of systematic review are described in detail in a companion article.16 As discussed for Step 2, identification of potentially relevant publications requires specific skill and effort, with expansion of search terms and full text searches for analyses of subgroups or effect modifiers (e.g. when searching for hypertension trials in individuals with CKD, the search may be conducted without restriction to CKD, in order to capture hypertension trials in the general population of hypertensive individuals and review their full text publications for valid CKD subgroup analyses.)42
Variable definitions of risk factors and comorbidities often hampers synthesis across studies, and there may be bias in the reporting of subgroup results. 42–44 Analyses can be challenging to interpret.11 If trial evidence for the target population or the outcomes of interest is limited, review of observational studies may yield additional insights, giving rise to the attendant challenges of assessing methodological rigor and synthesizing across different study designs. Further, extrapolation of evidence from people with single conditions to people with multimorbidity may be necessary, even though this decreases the certainty in the conclusions by introducing indirectness (or reducing applicability). Overall synthesis may entail extrapolation of evidence, with judgments about how coexisting conditions modify baseline risk and effectiveness due to treatment responsiveness, harms, or the opportunity for the treatment effect to manifest itself despite competing risks.
Step 7: Assessing Quality of Evidence for Each Outcome, Including Directness of Evidence or Applicability for Each Outcome
Assessment of how multimorbidity affects the appraisal of evidence by increasing indirectness or reducing applicability is challenging. The degree of directness or applicability of evidence is a measure of how the conclusions from studies relate to the target population in the target context. This requires a clear specification of the relevant setting, population, intervention, and comparator to allow appraisal of applicability with regards to what was prespecified.
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group includes an assessment of the directness of evidence as a component of assessing the quality of evidence for each outcome.45 GRADE considers important implications of any mismatch between the population studied and the population that is the target of inference, along with mismatches between interventions, comparators and outcomes.
The Evidence-based Practice Centers, which produce systematic reviews rather than CPGs, use a similar approach for assessing strength of evidence, but make judgments about applicability explicit and separate from assessments of other domains of strength of evidence.23,24,46,47 Their goal in assessing applicability separately is to enable decision-makers to take into account how well the evidence maps to the populations, settings, diseases or conditions, interventions, comparators, and outcomes that are most relevant to their decisions. Thus, for “applicability,” Evidence-based Practice Centers typically highlight noteworthy features of included studies and identify potential limitations to applicability.
In either system, directness of evidence or applicability may be impacted by specific comorbidities or burden of multimorbidity. The assessment of directness or applicability needs to be repeated for each distinct target population and for each important outcome, as well as across all outcomes (Step 8). The fact that a group of patients was study-eligible, does not necessarily imply that results apply directly to all individuals in this group, if such patients were not included in the study in sufficient numbers, appropriate subgroup analysis was not performed and/or there is reason to anticipate heterogeneity of effects.43,48 Persons with multimorbidity are likely to be under-represented in the evidence base, compounding this problem.12,44,49
Synthesis is more challenging when it has to account for a patchwork of evidence with varying directness (or applicability) and methodological quality. For example, evidence on the comparative effectiveness of different anti-hypertensive agents in individuals with CKD and hypertension is derived from trials in CKD patients with surrogates including kidney function and proteinuria, and from hypertension trials that examined CVD outcomes and mortality often without separately reporting results for those with CKD. Experimental evidence with surrogates and short duration of follow-up may need to be combined with observational studies that associate a risk factor or treatment exposure with patient-important outcomes. An understanding of how pathophysiology, baseline risks and risk relationships (i.e. direction and size of effect estimates) may vary across heterogeneous populations is necessary to support judgments about how to combine evidence and reconcile inconsistency across different sources of evidence. Referring back to the analytic framework (Steps 1 and 2) is helpful for evidence synthesis.
Step 8: Assessing Quality of Evidence Overall, Including Directness or Applicability
To estimate the overall quality of evidence across all important outcomes, the same considerations as in Step 7 apply. The context of comorbidity makes decision-making more complex, since the estimate for overall quality of evidence or applicability needs to consider the importance of each relevant outcome (Step 6), and how the evidence for it is altered as a result of indirectness or reduced applicability (Step 7). The deliberations and decisions should be described explicitly. For example, transparent deliberations about how directly evidence applies to certain populations are essential. The Antithrombotic Therapy for Atrial Fibrillation CPG discusses how older age increases bleeding risk, but explains that they refrained from making separate recommendations depending on bleeding risk because of the lack of high-quality, precise and validated bleeding risk scores.52
When applicability is assessed separately from the quality of evidence, as in the approach followed by the Evidence-based Practice Centers, noteworthy features of the overall body of evidence need to be highlighted to allow users to apply the findings to persons with multimorbidity.
Step 9: Trading Off Benefits and Harms
A CPG workgroup that considers a combination of conditions rather than a single condition will need to assess how comorbidity and prognosis may impact on the expected balance of benefits and harms and perceived treatment burden. In GRADE, this step is part of formulating recommendations and grading their strength. For persons with multimorbidity, effects across a spectrum of outcomes need to be summarized, including some that are more difficult to harmonize, such as scales for functional outcomes.50 The estimated time horizon for the net effects, both in absolute and relative terms, and important trade-offs over time need specification.
Recognizing that it is impossible to make evidence-based recommendations without some judgments and assumptions about preferences, CPG developers should incorporate what is known about values and preferences of individuals with the relevant coexisting conditions through the methods described above (Step 5), explaining judgments they have made on behalf of patients.20,41 Existing guidance for transparent decision modeling may prove useful to quantitatively address the impact of such judgments.12,51
Step 10: Formulating Recommendations and Grading Their Strength
CPG workgroups must determine whether to formulate recommendations for overarching groups with modification of strength for certain populations or whether to formulate specific recommendations for specific populations. This should be decided early in the development process. The strength of a recommendation may need to be altered for subgroups of people with specific coexisting conditions, or stratified based on morbidity burden or a morbidity index. The Antithrombotic Therapy for Atrial Fibrillation CPG provides recommendations for patients with various stroke risks according to a comorbidity-based risk score.52 Similarly, treatment goals for glycemia, blood pressure, and dyslipidemia in older adults with diabetes may vary based on the degree of comorbidity.53
There should be an effort to highlight key CPG recommendations and to consider how the presence of multimorbidity or specific coexisting conditions impacts the priority of following a recommendation. Examples include identifying groups with specific coexisting conditions that are not likely to benefit or may be harmed from following a CPG recommendation, and those for whom trade-offs are uncertain. The CPG workgroup may identify scenarios of comorbidity or limitations of life expectancy, when a recommendation should not be or no longer be followed. If a recommendation does not need any modification and is also applicable for patients with specific coexisting conditions or multimorbidity, this should be explicitly stated.
ROADMAP FOR FUTURE RESEARCH
Future research is needed to advance the science of addressing multimorbidity in CPGs. CPGs build on primary evidence from trials and observational studies, and their synthesis through systematic reviews and meta-analyses. Thus, their ability to be tailored to individuals with multimorbidity depends on how trials and observational studies and evidence syntheses include people with multimorbidity and apply to them. Until recommendations for improving evidence generation and evidence synthesis for this purpose are implemented,11,16 the steps outlined above provide some practical guidance to help CPG developers assess currently available evidence to inform care of individuals with multimorbidity.
A major challenge is to explore and describe risk stratification and how it can be used to predict treatment response. Morbidity indices collapse a multitude of variables into one measure to characterize overall burden of disease or risk for specific outcomes. Morbidity indices can be used to explore treatment heterogeneity.48,54,55 In the context of CPGs for patients with comorbidities, prediction instruments with adequate validity, feasibility, and discriminatory performance may be useful when the independent variables include conditions other than the one for which the instrument is being developed. More prediction instruments may become available for particular disease clusters and may be considered in CPG development, but some have cautioned about the utility of instruments for decision-making for an individual patient.56,57 Not all indices transparently report the uncertainty surrounding the prediction estimates.58
A number of innovations are needed going forward. First, development and validation of instruments to improve risk prediction for multiple outcomes of importance to patients is needed. Second, risk-based exploration of treatment heterogeneity is needed for tailoring treatment recommendations, avoiding inappropriate subgroup analyses.42,59 Third, meaningful outcomes need to be assessed that comprehensively capture health states and mortality at defined time points.25,35,60 Treatment effects should be expressed in absolute and relative terms. A number of steps will require an expansion of our knowledge of patient values and judgments in the context of multimorbidity, to help weigh benefits and harms. Such advances will facilitate development of CPGs that can inform decision-making with people with multimorbidity in clinical practice. Future research is needed to support complex decision-making in multimorbid patients, through better tools for predicting risk, for communicating risks and for eliciting preferences in clinical practice.
CONCLUSION
We present a framework to craft CPGs that are more useful for application to the person with multimorbidity. Many of the recommended steps depend to a large degree on the best effort and ability of a CPG workgroup to make complex judgments, often in the absence of high quality evidence. As trials and systematic reviews better address multimorbidity over time, the usefulness of the evidence base will improve. Although our recommendations are based on the expertise of CPG developers, clinicians for patients with multimorbidity, clinical researchers and with input from a wide panel of experts and stakeholders, these recommendations will require validation through implementation, evaluation and refinement.
Acknowledgments
The expert panel included Klara Brunnhuber, Jako S. Burgers, Sheldon Greenfield, Gordon Guyatt, Kevin High, Rosanne Leipzig, Cynthia Mulrow, Kenneth Schmader, Holger Schunemann, Louise C. Walter, and James Woodcock.
We acknowledge Anand Parekh and Kay Dickersin for their attendance for part of the ‘Improving Guidelines for Multimorbid Patients Stakeholder Conference.’
We acknowledge the Participants in the Guideline Breakout Groups who attended the ‘Improving Guidelines for Multimorbid Patients Stakeholder Conference’ (See below), Baltimore, Maryland, Fall 2010
Bass, Eric
Blaum, Caroline
Bruce, Stephanie
Hadley, Evan
Nici, Linda
Noronha, Gary
Green, Lee
Lewis, Sandra Zelman
Wolff, Tracy
Shiffman, Richard
Schmader, Ken
Walter, Louise
Barton, Mary
Greenfield, Sheldon
Grimshaw, Jeremy
High, Kevin
Lau, Joseph
Dolter, Kathryn
Mukherjee, Debjani
Nix, Mary
ZuWallack, Richard
Stokes, Tim
Schunemann, Holger
Funding
This work was funded by AHRQ R21 HS018597-01 (PI Boyd) and AHRQ R21 HS017653. Dr. Boyd’s effort was supported in part by the Johns Hopkins Bayview Center for Innovative Medicine, The Robert Wood Johnson Foundation Physician Faculty Scholars Program, and the Paul Beeson Career Development Award Program (NIA K23 AG032910, AFAR, The John A. Hartford Foundation, The Atlantic Philanthropies, The Starr Foundation, and an anonymous donor). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Dr. Walter’s effort was supported by the National Cancer Institute (grant number R01 CA134425) and the National Institute on Aging (grant number K24 AG041180).
Improving Guidelines for Multimorbid Patients Investigator Group
Cynthia Boyd, Johns Hopkins Medical Institutions (JHMI), Sydney Dy, Johns Hopkins Bloomberg School of Public Health (JHSPH), David M. Kent, Tufts Medical Center (TMC), Bruce Leff, JHMI, Jodi Segal (JHMI) Thomas A. Trikalinos, Brown University, Katrin Uhlig, TMC, Ravi Varadhan, JHMI, Carlos Weiss, JHMI.
Conflict of Interest
Dr. Boyd is a co-author of an article on multimorbidity for UpToDate.
REFERENCES
- 1.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 2.Patient-centered care for older adults with multiple chronic conditions: a stepwise approach from the american geriatrics society: american geriatrics society expert panel on the care of older adults with multimorbidity. J Am Geriatr Soc. 2012;60(10):1957–68. [DOI] [PMC free article] [PubMed]
- 3.Tinetti ME, Bogardus ST, Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med. 2004;351(27):2870–4. doi: 10.1056/NEJMsb042458. [DOI] [PubMed] [Google Scholar]
- 4.Fabbri LM, Boyd C, Boschetto P, Rabe KF, Buist AS, Yawn B, et al. How to integrate multiple comorbidities in guideline development: article 10 in Integrating and coordinating efforts in COPD guideline development. An official ATS/ERS workshop report. Proc Am Thorac Soc. 2012;9(5):274–81. doi: 10.1513/pats.201208-063ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294(6):716–24. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 6.Schoen C, Osborn R, Doty MM, Bishop M, Peugh J, Murukutla N. Toward higher-performance health systems: adults’ health care experiences in seven countries, 2007. Health Aff (Millwood) 2007;26(6):w717–34. doi: 10.1377/hlthaff.26.6.w717. [DOI] [PubMed] [Google Scholar]
- 7.Lugtenberg M, Burgers JS, Clancy C, Westert GP, Schneider EC. Current guidelines have limited applicability to patients with comorbid conditions: a systematic analysis of evidence-based guidelines. PLoS ONE. 2011;6(10):e25987. doi: 10.1371/journal.pone.0025987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown AF, Mangione CM, Saliba D, Sarkisian CA. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc. 2003;51(5 Suppl Guidelines):S265–80. doi: 10.1046/j.1532-5415.51.5s.1.x. [DOI] [PubMed] [Google Scholar]
- 9.Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuunemann HJ. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):7S–47S. doi: 10.1378/chest.1412S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Department of Health and Human Services Strategic Framework on Multiple Chronic Conditions, 2010.
- 11.Weiss CO, Varadhan R, Puhan M, Vickers A, Bandeen-Roche K, Boyd C, et al. Multimorbidity and Evidence Generation. JGIM. 2013, doi:10.1007/s11606-013-2555-5. [DOI] [PMC free article] [PubMed]
- 12.Van Spall HG, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA. 2007;297(11):1233–40. doi: 10.1001/jama.297.11.1233. [DOI] [PubMed] [Google Scholar]
- 13.Nouwen A, Winkley K, Twisk J, Lloyd CE, Peyrot M, Ismail K, et al. Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia. 2010;53(12):2480–6. doi: 10.1007/s00125-010-1874-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braithwaite RS, Fiellin D, Justice AC. The payoff time: a flexible framework to help clinicians decide when patients with comorbid disease are not likely to benefit from practice guidelines. Med Care. 2009;47(6):610–7. doi: 10.1097/MLR.0b013e31819748d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braithwaite RS, Concato J, Chang CC, Roberts MS, Justice AC. A framework for tailoring clinical guidelines to comorbidity at the point of care. Arch Intern Med. 2007;167(21):2361–5. doi: 10.1001/archinte.167.21.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trikalinos T, Segal J, Boyd CM. Addressing Multimorbidity in Evidence Integration and Synthesis. JGIM. 2013, doi:10.1007/s11606-013-2661-4. [DOI] [PMC free article] [PubMed]
- 17.Boyd C, Kent D. Evidence Based Medicine and the Hard Problem of Multimorbidity. JGIM. 2013, doi:10.1007/s11606-013-2658-z. [DOI] [PMC free article] [PubMed]
- 18.Lunney JR, Lynn J, Foley DJ, Lipson S, Guralnik JM. Patterns of functional decline at the end of life. JAMA. 2003;289(18):2387–92. doi: 10.1001/jama.289.18.2387. [DOI] [PubMed] [Google Scholar]
- 19.Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians? BMJ. 2008;336(7651):995–8. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schunemann HJ, Jaeschke R, Cook DJ, Bria WF, El-Solh AA, Ernst A, et al. An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. Am J Respir Crit Care Med. 2006;174(5):605–14. doi: 10.1164/rccm.200602-197ST. [DOI] [PubMed] [Google Scholar]
- 22.Barton MB, Miller T, Wolff T, Petitti D, LeFevre M, Sawaya G, et al. How to read the new recommendation statement: methods update from the U.S. Preventive Services Task Force. Ann Intern Med. 2007;147(2):123–7. doi: 10.7326/0003-4819-147-2-200707170-00171. [DOI] [PubMed] [Google Scholar]
- 23.Petitti DB, Teutsch SM, Barton MB, Sawaya GF, Ockene JK, DeWitt T. Update on the methods of the U.S. Preventive Services Task Force: insufficient evidence. Ann Intern Med. 2009;150(3):199–205. doi: 10.7326/0003-4819-150-3-200902030-00010. [DOI] [PubMed] [Google Scholar]
- 24.Owens DK, Lohr KN, Atkins D, Treadwell JR, Reston JT, Bass EB, et al. AHRQ Series Paper 5: grading the strength of a body of evidence when comparing medical interventions—Agency for Healthcare Research and Quality and the Effective Health-Care Program. J Clin Epidemiol. 2010;63(5):513–23. doi: 10.1016/j.jclinepi.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Institute of Medicine. Clinical Practice Guidelines We Can Trust: The National Academies Press, 2011. [PubMed]
- 26.Guideline Development Methods: Information for National Collaborating Centres and Guideline Developers. London: National Institute for Clinical Excellence; 2004. [Google Scholar]
- 27.U.S. Preventive Services Task Force (USPSTF) Procedure Manual. http://www.uspreventiveservicestaskforce.org/uspstf08/methods/procmanual.htm, Accessed on November 1, 2013.
- 28.Gupta SK, Eustace JA, Winston JA, Boydstun II, Ahuja TS, Rodriguez RA, et al. Guidelines for the management of chronic kidney disease in HIV-infected patients: recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2005;40(11):1559–85. doi: 10.1086/430257. [DOI] [PubMed] [Google Scholar]
- 29.KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis 2007;49(2 Suppl 2):S12–154. [DOI] [PubMed]
- 30.Guyatt G, Akl EA, Hirsh J, Kearon C, Crowther M, Gutterman D, et al. The vexing problem of guidelines and conflict of interest: a potential solution. Ann Intern Med. 2010;152(11):738–41. doi: 10.7326/0003-4819-152-11-201006010-00254. [DOI] [PubMed] [Google Scholar]
- 31.Uhlig K, Boyd C. Guidelines for the older adult with CKD. Am J Kidney Dis. 2011;58(2):162–5. doi: 10.1053/j.ajkd.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Parker MG, Atkins MB, Ucci AA, Levey AS. Rapidly progressive glomerulonephritis after immunotherapy for cancer. J Am Soc Nephrol. 1995;5(10):1740–4. doi: 10.1681/ASN.V5101740. [DOI] [PubMed] [Google Scholar]
- 33.Giovannetti ER, Wolff JL, Xue QL, Weiss CO, Leff B, Boult C, et al. Difficulty assisting with health care tasks among caregivers of multimorbid older adults. J Gen Intern Med. 2012;27(1):37–44. doi: 10.1007/s11606-011-1831-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrett BJ, Parfrey PS. Clinical practice. Preventing nephropathy induced by contrast medium. N Engl J Med. 2006;354(4):379–86. doi: 10.1056/NEJMcp050801. [DOI] [PubMed] [Google Scholar]
- 35.Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64(4):395–400. doi: 10.1016/j.jclinepi.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 36.KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2009(113):S1–130. [DOI] [PubMed]
- 37.Rahn DD, Abed H, Sung VW, Matteson KA, Rogers RG, Morrill MY, et al. Systematic review highlights difficulty interpreting diverse clinical outcomes in abnormal uterine bleeding trials. J Clin Epidemiol. 2011;64(3):293–300. doi: 10.1016/j.jclinepi.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Legare F, Boivin A, van der Weijden T, Pakenham C, Burgers J, Legare J, et al. Patient and public involvement in clinical practice guidelines: a knowledge synthesis of existing programs. Med Dec Making. 2011;31(6):E45–74. doi: 10.1177/0272989X11424401. [DOI] [PubMed] [Google Scholar]
- 39.den Breejen EM, Nelen WL, Knijnenburg JM, Burgers JS, Hermens RP, Kremer JA. Feasibility of a wiki as a participatory tool for patients in clinical guideline development. J Med Internet Res. 2012;14(5):e138. doi: 10.2196/jmir.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacLean S, Mulla S, Akl EA, Jankowski M, Vandvik PO, Ebrahim S, et al. Patient values and preferences in decision making for antithrombotic therapy: a systematic review: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e1S–23S. doi: 10.1378/chest.11-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butler M, Talley KMC, Burns R, Ripley A, Rothman A, Johnson P, et al. Values of Older Adults Related to Primary and Secondary Prevention. Rockville (MD): Agency for Healthcare Research and Quality (US); 2011. [PubMed] [Google Scholar]
- 42.Sun X, Briel M, Busse JW, You JJ, Akl EA, Mejza F, et al. Credibility of claims of subgroup effects in randomised controlled trials: systematic review. BMJ. 2012;344:e1553. doi: 10.1136/bmj.e1553. [DOI] [PubMed] [Google Scholar]
- 43.Vollenweider D, Boyd CM, Puhan MA. High prevalence of potential biases threatens the interpretation of trials in patients with chronic disease. BMC Med. 2011;9:73. doi: 10.1186/1741-7015-9-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boyd CM, Vollenweider D, Puhan MA. Informing evidence-based decision-making for patients with comorbidity: availability of necessary information in clinical trials for chronic diseases. PLoS One. 2012;7(8):e41601. doi: 10.1371/journal.pone.0041601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence–indirectness. J Clin Epidemiol. 2011;64(12):1303–10. doi: 10.1016/j.jclinepi.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 46.Guyatt GH, Helfand M, Kunz R. Comparing the USPSTF and GRADE approaches to recommendations. Ann Intern Med. 2009;151(5):363. doi: 10.7326/0003-4819-151-5-200909010-00016. [DOI] [PubMed] [Google Scholar]
- 47.Petitti DB, Teutsch SM, Barton MB, Sawaya GF, Ockene JK, DeWitt T. Comparing the USPSTF and GRADE Approaches to Recommendations. Ann Intern Med. 2009;151(5):363–64. doi: 10.7326/0003-4819-151-5-200909010-00017. [DOI] [PubMed] [Google Scholar]
- 48.Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: the need for risk stratification. JAMA. 2007;298(10):1209–12. doi: 10.1001/jama.298.10.1209. [DOI] [PubMed] [Google Scholar]
- 49.Zulman DM, Sussman JB, Chen X, Cigolle CT, Blaum CS, Hayward RA. Examining the evidence: a systematic review of the inclusion and analysis of older adults in randomized controlled trials. J Gen Intern Med. 2011;26(7):783–90. doi: 10.1007/s11606-010-1629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leipzig RM, Whitlock EP, Wolff TA, Barton MB, Michael YL, Harris R, et al. Reconsidering the approach to prevention recommendations for older adults. Ann Intern Med. 2010;153(12):809–14. doi: 10.7326/0003-4819-153-12-201012210-00007. [DOI] [PubMed] [Google Scholar]
- 51.Trikalinos TA, Kulasingam S, Lawrence WF. Chapter 10: deciding whether to complement a systematic review of medical tests with decision modeling. J Gen Intern Med. 2012;27(Suppl 1):S76–82. doi: 10.1007/s11606-012-2019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.You JJ, Singer DE, Howard PA, Lane DA, Eckman MH, Fang MC, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e531S–75S. doi: 10.1378/chest.11-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, et al. Diabetes in older adults. Diabetes Care. 2012;35(12):2650–64. doi: 10.2337/dc12-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greenfield S, Billimek J, Pellegrini F, Franciosi M, De Berardis G, Nicolucci A, et al. Comorbidity affects the relationship between glycemic control and cardiovascular outcomes in diabetes: a cohort study. Ann Intern Med. 2009;151(12):854–60. doi: 10.7326/0003-4819-151-12-200912150-00005. [DOI] [PubMed] [Google Scholar]
- 55.Justice AC, McGinnis KA, Skanderson M, Chang CC, Gibert CL, Goetz MB, et al. Towards a combined prognostic index for survival in HIV infection: the role of 'non-HIV' biomarkers. HIV Med. 2010;11(2):143–51. doi: 10.1111/j.1468-1293.2009.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Minne L, Ludikhuize J, de Rooij SE, Abu-Hanna A. Characterizing predictive models of mortality for older adults and their validation for use in clinical practice. J Am Geriatr Soc. 2011;59(6):1110–5. doi: 10.1111/j.1532-5415.2011.03411.x. [DOI] [PubMed] [Google Scholar]
- 57.Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: a systematic review. JAMA. 2012;307(2):182–92. doi: 10.1001/jama.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Framingham Heart Study, http://www.framinghamheartstudy.org/risk/coronary.html, Accessed on September 17, 2013.
- 59.Kent DM, Rothwell PM, Ioannidis JP, Altman DG, Hayward RA. Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal. Trials. 2010;11:85. doi: 10.1186/1745-6215-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tinetti ME, McAvay GJ, Chang SS, Newman AB, Fitzpatrick AL, Fried TR, et al. Contribution of multiple chronic conditions to universal health outcomes. J Am Geriatr Soc. 2011;59(9):1686–91. [DOI] [PMC free article] [PubMed]