Abstract
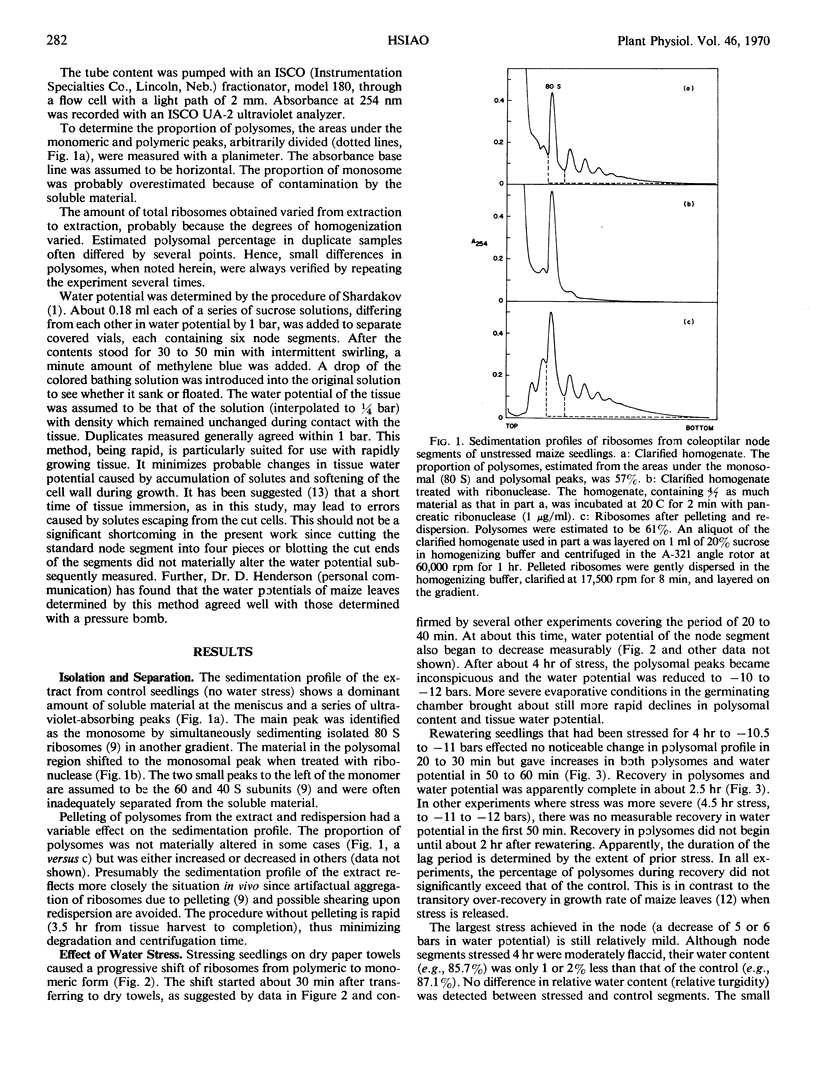
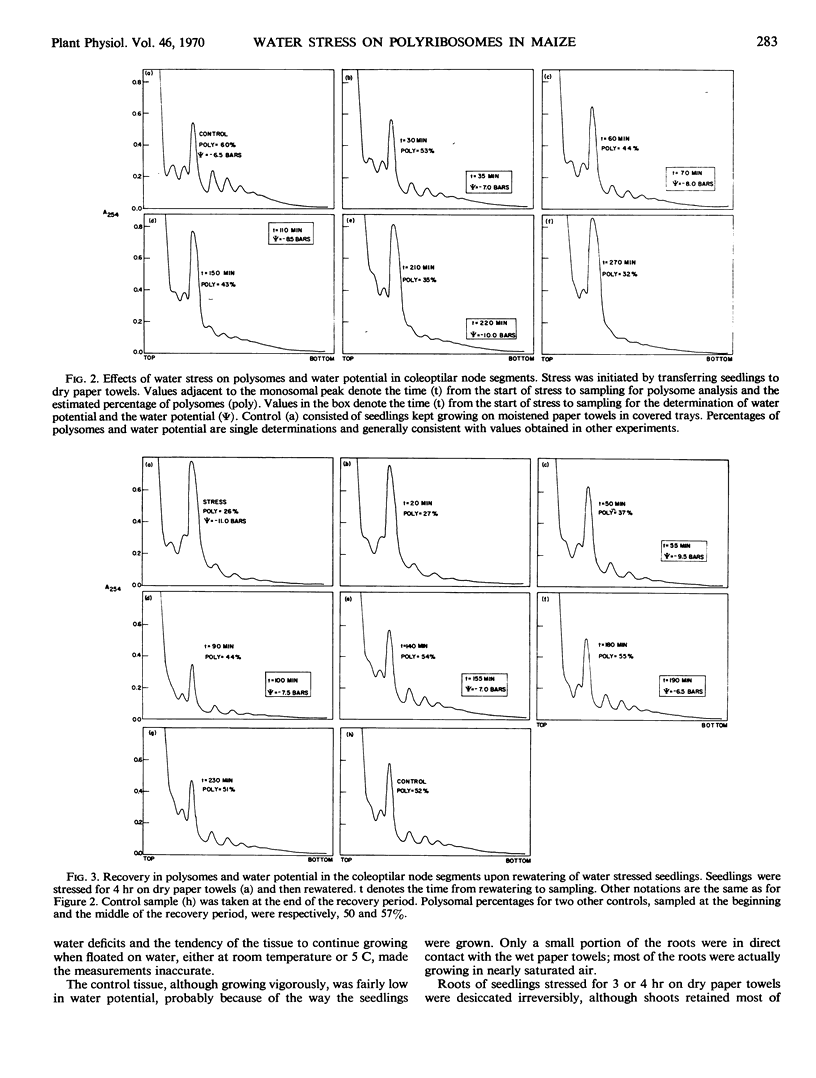
Sucrose gradient profiles of polyribosomes from the coleoptilar node region of seedlings of Zea mays L. were obtained without pelleting and redispersion of the particles. Water stress caused a shift of ribosomes from the polymeric to the monomeric form, starting about 30 minutes after stress initiation and when the water potential of the tissue began to decrease measurably. After about 4 hours of stress (a decrease in tissue water potential of about 5 bars), most of the higher polymers of ribosomes had shifted to monoribosomes. Release of stress caused the ribosomes to revert from monomeric to polymeric form after a lag period apparently determined by the extent of prior stress. Use of bentonite and isolation of polyribosomes from combined stressed and control tissue gave results indicating that the reduced polyribosomal level was not an artifact caused by ribonuclease during isolation.
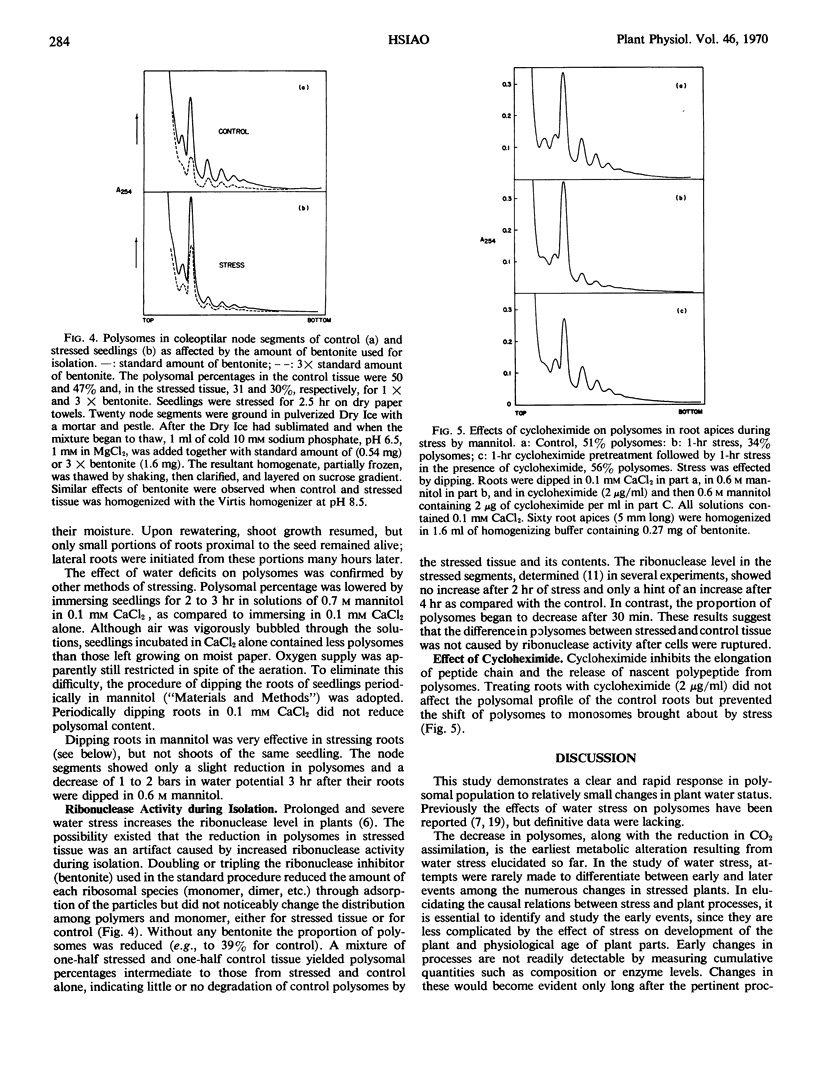
Incubating roots in cycloheximide (2 micrograms per milliliter) had no effect on the proportion of polyribosomes in control roots, but it prevented the loss of polyribosomes caused by stress. Since cycloheximide inhibits the release of nascent polypeptide from polyribosomes, it appears possible that stress-effected loss in polyribosomes occurs only if polypeptides can be terminated and released.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Zioni A., Itai C., Vaadia Y. Water and salt stresses, kinetin and protein synthesis in tobacco leaves. Plant Physiol. 1967 Mar;42(3):361–365. doi: 10.1104/pp.42.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK M. F., MATTHEWS R. E., RALPH R. K. RIBOSOMES AND POLYRIBOSOMES IN BRASSICA PEKINENSIS. Biochim Biophys Acta. 1964 Oct 16;91:289–304. doi: 10.1016/0926-6550(64)90253-1. [DOI] [PubMed] [Google Scholar]
- Chen D., Sarid S., Katchalski E. The role of water stress in the inactivation of messenger RNA of germinating wheat embryos. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1378–1383. doi: 10.1073/pnas.61.4.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove L. D. Ribonuclease activity of stressed tomato leaflets. Plant Physiol. 1967 Sep;42(9):1176–1178. doi: 10.1104/pp.42.9.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. B. Growth Physics in Nitella: a Method for Continuous in Vivo Analysis of Extensibility Based on a Micro-manometer Technique for Turgor Pressure. Plant Physiol. 1968 Aug;43(8):1169–1184. doi: 10.1104/pp.43.8.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao T. C., Acevedo E., Henderson D. W. Maize leaf elongation: continuous measurements and close dependence on plant water status. Science. 1970 May 1;168(3931):590–591. doi: 10.1126/science.168.3931.590. [DOI] [PubMed] [Google Scholar]
- Hsiao T. C. Ribonuclease Activity Associated With Ribosomes of Zea mays. Plant Physiol. 1968 Sep;43(9):1355–1361. doi: 10.1104/pp.43.9.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. Y., Key J. L. Dissocation and reassembly of polyribosomes in relation to protein synthesis in the soybean root. J Mol Biol. 1967 Jun 14;26(2):237–247. doi: 10.1016/0022-2836(67)90294-x. [DOI] [PubMed] [Google Scholar]
- Noll H. Characterization of macromolecules by constant velocity sedimentation. Nature. 1967 Jul 22;215(5099):360–363. doi: 10.1038/215360a0. [DOI] [PubMed] [Google Scholar]


