Abstract
Various disease models have been shown to alter hepatic drug-metabolizing enzyme (DME) and transporter expression and to induce cholestasis through altered enzyme and transporter expression. Previously, we detailed the regulation of hepatic DMEs during infectious colitis caused by Citrobacter rodentium infection. We hypothesized that this infection would also modulate hepatic drug transporter expression and key genes of bile acid (BA) synthesis and transport. Mice lacking Toll-like receptor 4 (TLR4), interleukin-6 (IL-6), or interferon-gamma (IFNγ) and appropriate wild-type animals were orally infected with C. rodentium and sacrificed 7 days later. In two wild-type strains, drug transporter mRNA expression was significantly decreased by infection for Slc22a4, Slco1a1, Slco1a4, Slco2b1, and Abcc6, whereas the downregulation of Abcc2, Abcc3, and Abcc4 were strain-dependent. In contrast, mRNA expressions of Slco3a1 and Abcb1b were increased in a strain-dependent manner. Expression of Abcb11, Slc10a1, the two major hepatic BA transporters, and Cyp7a1, the rate-limiting enzyme of BA synthesis, was also significantly decreased in infected animals. None of the above effects were caused by bacterial lipopolysaccharide, since they still occurred in the absence of functional TLR4. The downregulation of Slc22a4 and Cyp7a1 was absent in IFNγ-null mice, and the downregulation of Slco1a1 was abrogated in IL-6-null mice, indicating in vivo roles for these cytokines in transporter regulation. These data indicate that C. rodentium infection modulates hepatic drug processing through alteration of transporter expression as well as DMEs. Furthermore, this infection downregulates important genes of BA synthesis and transport and may increase the risk for cholestasis.
Introduction
Many endogenous and exogenous chemical substances are eliminated primarily by hepatobiliary efflux. The hepatic transporters responsible for this route of elimination belong to two gene families, ATP-binding cassette (ABC) and solute carrier (SLC). Members of these families often share overlapping substrate pools, resulting in the vectorial transport of target chemicals from the sinusoidal blood into the bile (Klaassen and Aleksunes, 2010).
As well as sharing overlapping substrate specificities, hepatic transporters often share common regulatory mechanisms both with other transporters and with hepatic drug-metabolizing enzymes (DMEs). Congiu et al. (2009) reported significant correlations between hepatic expression of DMEs, drug transporters, and transcription factors during viral hepatitis. Understanding the mechanisms and results of this coordinate regulation of DME and transporter expression would provide an invaluable tool in the prediction of altered drug and toxicant disposition in disease states.
Infection and inflammation have been shown to significantly impact hepatic DMEs and drug transporters, in both the clinical setting and a number of experimental models (Teng and Piquette-Miller, 2005, 2008; Morgan et al., 2008; Cressman et al., 2012). Because of the vital role these proteins play in both the clearance of drugs and toxicants and the metabolism of physiologic molecules, changes in their expression or activity may lead to adverse effects (Saab et al., 2013). However, despite their overlapping substrate specificities, DME and drug transporters have many distinct and specific substrates that they recognize and act upon. Therefore, detailing the effects of inflammation on specific genes (rather than on the class as a whole) is vital to understanding the potential risks and toxicity that may accompany disease and inflammation.
We have previously demonstrated that models of live infection may exhibit distinct and specific patterns of gene expression when compared with lipopolysaccharide (LPS) administration, a standard model of inflammation (Richardson and Morgan, 2005; Chaluvadi et al., 2009). Citrobacter rodentium is a rodent-specific intestinal pathogen, producing colitis similar to that seen in enteropathogenic Escherichia coli (EPEC) in humans. C. rodentium infection produces selective, mostly reversible effects on expression of cytochrome P450s (P450s) and other DMEs (Richardson and Morgan, 2005; Chaluvadi et al., 2009). Interestingly, though this Gram-negative bacterium produces LPS, the P450 gene expression changes appear to be LPS-independent, as animals lacking the LPS receptor Toll-like receptor 4 (TLR4) exhibited the same effects (Richardson et al., 2006).
Just as this model of live infection exhibits distinct and specific patterns of gene expression when compared with lipopolysaccharide administration, so different disease states may present divergent impacts on gene expression through distinct mechanisms. As yet, nothing is known about the regulation of hepatic drug transporters during infectious colitis. Therefore, we here report our findings on the impact of C. rodentium infection on hepatic transporter expression. To determine whether the effects of infection are attributable to bacterial lipopolysaccharide, we performed these studies in wild-type mice and mice lacking a functional TLR4.
To properly predict the effects of a similar disease state in humans, it is necessary to understand the mechanisms underlying the modulation of these genes. We have previously investigated several potential mechanisms behind the effects of C. rodentium infection on hepatic cytochrome P450 expression, including the role of cytokines interleukin-6 (IL-6), interleukin-1 (IL-1), interferon-gamma (IFNγ), and tumor necrosis factor-alpha (TNFα) in altering DME expression (Nyagode et al., 2010; Kinloch et al., 2011). A major goal of the present work was to examine the roles played by cytokines IL-6 and IFNγ in observed changes in hepatic transporter expression. To do this, we employed samples from the same IL-6 and IFNγ-null mice investigated in the previous study (Nyagode et al., 2010).
In addition to the impact of sepsis on elimination of drugs and other exogenous compounds, these changes in transporter gene expression are responsible for alterations in the elimination of endogenous substrates in the bile (Geier et al., 2007). Retention of biliary constituents in the liver (cholestasis) can be a serious source of hepatic damage. Though some hepatic transporters (Abcc and Slco subfamily members) transport bile acids (BA) in addition to drugs and toxicants, specific transporters of BA are expressed in the liver and are affected during experimental sepsis (Geier et al., 2007; Lickteig et al., 2007; Bodeman et al., 2013). While experimental sepsis has been shown to drastically reduce genes associated with BA transport and regulation, relatively few studies have reported on the impact of colitis on these genes (Jahnel et al., 2009). Therefore, to investigate whether the processes of hepatobiliary transport and BA synthesis are modulated in our model of infectious colitis, we measured the expression of specific bile constituent transporters Slc10a1 (Ntcp), Abcb11 (Bsep), and Abcb4 (Mdr2); the principal enzyme responsible for the rate of BA synthesis (Cyp7a1); and two nuclear receptors known to regulate the expression of these genes (Nr1h4, farnesoid X receptor; Nr1h3, liver X receptor).
Materials and Methods
The results detailed in this article were obtained from two separate experiments. Samples from one experiment, in the C57BL/6J background, have been analyzed and results published with respect to disease parameters and DME expression (Nyagode et al., 2010). Experimental design, including age, sex, animal treatment, tissue collection, and sample analysis were identical between the two experiments and are detailed below.
Unless otherwise specified, all reagents and chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Animals and Treatments.
Nine-week-old female mice were purchased from The Jackson Laboratory (Bar Harbor, ME). C3H/HeJ mice carry a spontaneous mutation of the TLR4 gene, while C3H/HeOuJ mice remain endotoxin-sensitive and were used as wild-type (WT) controls. IL-6- and IFNγ-deficient mice have been back-crossed for more than 10 generations to a C57BL/6J background, and C57BL/6J mice were used as WT controls (Dalton et al., 1993; Kopf et al., 1994). Mice were housed in groups of four or six to a cage and were acclimatized for at least one week in the animal facility before the beginning of the studies. All protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Emory University.
Animals of each background were infected with the same bacterial preparation. Infections of C3H/HeJ and C3H/HeOuJ mice with C. rodentium were carried out in a single experiment with six animals per treatment group. Each treatment with cytokine-null mice was carried out in two separate experiments with a total of eight animals per treatment group. C. rodentium (#51116, wild-type strain) was obtained from the American Type Culture Collection (Manassas, VA). After overnight growth in Luria broth, without shaking, at 37°C, the culture was serially diluted in sterile phosphate-buffered saline, and the concentration calculated spectrophotometrically. Infection was achieved by replacing food and drinking water with a 20% sucrose solution that had been inoculated with C. rodentium, while control animals received a sterile 20% sucrose solution. Food and water were restored after 24 hours. The volume of liquid consumed by the mice was measured and actual bacterial concentrations in the solution were determined by retrospective plating on MacConkey agar, on which C. rodentium forms small pink colonies with white rims. Infected mice were housed in a biosafety level 2 facility to prevent transmission of infection to other mouse colonies. Changes in body weight were monitored daily, and animals were sacrificed 7 days after administration of sucrose or bacteria.
Tissue Collection.
Liver and spleen were dissected from the abdominal cavity and rinsed in cold 1.15% potassium chloride, then weighed. The liver was then portioned, flash-frozen, and stored at –80°C for subsequent RNA preparation or kept on ice for the determination of viable bacteria. The colon was removed, washed of fecal matter by using cold 1.15% potassium chloride, sectioned, and kept on ice for the determination of viable bacteria. Blood was also collected from the animals at sacrifice and 50 μl was plated to determine approximate bacterial load.
Determination of Tissue Bacterial Loads.
The number of viable bacteria in the infected animals was determined from organ homogenates and blood. Liver and colon were weighed and homogenized in 1 ml of phosphate-buffered saline at low speed with a Tissuemizer (IKA Works, Inc., Wilmington, NC). Liver homogenate, blood, or serial dilutions of the colon homogenates were plated onto MacConkey agar and the number of colony forming units was determined following overnight incubation at 37°C.
RNA Extraction, cDNA Synthesis, and mRNA Measurement.
Total liver RNA was prepared using RNA-Bee isolation reagent (Tel-Test Inc., Friendswood TX), per manufacturer’s instructions. RNA concentration was determined spectrophotometrically, and RNA purity and integrity were confirmed by gel electrophoresis followed by visualization with ethidium bromide. Purified total RNA was reverse-transcribed with a SuperScript First-Strand Synthesis System kit (Life Technologies, Carlsbad, CA), according to the manufacturer’s protocol. Primers were custom-synthesized by Operon Biotechnologies, Inc. (Huntsville, AL) and have been published previously or are described below. Relative mRNA expression was measured by reverse transcriptase real-time PCR (RT-qPCR) using the Eppendorf Mastercycler Realplex (Eppendorf, Hamburg, Germany) and SYBR Green Master Mix reagent (Applied Biosystems, Foster City, CA). For each gene, expression data of all samples was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression using the ΔΔCt method described by Livak and Schmittgen (2001).
RT-qPCR Primers.
Primers for mouse drug transporters, cytochrome P450 enzymes, and nuclear receptors were designed using the Primer-BLAST program through the National Center for Biotechnology Information. Primers with potentially unintended templates were discarded and the amplicons from each primer pair were sequenced to ensure specific amplification of the correct gene. The primer sequences are found below (Table 1).
TABLE 1.
Primer Sequences used in qRT-PCR
| Gene Names | Forward Primer | Reverse Primer |
|---|---|---|
| Abcb1a (Mdr1a) | AGGAGCTGCTGGTCCCATCTTCC | TCCATCACGACCTCACGTGTCTCT |
| Abcb1b (Mdr1b) | AGCCGTAAGAGGCTGAGGCCG | CATCACCACCTCACGTGCCACC |
| Abcb4 (Mdr2) | GCGGCGAGCAAAGTCCAGGTC | CTGCCTTGGTTGCTGATGCTGCC |
| Abcb11 (Bsep) | AGACAGGCAACCCGTCATGGACT | ACGAACGCCGTCGTTTCCCC |
| Abcc2 (Mrp2) | TAATGAGGCGCCGTGGGTGAC | GTCCTGCCCACCACACCGAC |
| Abcc3 (Mrp3) | GGGCTGCCTTGCCCTGCTAC | CCGAGGGCCGTCTTGAGCCT |
| Abcc4 (Mrp4) | CCGAGGTGAAACCCAACCCGC | CGGGTTGAGCCACCAGAAGAACA |
| Abcc6 (Mrp6) | AGCAGGAGCCTGCGGCCTAT | GGGCCAAGCCCAGCACCATT |
| Abcg2 (Bcrp) | GCCGTTAGGACGCTCGCAGA | TAGCAACGAAGACTTGCCTCCGC |
| Cyp7a1 | TGTCTGCGAGGGCTGGAGCA | CCAGCCTGGGATGCTATGGGC |
| Nr1h3 (Lxr) | CAAAGCAGGGCTGCAGGTGGA | TGGGTCGTGGGGGTGGTTGAT |
| Nr1h4 (Fxr) | ACGGGGGCAACTGCGTGATG | CGCCCTTCGCTGTCGTCCTC |
| Slc10a1 (Ntcp) | AATCCAAGCTGCAGACGCACC | GCATCTTCTGTTGCAGCAGCCTT |
| Slc22a1 (Oct1) | GTGGGGCTAGTGGGGCTTGC | GCACATCATCTTCAGGTCAGCAGGG |
| Slc22a4 (Octn1) | GGGCAGCATCATTGCCCCCT | TTTCCCACATCTGAACCCTCTCACT |
| Slc22a5 (Octn2) | TACCTAGGTGCCTATGATCGCTTCC | GCTGTGCTCTTTAGGACTGTTGGGC |
| Slc22a7 (Oat2) | TACCCCGGGAGACTGACGGC | TGCTGACACACCAGATCCCACTCA |
| Slco1a1 (Oatp1a1) | GCCAACGCAAGATCCAACAGAGTG | TCGGGCCAACAATCTTCCCCAT |
| Slco1a4 (Oatp1a4) | GCCAGTGCAAGGCCAGAACC | AGGGTGGGGGTTTGCTACAGAA |
| Slco1b2 (Oatp1b2) | TGGAAGGCATAGGGTAGGCGGT | TGGGCAGCTTTGCTTGGATGCT |
| Slco2b1 (Oatp2b1) | TTGGCATCGGTGGTGTGCCC | ATGCCTCCTTCTGGCATCCGGT |
| Slco3a1 (Oatp3a1) | CGCTGCCTTCCTCGGCAAGT | GATCGCCGTCATCCCGAGCAG |
Immunoblotting.
Transporter protein levels in mouse whole-cell lysates were measured by Western blotting and chemiluminescent detection. Fifty micrograms of whole-cell lysate were mixed with Laemmli Sample Buffer (Bio-Rad, Hercules, CA) and treated under conditions described below for each protein. After treatment, samples were separated on 7.5% SDS-PAGE and transferred to polyvinyladine fluoride (PVDF) membranes. Nonfat dry milk (5%) in PBS containing 0.05% Tween was used to block nonspecific antibody binding. The following conditions and antibodies were used for detection of each protein: Abcg2, 5% 2-mercaptoethanol, 85°C for 10 minutes (BXP-21 clone; Abcam, Cambridge, MA); Abcb1, 5% 2-mercaptoethanol, 37°C for 30 minutes (H-241 clone; Santa Cruz Biotechnology, Dallas, TX); Abcc6, no 2-mercaptoethanol, 85°C for 10 minutes (M6II-31 clone; Abcam). ERK1 (C-16 clone; Santa Cruz Biotechnology) and ERK2 (C-14 clone; Santa Cruz Biotechnology,) were used as loading control for all blots. Images were captured and relative protein quantities were determined using Image Laboratory imaging system (Bio-Rad).
Statistical Analysis.
All data are presented as mean values ± S.E.M. Control and experimental groups were compared by unpaired Student’s t test. All statistical analysis was performed using SPSS software (SPSS Inc., Chicago, IL). Statistical significance was set at P < 0.05.
Results
C. rodentium Infection of Receptor- or Cytokine-Null Mice.
In our previously published studies using the C. rodentium infection model, we observed that the time course of hepatic P450 regulation mirrored the time course of intestinal colonization, reaching a peak at 7–10 days postinfection (Chaluvadi et al., 2009). As detailed above, experiments in the current study were performed in two distinct genetic backgrounds, with HeOuJ mice serving as control animals for their HeJ (TLR4-null) counterparts, and C57BL/6J mice serving as controls for the IL-6- and IFNγ-null groups. Despite previous reports of occasional lethality of C. rodentium infection in C3H strains (Vallance et al., 2003), all animals survived to the planned experimental endpoint. Additionally, no mice exhibited overt clinical signs of infection with the exception of slight diarrhea.
As previously reported, in the cytokine-null arm of the study the livers of infected mice were 14–25% larger and their spleens were 55–90% heavier than those of mice not infected with C. rodentium, though no change in total body weight was observed (Nyagode et al., 2010). In the TLR4-null study arm, total body weight was significantly decreased from starting body weight, liver weight normalized to whole body weight remained unchanged by infection, and spleen weight doubled in the infected animals (Table 2). Bacterial colonization of blood, liver, and colon of infected animals was also analyzed. As expected, colonization of the colon vastly overshadowed that of the other organs, with bacterial loads per microgram of tissue over a millionfold higher. Because of the high variability of the bacterial load in colon and blood tissues, no significant difference was found between WT control and knockout animals. However, bacterial colonization of the liver in mice lacking TLR4 trended toward a value higher than that of their WT counterparts (Table 2).
TABLE 2.
Disease parameters
Impact of C. rodentium infection on total body and relative organ weight of liver and spleen, as well as bacterial load in colon, blood, and liver. Values are mean ± S.E.M. (n = 6): body weight at sacrifice relative to body weight preinfection; organ weight at sacrifice relative to body weight at sacrifice; bacterial CFU per gram of tissue or per 50 μl of blood.
| HeOuJ |
HeJ |
|||
|---|---|---|---|---|
| Control | Infected | Control | Infected | |
| Body weight | 1.035 ± 0.009 | 0.921 ± 0.024* | 1.009 ± 0.009 | 0.886 ± 0.026* |
| Liver (% body weight) | 5.13 ± 0.16 | 5.21 ± 0.13 | 4.98 ± 0.08 | 5.13 ± 0.28 |
| Spleen (% body weight) | 0.466 ± 0.023 | 0.933 ± 0.137* | 0.539 ± 0.027 | 1.126 ± 0.078* |
| Colon bacterial CFU (×105) | n.a. | 1049.7 ± 250.0 | n.a. | 1451.2 ± 340.5 |
| Blood bacterial CFU | n.a. | 179.8 ± 164.1 | n.a. | 116.3 ± 35.5 |
| Liver bacterial CFU | n.a. | 441.7 ± 147.0 | n.a. | 4868.2 ± 2773.9 |
Significantly different than control, P < 0.05; n.a., not applicable.
Effect of C. rodentium Infection on Hepatic Uptake Transporters.
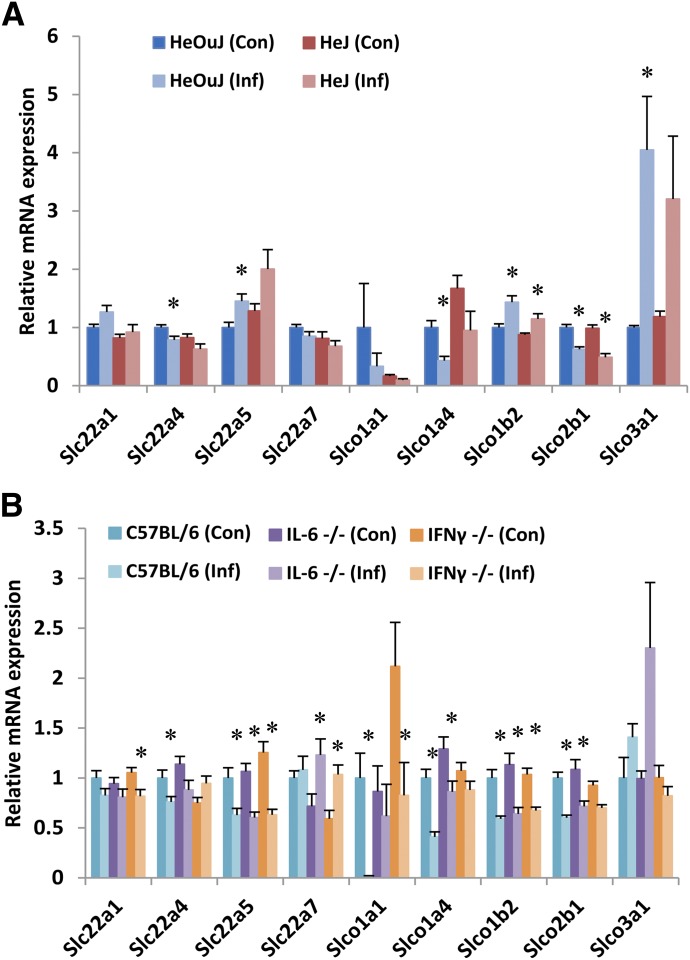
In both WT control groups (HeOuJ and C57BL/6J), significant downregulation of Slc22a4, Slco1a4, and Slco2b1 was observed in infected animals when compared with uninfected littermates (Fig. 1, A and B). Slco1a1 was dramatically downregulated in C57BL/6J mice, and a nonsignificant trend toward the same effect was observed in the HeOuJ strain. No change was seen in the expression of Slc22a1 or Slc22a7 in either WT group. However, significant upregulation in HeOuJ mice of transporters Slc22a5, Slco1b2, and Slco3a1 contrasted with observed downregulation in C57BL/6J mice of Slc22a5 and Slco1b2, with a trend toward induction of Slco3a1 failing to reach statistical significance. Additionally, C. rodentium infection in C57BL/6J mice markedly downregulated Slco1a1 expression, an effect that was not significant in HeOuJ mice.
Fig. 1.
Effect of C. rodentium infection on hepatic mRNA expression of uptake transporters. Seven days following oral infection with C. rodentium, female mice were sacrificed, livers harvested, and mRNA isolated from (A) HeOuJ and HeJ (TLR4-null) mice (n = 6) and (B) C57BL/6, IL-6-null, and IFNγ-null mice (n = 8). mRNA was quantified by qRT-PCR and resulting values are expressed as relative levels of mRNA expression after normalization to GAPDH, with uninfected wild-type (HeOuJ or C57BL/6) set to 1. Values represent mean ± S.E.M. Significant differences are in comparison with uninfected control groups. *P < 0.05 by t test.
In the absence of functional TLR4, the pattern of regulation during C. rodentium infection was highly similar to that in WT mice. The modulation of several transporters (Slc22a4, Slc22a5, Slco1a4, Slco3a1) failed to reach statistical significance in the TLR4-null animals, but in no case did the effects appear to be fully reversed (Fig. 1A). In mice lacking IL-6 the downregulation seen in the expression of Slco1a1 was blocked (Fig. 1B). Likewise, downregulation of Slc22a4 was fully reversed in IFNγ-null animals. C. rodentium infection in mice lacking IFNγ also failed to fully downregulate Slco1a1, Slco1a4, and Slco2b1 as compared with WT infected animals. With Slc22a1, infection in IFNγ-null mice downregulated expression though no effect was observed in WT animals. Similarly, Slc22a7 expression was induced by infection in both IL-6- and IFNγ-null mice, but not in WT controls.
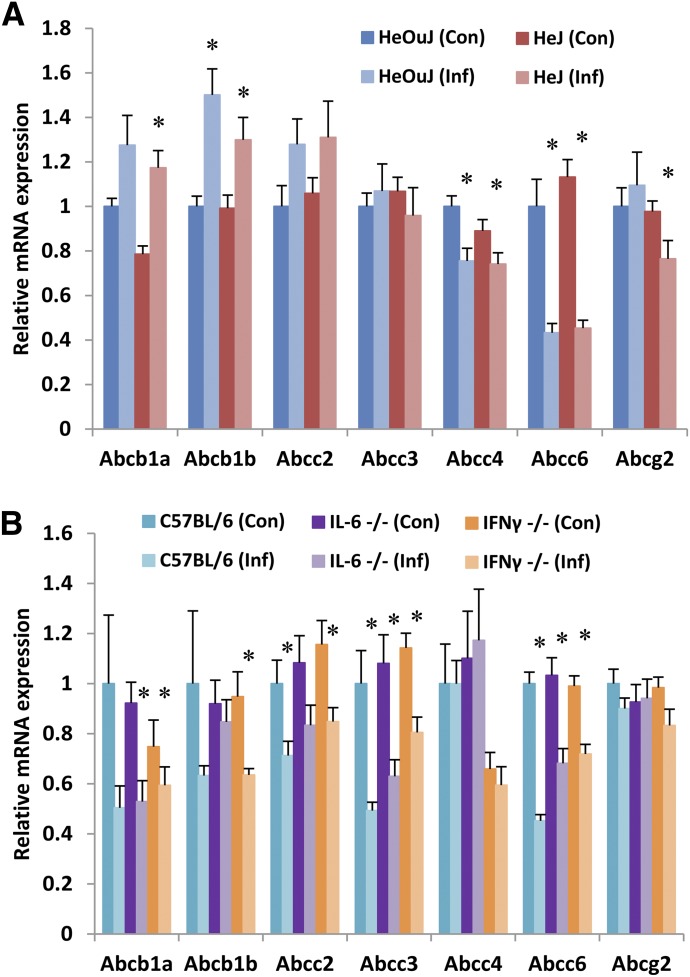
Effect of C. rodentium Infection on Hepatic Efflux Transporters.
As shown in Fig. 2, A and B, expression of efflux transporter Abcg2 was unaffected by infection in both HeOuJ and C57BL/6J mice, and any changes in expression of Abcb1a failed to reach statistical significance. Contrastingly, Abcc6 was downregulated in both WT groups. In HeOuJ but not C57BL/6J mice, Abcb1b was upregulated and Abcc4 downregulated by infection. In contrast, Abcc2 and Abcc3 were downregulated by infection in C57BL/6J mice, but unaffected in HeOuJ mice. Loss of TLR4 failed to modulate the expression changes observed in C. rodentium infection (HeJ mice, Fig. 2A). The downregulation of Abcc6 mRNA was partially reversed in both IL-6- and IFNγ-null mice (Fig. 2B).
Fig. 2.
Effect of C. rodentium infection on hepatic mRNA expression of efflux transporters. Seven days following oral infection with C. rodentium, female mice were sacrificed, livers harvested, and mRNA isolated from (A) HeOuJ and HeJ (TLR4-null) mice (n = 6) and (B) C57BL/6, IL-6-null, and IFNγ-null mice (n = 8). mRNA was quantified by real-time qRT-PCR and resulting values are expressed as relative levels of mRNA expression after normalization to GAPDH, with uninfected wild type (HeOuJ or C57BL/6) set to 1. Values represent mean ± S.E.M. Significant differences are in comparison with uninfected control groups. *P < 0.05 by t test.
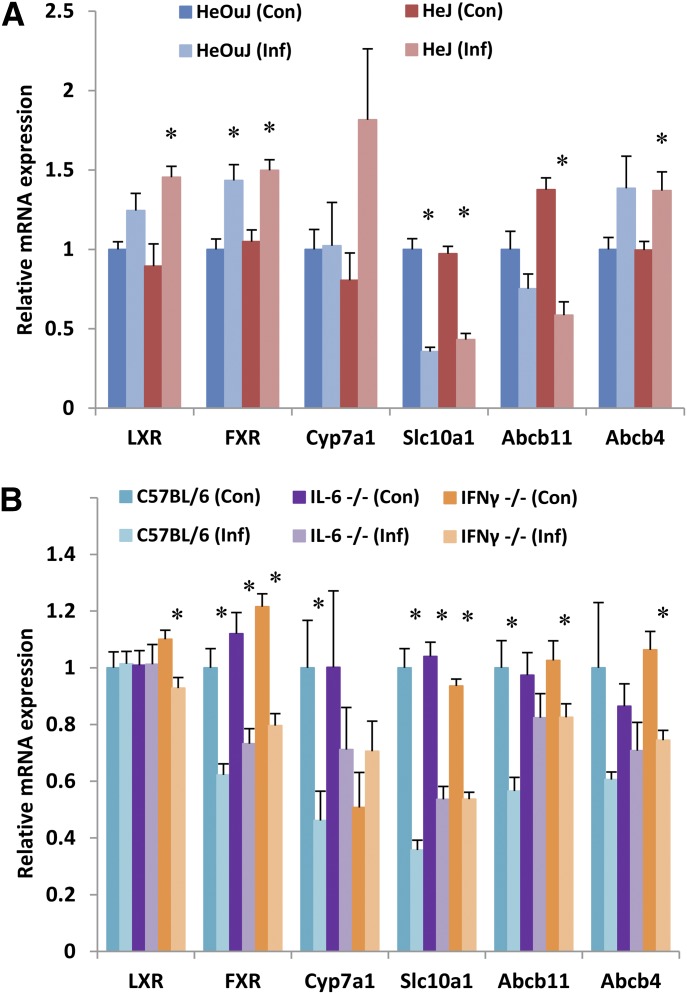
Effect of C. rodentium Infection on Genes Involved in Bile Synthesis and Transport.
C. rodentium infection significantly altered the expression of several genes associated with BA transport, synthesis, and signaling within the liver (Fig. 3, A and B). Expression of the farnesoid X receptor (FXR) was significantly upregulated in HeOuJ infected animals, and significantly downregulated in C57BL/6J mice. Abcb4, a phosphatidylcholine transporter associated with progressive familial intrahepatic cholestasis, exhibited expression changes of a similar magnitude to FXR (increased in HeOuJ and decreased in C57BL/6J) but failed to reach statistical significance in either strain. Cyp7a1, the rate-limiting enzyme in BA synthesis, and Abcb11, the mRNA encoding the bile salt export pump, were both significantly downregulated in C57BL/6J mice, but unchanged in HeOuJ mice. Finally, the expression of Slc10a1, encoding the sodium/taurocholate cotransporting polypeptide, was downregulated in infected animals of both WT groups.
Fig. 3.
Effect of C. rodentium infection on hepatic mRNA expression of bile acid–associated genes. Seven days following oral infection with C. rodentium, female mice were sacrificed, livers harvested, and mRNA isolated from (A) HeOuJ and HeJ (TLR4-null) mice (n = 6) and (B) C57BL/6, IL-6-null, and IFNγ-null mice (n = 8). mRNA was quantified by real-time qRT-PCR and resulting values are expressed as relative levels of mRNA expression after normalization to GAPDH, with uninfected wild-type (HeOuJ or C57BL/6) set to 1. Values represent mean ± S.E.M. Significant differences are in comparison with uninfected control groups. *P < 0.05 by t test.
In IL-6-null mice, the effect of infection on Cyp7a1 and Abcb11 was partially reversed when compared with WT animals (Fig. 3B). IFNγ-null mice similarly exhibited a smaller downregulation of Abcb11 during infection; however, the effect on Cyp7a1 expression was fully reversed in these mice. Contrastingly, loss of TLR4 failed to modulate the effects of infection on the expression of these bile-associated genes (Fig. 3A).
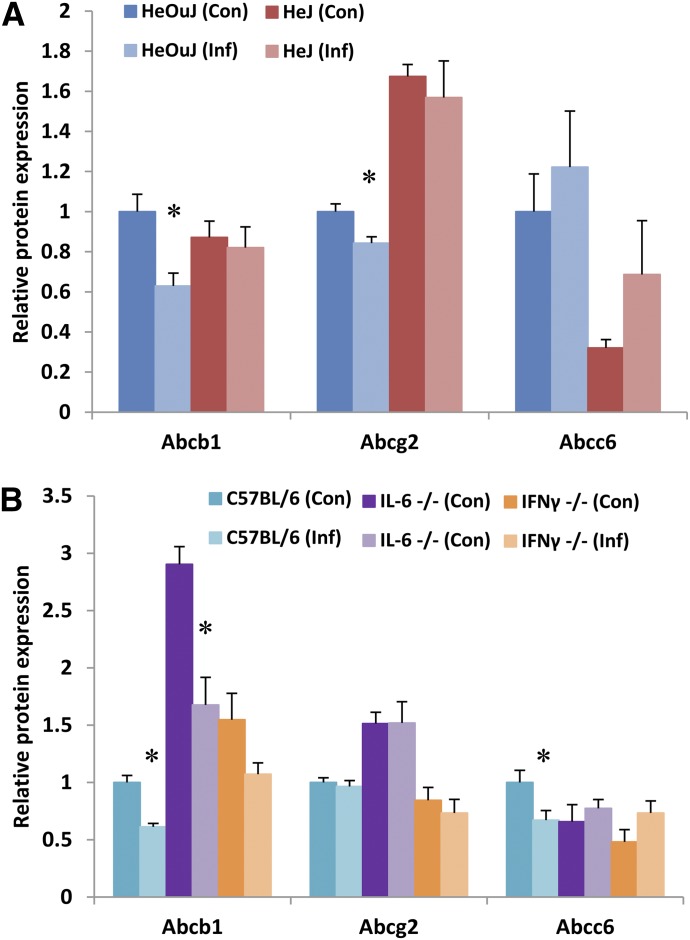
Effect of C. rodentium Infection on Transporter Protein Expression.
To investigate whether C. rodentium infection also caused changes in protein expression, western blotting was performed to measure relative changes in efflux transporter proteins. As seen in Fig. 4, A and B, infection decreased protein expression of Abcb1 in both HeOuJ and C57BL/6J mice. Abcg2 protein expression was slightly but significantly downregulated only in infected HeOuJ mice (Fig. 4A), while Abcc6 protein expression was downregulated only in infected C57BL/6J mice (Fig. 4B). Western blot images used for these analyses are shown in Supplemental Fig. 1. Absence of TLR4 in the HeJ mice appeared to reverse the downregulation of Abcb1 caused by infection (Fig. 4A). Similarly, loss of either IL-6- or IFNγ reversed the disease-induced decrease in Abcc6 protein expression (Fig. 4B).
Fig. 4.
Effect of C. rodentium infection on hepatic protein expression of efflux transports. Seven days following oral infection with C. rodentium, female mice were sacrificed, livers harvested, and whole-cell lysates prepared from (A) HeOuJ and HeJ (TLR4-null) mice (n = 6) and (B) C57BL/6, IL-6-null, and IFNγ-null mice (n = 8). Protein levels of specific transporters were detected by western blotting and quantified using Image Laboratory software (Bio-Rad). Resulting values are expressed as relative levels of protein expression after normalization to Erk1/2, with uninfected wild-type (HeOuJ or C57BL/6) set to 1. Values represent mean ± S.E.M. Significant differences are in comparison with uninfected control groups. *P < 0.05 by t test.
Discussion
This study demonstrates significant regulation of hepatic drug transporter genes, including Slc22a4, Slc22a5, Slco1a4, Slco1b2, Slco2b1, Slco3a1, Abcb1b, Abcc2, Abcc3, Abcc4, and Abcc6 during colonic inflammation caused by C. rodentium infection. C. rodentium also significantly alters the expression of BA-associated genes FXR, Cyp7a1, Slc10a1, and Abcb11. Regulation of these genes in mice lacking functional TLR4 was similar to that in WT mice, indicating that bacterial LPS is not the cause. The involvement of cytokines IL-6 and IFNγ in the regulation of Slc22a4, Slco1a1, Slco1a4, and Cyp7a1, as well as more minor involvement in the regulation of other genes, was implied by differences in the responses of IL-6 and IFNγ-null mice.
Slc22a4 [organic cation/carnitine transporter (Octn)1], Slco1a1 [organic anion transporting polypeptide (Oatp)1a1], Slco1a4 (Oatp1a4), Slco2b1 (Oatp2b1), Abcc6 (Mrp6), and Slc10a1 (Ntcp) was downregulated by infection in both WT strains of mice. Of these, expression of all but Slc22a4 was suppressed to one-half of WT values or lower, indicating a likelihood that these effects may have pharmacological significance in the transport of the respective drug substrates. Slco1a1 and Slco1a4 play important roles in the transport of estrogen conjugates, as well as several therapeutic drugs (Ose et al., 2010; Gong et al., 2011). While these transporters share substrates with human transporter SLCO1A2, they are not considered orthologs (Shitara et al., 2013).
The physiologic significances of the smaller effects seen with, e.g., Scl22a4 remain to be determined. In addition, many of the effects observed were strain-dependent. Thus, Slc22a5 (Octn2), Slco1b2 (Oatp1b2), Abcb1a (Mdr1a), Abcb1b (Mdr1b), and FXR were downregulated in HeOuJ mice and upregulated in the C57BL/6J strain. Abcc2 (Mrp2), Abcc3 (Bsep), and Cyp7a1 were downregulated in C57BL/6J but unaffected in HeOuJ; and Slco3A1 was specifically upregulated in HeOuJ mice only. At present there is no way to predict which of the strain-specific responses might be relevant to humans, but it does demonstrate the propensity for a wide spectrum of transporter genes to be regulated.
Altered responses to infection in mice lacking IL-6 or IFNγ were observed for a subset of the genes studied. The almost complete suppression of Slco1a1 expression, and the 50% decrease in Abcb11 mRNA seen in WT mice were blocked in the IL-6-null animals, indicating a likely in vivo regulation of these genes by IL-6. This complements the findings of Siewert et al. (2004) implicating IL-6 in the downregulation of Slco1a1 caused by LPS or turpentine administration. By the same criteria, partial roles for IL-6 in C. rodentium infection can be inferred for Slco1a4, Abcc6, and Cyp7a1. Attenuation of Slco1a1, Slco1a4, Abcb1a, Abcc6, and Cyp7a1 downregulation was also seen in the IFNγ-null mice; whereas, the downregulation of Slc22a4 was reversed. The overlap between genes apparently regulated by both IL-6 and IFNγ can to some extent be explained by the fact that the components of the inflammatory response are highly interdependent. IFNγ-null animals failed to mount plasma IL-6 or TNFα responses to infection, while IL-6-null mice had an impaired serum IFNγ, but not TNFα response (Nyagode et al., 2010). Interestingly, Geier et al. (2003) described the successful prevention of Oatp2 downregulation in LPS administration, using etanercept injections to block TNFα activity. That we observed no downregulation in IFNγ-null mice could be due to the impaired TNFα response in these animals.
To our knowledge, the downregulation of Slc22a4 and Slc22a5 mRNAs (in C57BL/6J mice) during C. rodentium infection is the first report of hepatic expression being altered in either Octn gene during inflammation. Conversely, hepatic downregulation of Slco1a4 (Oatp1a4) has been reported in fatty-liver disease, extrahepatic cancer, and experimental sepsis (Sharma et al., 2008; Fisher et al., 2009; Bodeman et al., 2013) as well as in the present study, but was not observed in necrotizing enterocolitis (NEC) or colitis caused by dextran sulfate sodium (DSS) treatment (Jahnel et al., 2009; Cherrington et al., 2013). C. rodentium infection also decreased Slco1a1 (Oatp1a1 or Oatp1) gene expression, which finding is consistent with reported downregulation after treatment with LPS or subcutaneous turpentine (Siewert et al., 2004; Bodeman et al., 2013).
Expression of Slco2b1 (Oatp2b1 or Oatp-B) was decreased in our infective model in animals of both backgrounds. Though previously reported to be decreased in several nonhepatic inflammatory models (Petrovic et al., 2008; Wojtal et al., 2009; Ohkura et al., 2012), to our knowledge this is the first in vivo report of hepatic downregulation of Slco2b1 during inflammation. Contrastingly, expression of Slco3a1 (Oatp3a1 or Oatp-D) was increased during infection in HeOuJ animals. This transporter has been reported to be increased in bile duct ligation (Klaassen and Aleksunes, 2010), but hepatic regulation during other models of inflammation has not been documented. Expression of SLCO3A1 in patients has been implicated in the accumulation or clearance of several antiretroviral drugs (Janneh et al., 2009; Molto et al., 2013). It is conceivable that increased expression in similar diseases could lead to increased clearance of these substrates, decreasing therapeutic efficacy.
ABCC family members, also known as Mrps, play a vital role in the transport of drugs and endogenous substrates (reviewed by Gu and Manautou, 2010). During live infection we observed a significant decrease in the mRNA expression of Abcc2 in C57BL/6J mice. Other inflammatory conditions have been reported to result in similar downregulation (Andrejko et al., 2008; Sharma et al., 2008; Cherrington et al., 2013). Multiple studies have indicated a role for IL-6 in the downregulation of Abcc2 (Siewert et al., 2004; Andrejko et al., 2008). In the current study, the decrease in Abcc2 expression in IL-6-null mice failed to reach statistical significance, but the decrease did not appear to be meaningfully different from that seen in their WT controls.
Protein expression of hepatic transporters was successfully analyzed for Abcb1, Abcg2, and Abcc6. The TLR4-dependent decrease observed in Abcb1 protein is unexpected both because it occurred in mice with increased Abcb1 mRNA, and because it was the only TLR4-dependent expression change observed. The potential for an LPS-responsive translational control of Abcb1 is an intriguing subject for future study.
Cholestasis often occurs in sepsis and bacterial infections. Due to limited sample volumes, we were unable to directly measure the levels of BA in either the blood or the liver. Instead we investigated expression changes in genes involved in BA transport, synthesis, and regulation (Geier et al., 2007). Downregulation of these genes has been reported in several inflammatory models, including necrotizing enterocolitis (Cherrington et al., 2013), LPS treatment (Feingold et al., 1996), IL-6 or turpentine treatment (Siewert et al., 2004), extrahepatic cancer (Sharma et al., 2008), and cecal puncture (Andrejko et al., 2008). We observed downregulation of hepatic transporters Slc10a1 (sodium/taurocholate cotransporter, Ntcp) and Abcb11 (bile salt export pump, Bsep). Also downregulated were the key enzyme of BA synthesis, Cyp7a1, and the BA-responsive nuclear receptor FXR. Our studies indicate involvement of both IL-6 and IFNγ in the regulation of Abcb11 and Cyp7a1, as downregulation was reversed in mice lacking those cytokines. While a role for IL-6 in regulating Abcb11 and Cyp7a1 had previously been identified (Geier et al., 2003; Kim et al., 2003; Siewert et al., 2004), we are unable to find any previous reports on a similar role for IFNγ.
Abcc3 and Abcc4 (Mrp3, Mrp4) are expressed at the apical membrane of hepatocytes and are responsible for effluxing substrates into the blood. Reports of their regulation during inflammation generally indicate decreased Abcc3 and Abcc4 expression, though induction during cholestasis is common (Siewert et al., 2004; Geier et al., 2007; Le Vee et al., 2011). Induction of Abcc3 during cholestasis is an important compensatory mechanism limiting the hepatocellular accumulation of toxic BAs (Keppler, 2011). We observed a decrease in Abcc3 expression during C. rodentium infection in conjunction with the other more cholestatic-like expression changes. It is unclear if this decrease in Abcc3 expression reflects an earlier timepoint in the cholestatic response, or if C. rodentium infection induces a noncholestatic condition that alters other BA-associated genes. Studies of the LPS-induced acute phase response have similarly found decreased Abcc3 expression in the context of other cholestatic-like expression changes (Siewert et al., 2004). It is also possible that induction of Abcc3 during cholestasis is regulated through mechanisms distinct from other BA-associated genes (Ruiz et al., 2013).
In an attempt to determine the impact of colitis on these same genes, Jahnel et al. (2009) found no significant changes in mRNA expression in mice treated with dextran sulfate sodium despite observable colitis. This suggests that the regulation seen in the current study may be dependent on both the infection and the resultant inflammatory response.
In conclusion, colonic infection by C. rodentium modulated mRNA expression of hepatic transporter genes. Several BA-associated genes were also significantly downregulated during infection. Expression changes of a subset of genes were ablated in IL-6- or IFNγ-null mice. These studies and others defining the mechanisms behind inflammation-induced changes in DME and transporter expression play an important role in understanding and preventing potential adverse reactions caused by altered drug/toxicant clearance during inflammatory disease states.
Supplementary Material
Abbreviations
- BA
bile acid
- CFU
colony-forming units
- DME
drug-metabolizing enzyme
- FXR
farnesoid X receptor
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IFNγ
interferon-gamma
- IL-6
interleukin-6
- LPS
lipopolysaccharide
- Mdr
multidrug resistance protein
- Mrp
multidrug resistance-associated protein
- Oatp
organic anion transporting polypeptide
- Octn
organic cation/carnitine transporter
- P450
cytochrome P450
- qRT-PCR
real-time reverse-transcription polymerase chain reaction
- SLC
solute carrier
- TLR4
Toll-like receptor 4
- TNFα
tumor necrosis factor-alpha
- WT
wild-type
Authorship Contributions
Participated in research design: Merrell, Nyagode, Morgan.
Conducted experiments: Nyagode, Clarke, Merrell.
Performed data analysis: Merrell.
Wrote or contributed to the writing of the manuscript: Merrell, Nyagode, Clarke, Cherrington, Morgan.
Footnotes
This work was supported by grants from the National Institutes of Health [DK072372, HD062489, AI083927] and National Institute of Environmental Health Sciences [ES012870, ES007091].
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Andrejko KM, Raj NR, Kim PK, Cereda M, Deutschman CS. (2008) IL-6 modulates sepsis-induced decreases in transcription of hepatic organic anion and bile acid transporters. Shock 29:490–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodeman CE, Dzierlenga AL, Tally CM, Mulligan RM, Lake AD, Cherrington NJ, McKarns SC. (2013) Differential regulation of hepatic organic cation transporter 1, organic anion-transporting polypeptide 1a4, bile-salt export pump, and multidrug resistance-associated protein 2 transporter expression in lymphocyte-deficient mice associates with interleukin-6 production. J Pharmacol Exp Ther 347:136–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaluvadi MR, Kinloch RD, Nyagode BA, Richardson TA, Raynor MJ, Sherman M, Antonovic L, Strobel HW, Dillehay DL, Morgan ET. (2009) Regulation of hepatic cytochrome P450 expression in mice with intestinal or systemic infections of citrobacter rodentium. Drug Metab Dispos 37:366–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrington NJ, Estrada TE, Frisk HA, Canet MJ, Hardwick RN, Dvorak B, Lux K, Halpern MD. (2013) The hepatic bile acid transporters Ntcp and Mrp2 are downregulated in experimental necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 304:G48–G56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congiu M, Mashford ML, Slavin JL, Desmond PV. (2009) Coordinate regulation of metabolic enzymes and transporters by nuclear transcription factors in human liver disease. J Gastroenterol Hepatol 24:1038–1044 [DOI] [PubMed] [Google Scholar]
- Cressman AM, Petrovic V, and Piquette-Miller M (2012) Inflammation-mediated changes in drug transporter expression/activity: implications for therapeutic drug response. Expert Rev Clin Pharmacol 5:69–89. [DOI] [PubMed]
- Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. (1993) Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 259:1739–1742 [DOI] [PubMed] [Google Scholar]
- Feingold KR, Spady DK, Pollock AS, Moser AH, Grunfeld C. (1996) Endotoxin, TNF, and IL-1 decrease cholesterol 7 alpha-hydroxylase mRNA levels and activity. J Lipid Res 37:223–228 [PubMed] [Google Scholar]
- Fisher CD, Lickteig AJ, Augustine LM, Oude Elferink RP, Besselsen DG, Erickson RP, Cherrington NJ. (2009) Experimental non-alcoholic fatty liver disease results in decreased hepatic uptake transporter expression and function in rats. Eur J Pharmacol 613:119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier A, Dietrich CG, Voigt S, Kim SK, Gerloff T, Kullak-Ublick GA, Lorenzen J, Matern S, Gartung C. (2003) Effects of proinflammatory cytokines on rat organic anion transporters during toxic liver injury and cholestasis. Hepatology 38:345–354 [DOI] [PubMed] [Google Scholar]
- Geier A, Wagner M, Dietrich CG, Trauner M. (2007) Principles of hepatic organic anion transporter regulation during cholestasis, inflammation and liver regeneration. Biochim Biophys Acta 1773:283–308 [DOI] [PubMed] [Google Scholar]
- Gong L, Aranibar N, Han YH, Zhang Y, Lecureux L, Bhaskaran V, Khandelwal P, Klaassen CD, and Lehman-McKeeman LD (2011) Characterization of organic anion-transporting polypeptide (Oatp) 1a1 and 1a4 null mice reveals altered transport function and urinary metabolomic profiles. Toxicological Sci 122:587–597. [DOI] [PubMed]
- Gu X, Manautou JE. (2010) Regulation of hepatic ABCC transporters by xenobiotics and in disease states. Drug Metab Rev 42:482–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnel J, Fickert P, Langner C, Högenauer C, Silbert D, Gumhold J, Fuchsbichler A, and Trauner M (2009) Impact of experimental colitis on hepatobiliary transporter expression and bile duct injury in mice. Liver Int 29:1316–1325. [DOI] [PubMed]
- Janneh O, Chandler B, Hartkoorn R, Kwan WS, Jenkinson C, Evans S, Back DJ, Owen A, Khoo SH. (2009) Intracellular accumulation of efavirenz and nevirapine is independent of P-glycoprotein activity in cultured CD4 T cells and primary human lymphocytes. J Antimicrob Chemother 64:1002–1007 [DOI] [PubMed] [Google Scholar]
- Keppler D. (2011) Cholestasis and the role of basolateral efflux pumps. Z Gastroenterol 49:1553–1557 [DOI] [PubMed] [Google Scholar]
- Kim MS, Shigenaga J, Moser A, Feingold K, Grunfeld C. (2003) Repression of farnesoid X receptor during the acute phase response. J Biol Chem 278:8988–8995 [DOI] [PubMed] [Google Scholar]
- Kinloch RD, Lee CM, van Rooijen N, Morgan ET. (2011) Selective role for tumor necrosis factor-α, but not interleukin-1 or Kupffer cells, in down-regulation of CYP3A11 and CYP3A25 in livers of mice infected with a noninvasive intestinal pathogen. Biochem Pharmacol 82:312–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen CD, Aleksunes LM. (2010) Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev 62:1–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Köhler G. (1994) Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368:339–342 [DOI] [PubMed] [Google Scholar]
- Le Vee M, Jouan E, Moreau A, Fardel O. (2011) Regulation of drug transporter mRNA expression by interferon-γ in primary human hepatocytes. Fundam Clin Pharmacol 25:99–103 [DOI] [PubMed] [Google Scholar]
- Lickteig AJ, Slitt AL, Arkan MC, Karin M, Cherrington NJ. (2007) Differential regulation of hepatic transporters in the absence of tumor necrosis factor-alpha, interleukin-1beta, interleukin-6, and nuclear factor-kappaB in two models of cholestasis. Drug Metab Dispos 35:402–409 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Moltó J, Xinarianos G, Miranda C, Pushpakom S, Cedeño S, Clotet B, Owen A, Valle M. (2013) Simultaneous pharmacogenetics-based population pharmacokinetic analysis of darunavir and ritonavir in HIV-infected patients. Clin Pharmacokinet 52:543–553 [DOI] [PubMed] [Google Scholar]
- Morgan ET, Goralski KB, Piquette-Miller M, Renton KW, Robertson GR, Chaluvadi MR, Charles KA, Clarke SJ, Kacevska M, Liddle C, et al. (2008) Regulation of drug-metabolizing enzymes and transporters in infection, inflammation, and cancer. Drug Metab Dispos 36:205–216 [DOI] [PubMed] [Google Scholar]
- Nyagode BA, Lee CM, Morgan ET. (2010) Modulation of hepatic cytochrome P450s by Citrobacter rodentium infection in interleukin-6- and interferon-gamma-null mice. J Pharmacol Exp Ther 335:480–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura N, Shigetani Y, Yoshiba N, Yoshiba K, Okiji T. (2012) Gene expression analysis of membrane transport proteins in normal and lipopolysaccharide-inflamed rat dental pulp. J Endod 38:648–652 [DOI] [PubMed] [Google Scholar]
- Ose A, Kusuhara H, Endo C, Tohyama K, Miyajima M, Kitamura S, Sugiyama Y. (2010) Functional characterization of mouse organic anion transporting peptide 1a4 in the uptake and efflux of drugs across the blood-brain barrier. Drug Metab Dispos 38:168–176 [DOI] [PubMed] [Google Scholar]
- Petrovic V, Wang JH, Piquette-Miller M. (2008) Effect of endotoxin on the expression of placental drug transporters and glyburide disposition in pregnant rats. Drug Metab Dispos 36:1944–1950 [DOI] [PubMed] [Google Scholar]
- Richardson TA, Morgan ET. (2005) Hepatic cytochrome P450 gene regulation during endotoxin-induced inflammation in nuclear receptor knockout mice. J Pharmacol Exp Ther 314:703–709 [DOI] [PubMed] [Google Scholar]
- Richardson TA, Sherman M, Antonovic L, Kardar SS, Strobel HW, Kalman D, Morgan ET. (2006) Hepatic and renal cytochrome p450 gene regulation during citrobacter rodentium infection in wild-type and toll-like receptor 4 mutant mice. Drug Metab Dispos 34:354–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz ML, Rigalli JP, Arias A, Villanueva S, Banchio C, Vore M, Mottino AD, Catania VA. (2013) Induction of hepatic multidrug resistance-associated protein 3 by ethynylestradiol is independent of cholestasis and mediated by estrogen receptor. Drug Metab Dispos 41:275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab L, Peluso J, Muller CD, and Ubeaud-Sequier G (2013) Implication of hepatic transporters (MDR1 and MRP2) in inflammation-associated idiosyncratic drug-induced hepatotoxicity investigated by microvolume cytometry. Cytometry Part A 83:403–408. [DOI] [PubMed]
- Sharma R, Kacevska M, London R, Clarke SJ, Liddle C, Robertson G. (2008) Downregulation of drug transport and metabolism in mice bearing extra-hepatic malignancies. Br J Cancer 98:91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitara Y, Maeda K, Ikejiri K, Yoshida K, Horie T, Sugiyama Y. (2013) Clinical significance of organic anion transporting polypeptides (OATPs) in drug disposition: their roles in hepatic clearance and intestinal absorption. Biopharm Drug Dispos 34:45–78 [DOI] [PubMed] [Google Scholar]
- Siewert E, Dietrich CG, Lammert F, Heinrich PC, Matern S, Gartung C, Geier A. (2004) Interleukin-6 regulates hepatic transporters during acute-phase response. Biochem Biophys Res Commun 322:232–238 [DOI] [PubMed] [Google Scholar]
- Teng S, Piquette-Miller M. (2005) The involvement of the pregnane X receptor in hepatic gene regulation during inflammation in mice. J Pharmacol Exp Ther 312:841–848 [DOI] [PubMed] [Google Scholar]
- Teng S, Piquette-Miller M. (2008) Regulation of transporters by nuclear hormone receptors: implications during inflammation. Mol Pharm 5:67–76 [DOI] [PubMed] [Google Scholar]
- Vallance BA, Deng W, Jacobson K, Finlay BB. (2003) Host susceptibility to the attaching and effacing bacterial pathogen Citrobacter rodentium. Infect Immun 71:3443–3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtal KA, Eloranta JJ, Hruz P, Gutmann H, Drewe J, Staumann A, Beglinger C, Fried M, Kullak-Ublick GA, Vavricka SR. (2009) Changes in mRNA expression levels of solute carrier transporters in inflammatory bowel disease patients. Drug Metab Dispos 37:1871–1877 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.