Abstract
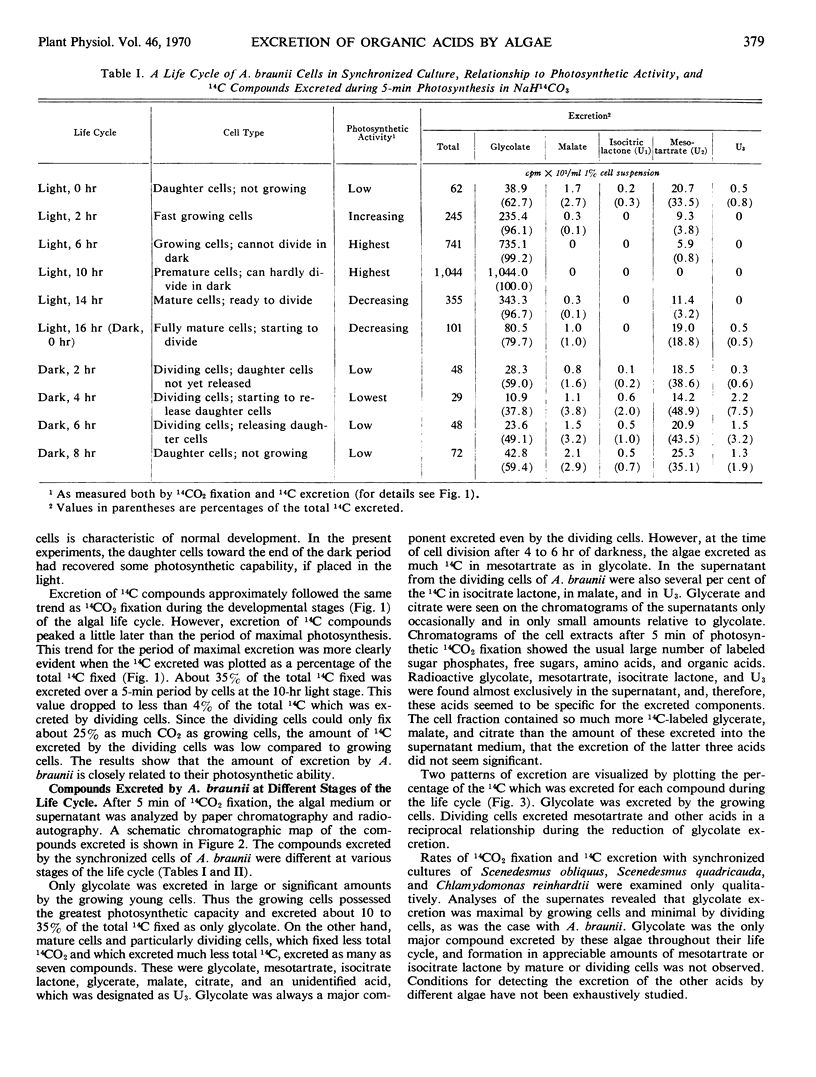
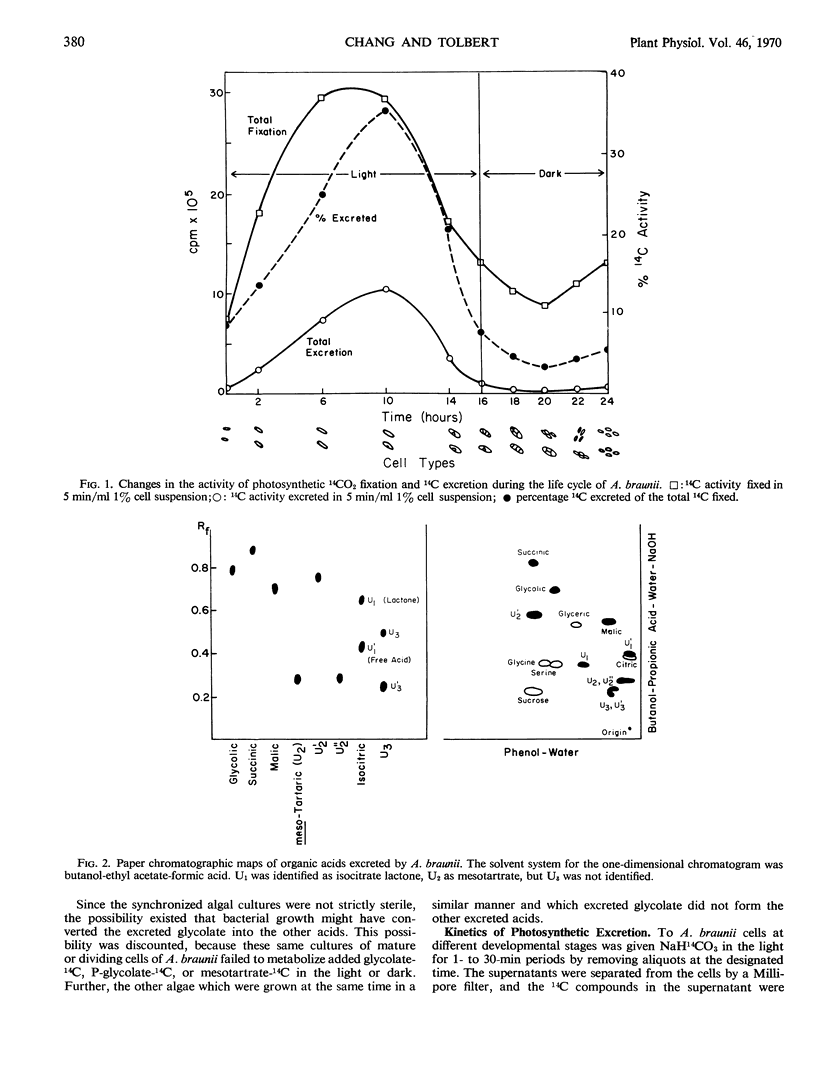
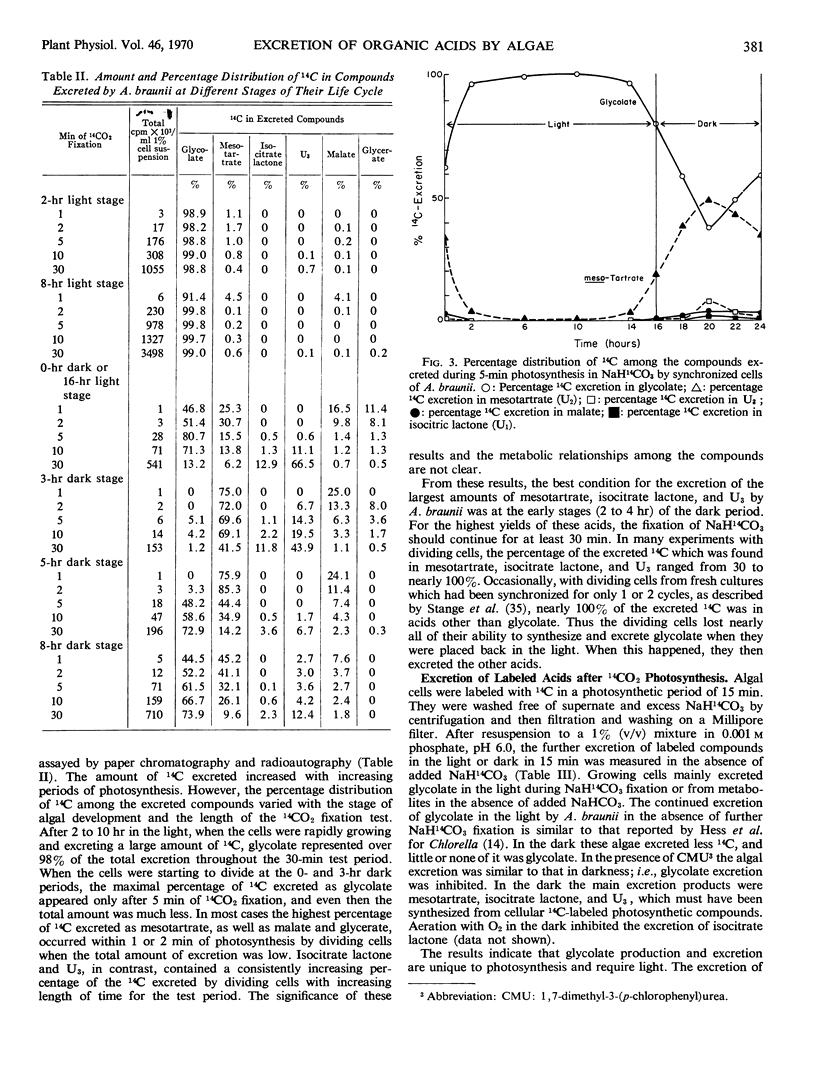
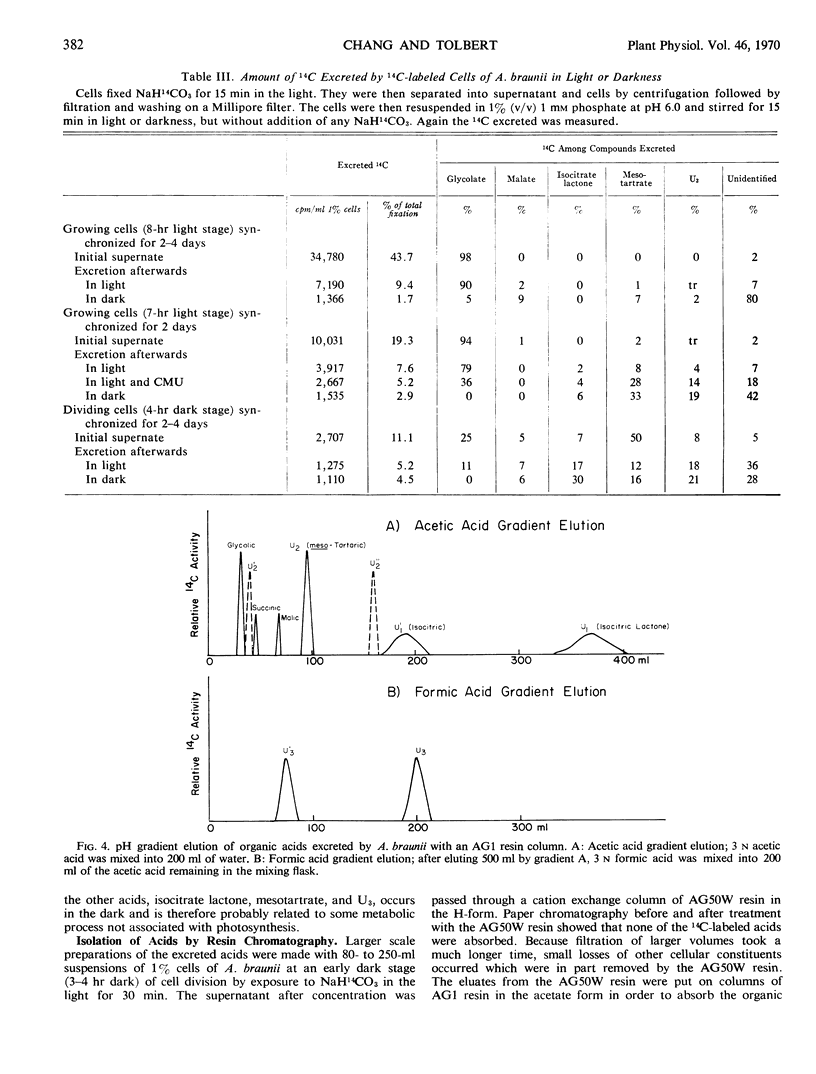
Fixation of 14CO2 by synchronized cultures of Ankistrodesmus braunii was highest for young growing cells, low for mature cells, and lowest for dividing cells. The amount of 14C excreted during photosynthesis followed the same trend. Cells at the end of the growing phase, after 10 hours of a 16-hour light phase, excreted nearly 35% of the total 14C fixed as one product, glycolate. Dividing cells from the dark phase, when tested in the light, excreted only 4% as much glycolate-14C as the young growing cells. Dividing cells also excreted as much mesotartrate as glycolate and also some isocitrate lactone and an unidentified acid. None of these excreted acids were found inside the cells in significant amounts. Methods for isolation and identification of the excreted acids are present. With 14C-labeled algae, it was shown that the excretion of glycolate was light-dependent and inhibited by 1,1-dimethyl-3-(p-chlorophenyl) urea. The excretion of labeled mesotartrate, isocitrate lactone, and an unknown acid, but not glycolate, also occurred in the dark. The excreted mesotartrate was predominantly carboxyl-labeled even after long periods of 14CO2 fixation. Since glycolate is known to be uniformly labeled, glycolate could not be the precursor of the carboxyl-labeled mesotartrate. The reason for the specific excretion of glycolate, mesotartrate, and isocitrate lactone is not known, but the metabolism of all three acids by the algae may be limited and each can form dilactides or lactones by dehydration. In this context isocitrate lactone was excreted rather than the free acid.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN M. B. Excretion of organic compounds by Chlamydomonas. Arch Mikrobiol. 1956;24(2):163–168. doi: 10.1007/BF00408630. [DOI] [PubMed] [Google Scholar]
- Bruin W. J., Nelson E. B., Tolbert N. E. Glycolate pathway in green algae. Plant Physiol. 1970 Sep;46(3):386–391. doi: 10.1104/pp.46.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W. H., Tolbert N. E. Distribution of C in Serine and Glycine after CO(2) Photosynthesis by Isolated Chloroplasts. Modification of Serine-C Degradation. Plant Physiol. 1965 Nov;40(6):1048–1052. doi: 10.1104/pp.40.6.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAFFLIN A. L., OCHOA S. Partial purification of isocitric dehydrogenase and oxalosuccinic carboxylase. Biochim Biophys Acta. 1950 Jan;4(1-3):205–210. doi: 10.1016/0006-3002(50)90025-4. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R., Johnson H. S. Further studies on a new pathway of photosynthetic carbon dioxide fixation in sugar-cane and its occurrence in other plant species. Biochem J. 1967 Feb;102(2):417–422. doi: 10.1042/bj1020417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J. L., Tolbert N. E. Glycolate pathway in algae. Plant Physiol. 1967 Mar;42(3):371–379. doi: 10.1104/pp.42.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J. R., Jones R. F. Pattern of CO-2 fixation during vegetative development and gametic differentiation in Chlamydomonas reinhardtii. J Cell Physiol. 1966 Feb;67(1):101–105. doi: 10.1002/jcp.1040670112. [DOI] [PubMed] [Google Scholar]
- Miller R. M., Meyer C. M., Tanner H. A. Glycolate Excretion & Uptake by Chlorella. Plant Physiol. 1963 Mar;38(2):184–188. doi: 10.1104/pp.38.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E. B., Tolbert N. E. The regulation of glycolate metabolism in Chlamydomonas reinhardtii. Biochim Biophys Acta. 1969 Jul 30;184(2):263–270. doi: 10.1016/0304-4165(69)90028-2. [DOI] [PubMed] [Google Scholar]
- Orth G. M., Tolbert N. E., Jimenez E. Rate of Glycolate Formation During Photosynthesis at High pH. Plant Physiol. 1966 Jan;41(1):143–147. doi: 10.1104/pp.41.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANGE L., BENNETT E. L., CALVIN M. Short-time radiocarbon-labelled carbon dioxide incorporation experiments with synchronously growing Chlorella cells. Biochim Biophys Acta. 1960 Jan 1;37:92–100. doi: 10.1016/0006-3002(60)90082-2. [DOI] [PubMed] [Google Scholar]
- Stafford H. A., Loewus F. A. The Fixation of CO(2) into Tartaric and Malic Acids of Excised Grape Leaves. Plant Physiol. 1958 May;33(3):194–199. doi: 10.1104/pp.33.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOLBERT N. E., ZILL L. P. Excretion of glycolic acid by algae during photosynthesis. J Biol Chem. 1956 Oct;222(2):895–906. [PubMed] [Google Scholar]
- VICKERY H. B., PALMER J. K. The metabolism of the organic acids of tobacco leaves. VII. Effect of culture of excised leaves in solutions of (+)-tartrate. J Biol Chem. 1954 Mar;207(1):275–285. [PubMed] [Google Scholar]
- WOELLER F. H. Liquid scintillation counting of C-14-labelled CO2 with phenethylamine. Anal Biochem. 1961 Oct;2:508–511. doi: 10.1016/0003-2697(61)90056-2. [DOI] [PubMed] [Google Scholar]


