Abstract
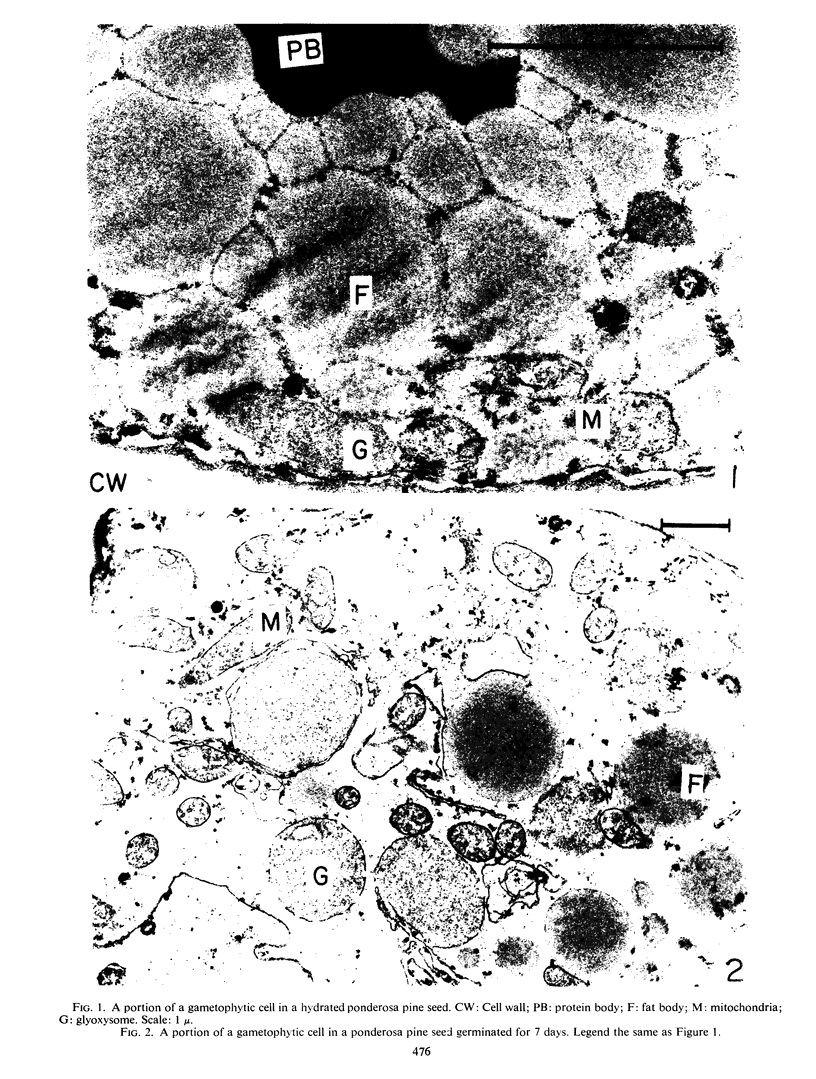
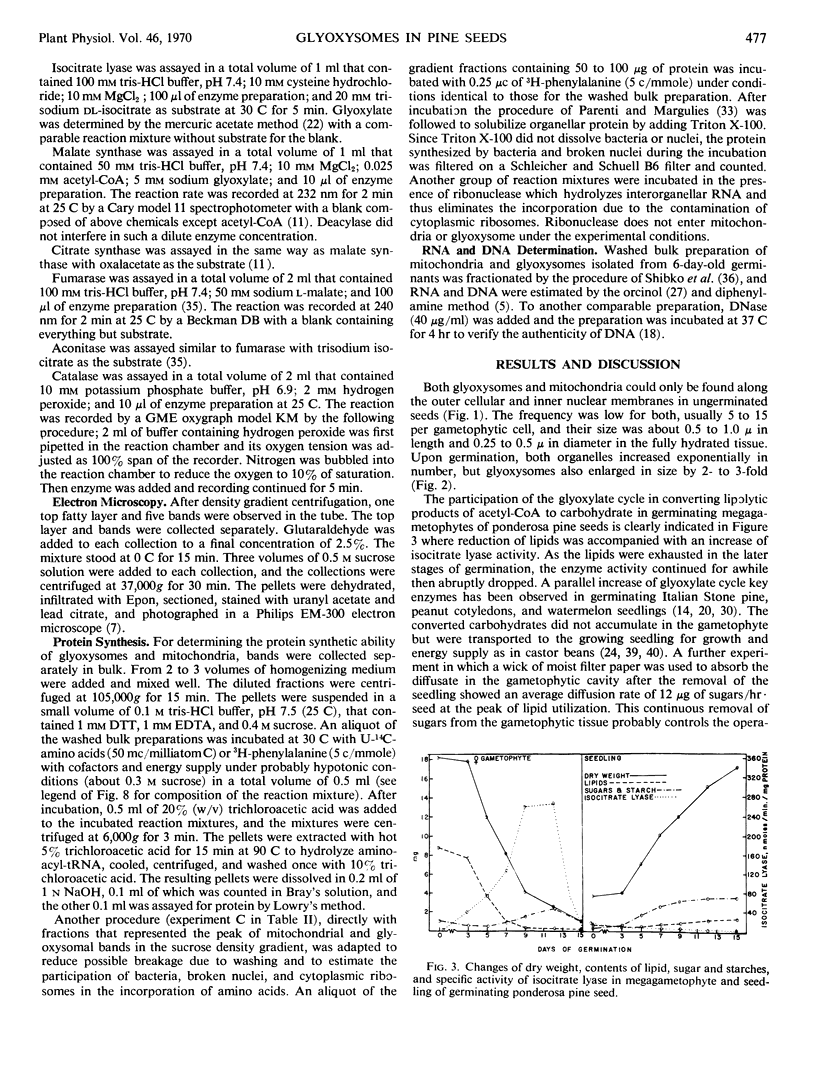
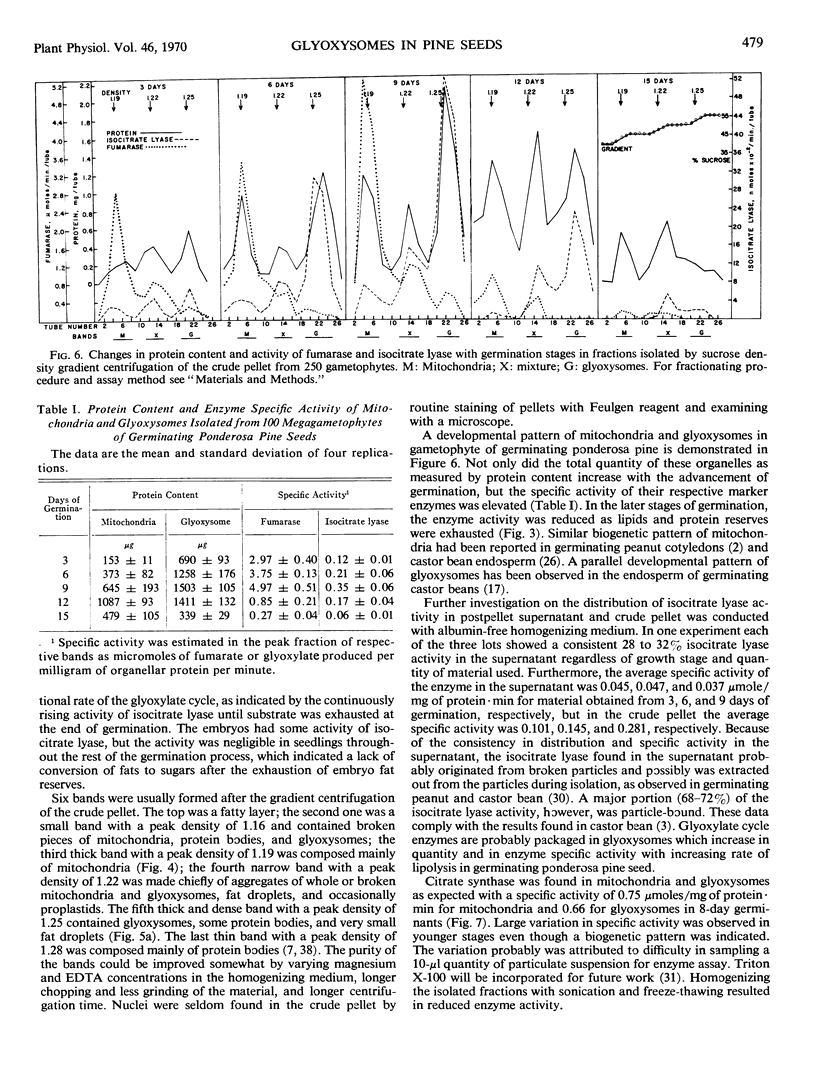
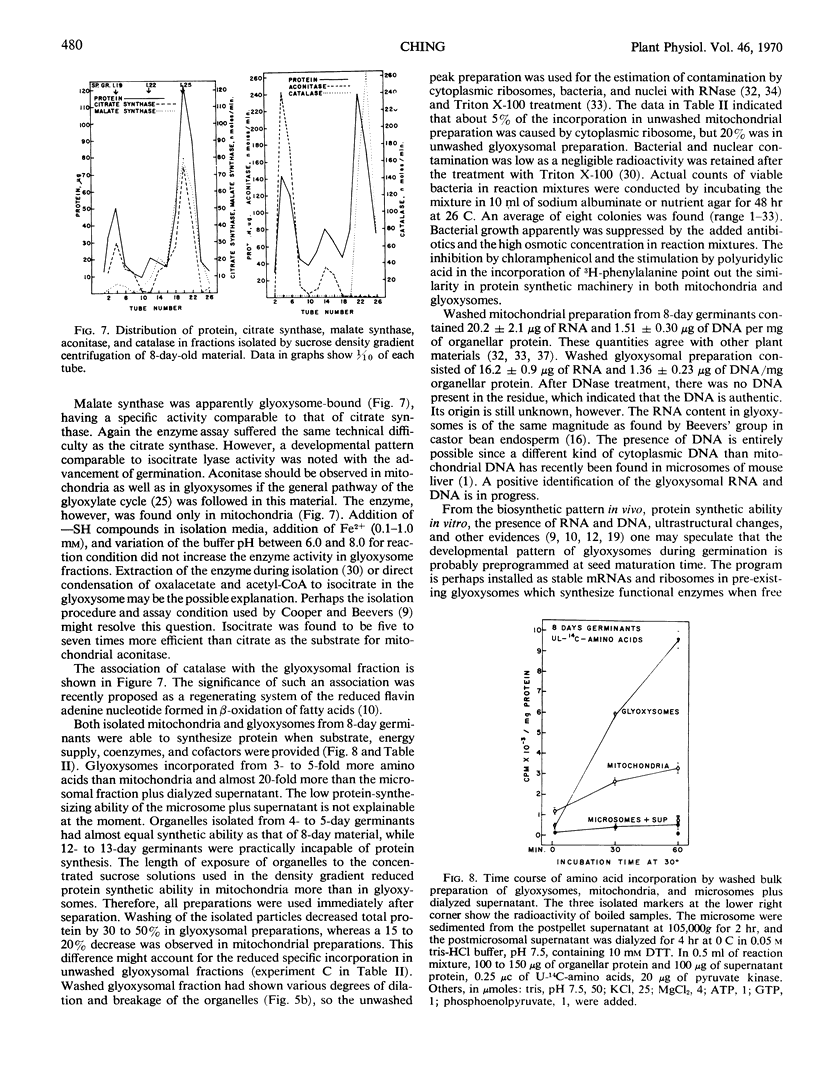
Decoated ponderosa pine (Pinus ponderosa Laws) seeds contained 40% lipids, which were mainly stored in megagametophytic tissue and were utilized or converted to sugars via the glyoxylate cycle during germination. Mitochondria and glyoxysomes were isolated from the tissue by sucrose density gradient centrifugation at different stages of germination. It was found that isocitrate lyase, malate synthase, and catalase were mainly bound in glyoxysomes. Aconitase and fumarase were chiefly localized in mitochondria, whereas citrate synthase was common for both. Both organelles increased in quantity and specific activity of their respective marker enzymes with the advancement of germination. When the megagametophyte was exhausted at the end of germination, the quantity of these organelles and the activity of their marker enzymes decreased abruptly. At the stage of highest lipolysis, the isolated mitochondria and glyoxysomes were able to synthesize protein from labeled amino acids. Both organellar fractions contained RNA and DNA. Some degree of autonomy in glyoxysomes is indicated.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond H. E., Cooper J. A., 2nd, Courington D. P., Wood J. S. Microsome-associated DNA. Science. 1969 Aug 15;165(3894):705–706. doi: 10.1126/science.165.3894.705. [DOI] [PubMed] [Google Scholar]
- Breidenbach R. W., Beevers H. Association of the glyoxylate cycle enzymes in a novel subcellular particle from castor bean endosperm. Biochem Biophys Res Commun. 1967 May 25;27(4):462–469. doi: 10.1016/s0006-291x(67)80007-x. [DOI] [PubMed] [Google Scholar]
- Breidenbach R. W., Castelfranco P., Peterson C. Biogenesis of mitochondria in germinating peanut cotyledons. Plant Physiol. 1966 May;41(5):803–809. doi: 10.1104/pp.41.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breidenbach R. W., Kahn A., Beevers H. Characterization of glyoxysomes from castor bean endosperm. Plant Physiol. 1968 May;43(5):705–713. doi: 10.1104/pp.43.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching T. M. Compositional changes of douglas fir seeds during germination. Plant Physiol. 1966 Oct;41(8):1313–1319. doi: 10.1104/pp.41.8.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching T. M. Intracellular distribution of lipolytic activity in the female gametophyte of germinating Douglas fir seeds. Lipids. 1968 Nov;3(6):482–488. doi: 10.1007/BF02530890. [DOI] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Beta oxidation in glyoxysomes from castor bean endosperm. J Biol Chem. 1969 Jul 10;244(13):3514–3520. [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- Filner P., Varner J. E., Wray J. L. Environmental or developmental changes cause many enzyme activities of higher plants to rise or fall. Science. 1969 Jul 25;165(3891):358–367. doi: 10.1126/science.165.3891.358. [DOI] [PubMed] [Google Scholar]
- Firenzuoli A. M., Vanni P., Mastronuzzi E., Zanobini A., Baccari V. Enzymes of glyoxylate in conifers. Plant Physiol. 1968 Jul;43(7):1125–1128. doi: 10.1104/pp.43.7.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firenzuoli A. M., Vanni P., Mastronuzzi E., Zanobini A., Baccari V. Participation of the glyoxylate cycle in the metabolism of germinating seed of Pinus pinca. Life Sci. 1968 Dec 15;7(24):1251–1258. doi: 10.1016/0024-3205(68)90253-1. [DOI] [PubMed] [Google Scholar]
- GIBOR A., IZAWA M. THE DNA CONTENT OF THE CHLOROPLASTS OF ACETABULARIA. Proc Natl Acad Sci U S A. 1963 Dec;50:1164–1169. doi: 10.1073/pnas.50.6.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt B. P., Beevers H. Developmental studies on glyoxysomes in Ricinus endosperm. J Cell Biol. 1970 Jan;44(1):94–102. doi: 10.1083/jcb.44.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt B. P., Beevers H. Occurrence of RNA in glyoxysomes from castor bean endosperm. Plant Physiol. 1969 Oct;44(10):1475–1477. doi: 10.1104/pp.44.10.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt B., Beevers H. Influence of sucrose on protein determination by the Lowry procedure. Anal Biochem. 1968 Aug;24(2):337–339. doi: 10.1016/0003-2697(68)90187-5. [DOI] [PubMed] [Google Scholar]
- Gientka-Rychter A., Cherry J. H. De Novo Synthesis of Isocitritase in Peanut (Arachis hypogaea L.) Cotyledons. Plant Physiol. 1968 Apr;43(4):653–659. doi: 10.1104/pp.43.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton D., Stumpf P. K. Fat Metabolism in Higher Plants. XXXVII. Characterization of the beta-Oxidation Systems From Maturing and Germinating Castor Bean Seeds. Plant Physiol. 1969 Apr;44(4):508–516. doi: 10.1104/pp.44.4.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. V., Evans H. J., Ching T. Enzymes of the glyoxylate cycle in rhizobia and nodules of legumes. Plant Physiol. 1966 Oct;41(8):1330–1336. doi: 10.1104/pp.41.8.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg H. L. The role and control of the glyoxylate cycle in Escherichia coli. Biochem J. 1966 Apr;99(1):1–11. doi: 10.1042/bj0990001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lado P., Schwendimann M. Changes of mitochondrial RNA level during the transition from rest to growth in the endosperm of germinating castor beans. Life Sci. 1967 Aug 15;6(16):1681–1690. doi: 10.1016/0024-3205(67)90136-1. [DOI] [PubMed] [Google Scholar]
- Lin R. I., Schjeide O. A. Micro estimation of RNA by the cupric ion catalyzed orcinol reaction. Anal Biochem. 1969 Mar;27(3):473–483. doi: 10.1016/0003-2697(69)90061-x. [DOI] [PubMed] [Google Scholar]
- Longo C. P. Evidence for de novo synthesis of isocitratase and malate synthesis in germinating peanut cotyledons. Plant Physiol. 1968 Apr;43(4):660–664. doi: 10.1104/pp.43.4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCUS A., VELASCO J. Enzymes of the glyoxylate cycle in germinating peanuts and castor beans. J Biol Chem. 1960 Mar;235:563–567. [PubMed] [Google Scholar]
- Müller M., Hogg J. F., De Duve C. Distribution of tricarboxylic acid cycle enzymes and glyoxylate cycle enzymes between mitochondria and peroxisomes in Tetrahymena pyriformis. J Biol Chem. 1968 Oct 25;243(20):5385–5395. [PubMed] [Google Scholar]
- Nass M. M. Mitochondrial DNA: Advances, Problems, and Goals. Science. 1969 Jul 4;165(3888):25–35. doi: 10.1126/science.165.3888.25. [DOI] [PubMed] [Google Scholar]
- Parenti F., Margulies M. M. In Vitro Protein Synthesis by Plastids of Phaseolus vulgaris. I. Localization of Activity in the Chloroplasts of a Chloroplast Containing Fraction from Developing Leaves. Plant Physiol. 1967 Sep;42(9):1179–1186. doi: 10.1104/pp.42.9.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard C. J., Stemler A., Blaydes D. F. Ribosomal ribonucleic acids of chloroplastic and mitochondrial preparations. Plant Physiol. 1966 Oct;41(8):1323–1329. doi: 10.1104/pp.41.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Shibko S., Koivistoinen P., Tratnyek C. A., Newhall A. R., Friedman L. A method for sequential quantitative separation and determination of protein, RNA, DNA, lipid, and glycogen from a single rat liver homogenate or from a subcellular fraction. Anal Biochem. 1967 Jun;19(3):514–528. doi: 10.1016/0003-2697(67)90242-4. [DOI] [PubMed] [Google Scholar]
- Suyama Y., Bonner W. D. DNA from plant mitochondria. Plant Physiol. 1966 Mar;41(3):383–388. doi: 10.1104/pp.41.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombs M. P. Protein bodies of the soybean. Plant Physiol. 1967 Jun;42(6):797–813. doi: 10.1104/pp.42.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]






