Abstract
Laboratory mice preferentially rear their offspring in communal nests (CN), with all mothers contributing to maternal care and feeding of all the pups. Previous studies using primarily outbred mice have shown that offspring reared under CN conditions may display increased preweaning growth rates and differences in adult behavior and neurobiology compared with mice reared under single-nesting (SN; one dam with her litter) conditions. Here we compared pup mortality; weaning and adult body weights; adult behavior; and gene expression in the hippocampus and frontal cortex between C57BL/6J, DBA/2J and 129x1/SvJ mice reared by using CN (3 dams and their litters sharing a single nest) or SN. Male and female pups of all 3 strains reared in CN cages showed higher body weight at weaning than did SN pups of the same strain, with no significant difference in pup mortality between groups. Adult male offspring reared in CN showed no differences in any behavioral test when compared with SN offspring. Combining CN dams and litters after parturition revealed greater cortical brain-derived neurotropic factor expression in adult male C57BL/6J offspring and cortical glucocorticoid receptor expression in adult male C57BL/6J and 129x1/SvJ offspring as compared with SN offspring of the same strain. Communal rearing can enhance juvenile growth rates but does not change adult behavior in inbred mouse strains, although potential effects on adult neurophysiology are possible.
Abbreviation: BDNF, brain-derived neurotrophic factor; CN, communal nesting; EPM, elevated plus-maze test; GR, glucocorticoid receptor; HB, holeboard test; LM, Lashley III maze test; NGF, nerve growth factor; SIT, social interest test; SN, single nesting
Mice (Mus musculus) are social animals and when housed together, under a variety of conditions, female mice preferentially rear their litters in a communal nest,35,46 in which dams combine all the pups into one nest site and share maternal duties, including nursing, among the offspring. As is shown in the present study and others31,41,45 communal nesting occurs regardless of genetic background or relatedness of the dams, and both familiar and unfamiliar female mice will combine their litters soon after introduction into the same cage. Previous studies also have shown that mouse pups reared in communal nests demonstrate equivalent or increased pup survival rates and increased body weight at weaning16,35,40,41 and as adults.16,26 In contrast to the premise that increasing litter size in the presence of a single dam results in decreased offspring body weight at weaning,30 the communal nesting phenomenon demonstrates that the presence of multiple dams and litters more than compensates for the increased competition inherent in a large litter in the same cage and may provide health and welfare benefits for growing mice.
Some studies have indicated that communal nesting (CN) is associated with significant differences in offspring behavior and neurophysiology when compared with mice reared in cages containing only their biologic mother and siblings (that is, single nesting; SN). These studies have shown that outbred male mice reared under CN conditions exhibited increased anxiety in the elevated plus-maze test, increased thigmotaxis in the open-field test,3,4 and increased brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) gene expression in the hippocampus and hypothalamus,5,6 when compared with mice reared in SN cages. In contrast, other studies using a variety of inbred strains and outbred stock have found that compared with mice reared in SN cages, CN mice showed either no significant behavioral differences17,24,41 or evidence of decreased anxiety12,14 in standard testing procedures, such as the open-field, zero-maze, and water-maze tests. In addition, another study11 indicated that variations in the periparturient social environment of the mouse dam (for example, the timing of the introduction of social housing of dams) may influence the development of adult offspring behavior and neurophysiology.
In light of these various findings, the current study examined how early-life exposure to a complex and high-density social environment such as a communal nest may interact with genetic background to affect pup welfare and adult offspring behavior and neurophysiology. To this end, we evaluated 3 commonly used inbred strains (C57Bl/6J, 129x1/SvJ, and DBA/2J) reared under CN or SN conditions by assessing pup survival prior to weaning; weaning and adult body weights; adult male behavior in a battery of tests that evaluate rodent sociability, anxiety, locomotion, and cognition; and the expression in specific areas of the brain of genes critical to emotion and stress response. In addition, communal conditions were varied between experiments to determine whether placing dams in communal cages before compared with after parturition differentially affected offspring development.
Materials and Methods
Experiment 1: CN with synchronized births. Animal care and use.
Animal procedures were approved by the Pennsylvania State University IACUC and took place in laboratory animal facilities that are fully AAALAC-accredited.
Mice (age, 6 wk; male, n = 20 each strain; female, n = 40 each strain) of 129x1/SvJ (129), C57BL/6J (B6), and DBA/2J (D2) strains were purchased from The Jackson Laboratory (Bar Harbor, ME). On arrival, male mice were housed individually and female mice were housed 4 per cage in animal rooms maintained within a temperature range of 18 to 24 °C, relative humidity of 30% to 70%, and a 12:12-h reverse light:dark cycle in polypropylene cages (27 cm × 15 cm × 13 cm) with corncob bedding (Bed-o'Cobs 1/4-in. , The Andersons, Maumee, OH) and stainless steel wire lids. Food (Labdiet 5001, PMI, St Louis, MO) and water were provided ad libitum. Dirty cages and bedding were replaced weekly. The SPF health status of mice in this facility was monitored by using sentinel surveillance. Mice remained negative for common mouse parasites and pathogens, including ectoparasites, pinworms, pathogenic enteric protozoa, mouse hepatitis virus, Sendai virus, Theiler murine encephalomyelitis virus (GD VII), Mycoplasma pulmonis, mouse parvovirus, minute virus of mice, epizootic diarrhea of infant mice virus, pneumonia virus of mice, reovirus 3, ectromelia virus, and lymphocytic choriomeningitis virus throughout the study period.
Seven days after arrival, breeding trios of 2 female mice and one male mouse were established per cage, with the addition of Enviro-dri (Shepherd Specialty Papers, Watertown, TN) as nesting material. Male mice were removed from the breeding cages 5 d later. Female mice remained housed in pairs until one or both mice showed visible signs of pregnancy, at which time they were housed individually.
Neonatal manipulation.
On the day of birth, for each viable litter of at least 4 pups, the dam was placed in a clean cage while the pups were sexed and weighed. SN pups and nesting material from the original cage then were placed in the clean cage with the dam. SN litters consisted of 5 to 7 mixed-sex pups (pups were culled or added from a single unassigned litter as needed), with an average of 5.8 per cage (dam:pup ratio = 1:5.8). Pups from a single unassigned litter were not added to more than one SN cage.
For CN litters, a similar procedure was followed, with the exception that 3 dams with viable litters of at least 4 pups were placed together in a clean cage. Pups from each litter were sexed, weighed, and then placed with nesting material from their original cage into a clean communal cage. Pups from a single unassigned litter were added, as needed, to make a total of 15 to 19 mixed-sex pups, with an average of 17.5 pups per CN cage (dam:pup ratio = 1:5.8). The 3 dams then were placed into the CN cage. Litters in cages used to calculate body weight data were born within a 24-h time span; litters in other CN cages were born within a 3-d time span.
The following day, pup numbers were recounted. Pups were then left undisturbed except for routine husbandry procedures until PND 12 or 13, at which point the pups and dams in each CN and SN cage were transferred to a larger cage (44.5 cm × 24 cm × 15 cm) of similar composition and having a stainless steel wire lid and corncob bedding. Nest material from each cage was transferred with the pups. SN pups were weighed and weaned on PND 25. Pups in CN cages were weighed and weaned on PND 25 of the youngest litter in the cage.
Experimental animals.
For both CN and SN groups, 2 male weanlings were chosen randomly from each cage and housed together as ‘sibling’ (that is, originating from the same CN or SN cage) pairs throughout the experiment until the start of Lashley maze testing, at which point they were transferred to individual caging for the remainder of the experimental period. Body weights were recorded at 62 d of age. Sibling pairs were housed in polypropylene cages (27 cm × 15 cm × 13 cm) with corncob bedding (Bed-o'Cobs 1/4-in., The Andersons), Enviro-dri nesting material (Shepherd Specialty Papers), and stainless steel wire lids except when behavioral testing required other housing (see individual test descriptions). Food (Labdiet 5001, PMI) and water were provided ad libitum. Excess weanling mice were used for other research studies or were euthanized.
The SN experimental group consisted of a total of 22 male mice (129x1/SvJ, n = 6; DBA/2J and C57BL/6J, n = 8 each) originating from 11 different SN cages. One male 129x1/SvJ mouse died of unknown cause at 50 d of age. The remaining sibling from that pair was housed individually for the remainder of the experimental period. The CN experimental group consisted of a total of 22 male mice (129x1/SvJ, n = 6; DBA/2J and C57BL/6J, n = 8 each) originating from 11 CN cages. No more than 2 male mice were used from any one SN or CN cage.
Behavioral testing.
Behavioral testing began at approximately 90 d of age and was performed in the sequence of social interest (SIT), elevated plus-maze (EPM), holeboard (HB), and Lashley III maze (LM) tests. Using the day of the SIT test as test day 1, the EPM test was performed on day 5 and the HB test on day 7 of testing for each mouse. The LM test followed on testing days 13 to 17 (for one male from each experimental pair) or days 19 to 22 (for the second male from each experimental pair). For all tests, the testing order was varied according to mouse, cage (CN or SN), and strain to balance potential order effects across groups. All testing occurred during the first 4 h of the dark phase in the same room within the animal facility. For the SIT and HB tests, the room was under dim illumination (11 to 16 lx at the level of the test cage floor). For the EPM test, the testing room was under bright illumination (215 to 269 lx at the level of the maze floor). For the LM test, the room was illuminated by dim red light. Test devices were wiped with 70% alcohol and allowed to dry between animals. Mice were brought from the animal room to the testing room on a laboratory cart with an opaque plastic bag covering the cages during the brief journey, to prevent exposure to bright illumination. In the test room, the plastic bag was removed, and the cages remained on the cart until testing was completed. During use, all test apparatuses were surrounded by opaque, white curtains that separated the apparatus from the computer equipment, other mice, and experimenter. The SIT and EPM tests were recorded by using an ANY-maze video tracking system (Stoelting, Wood Dale, IL).
SIT testing.
The SIT test was used to evaluate the tendency of a mouse to either investigate or avoid an unfamiliar mouse confined within the test mouse's home cage without allowing direct contact. Sibling pairs of experimental mice were housed in the animal room in large polypropylene cages (44.5 cm × 24 cm × 15 cm) with corncob bedding (Bed-o'Cobs 1/4-in., The Andersons), Enviro-dri nesting material (Shepherd Specialty Papers), and stainless steel wire lids. Mice were housed in one of these cages for a minimum of 24 h prior to testing. Food (Labdiet 5001, PMI) and water were provided ad libitum. This cage was considered to be the ‘home’ cage, and all SIT testing took place within each mouse's home cage. Immediately prior to the testing session, sibling pairs were placed together into a clean holding cage with wire lid for transport to the testing room.
The subject mouse's home cage (food, water, and wire lid removed) was placed on a table within the test room for videotaping. Two home cages were placed side-by-side on the table for simultaneous videotaping. An opaque barrier was placed between cages to prevent subject mice from seeing the other cage. A small wire cage (Galaxy Pencil/Utility Cup, Spectrum Diversified Designs, Streetsboro, OH) was placed (inverted) within each half of the home cage.
The 9-cm-diameter open bottom of the small wire cages rested directly on the bedding. Each wire cage was 11 cm tall, with 6 mm spacing between vertical wires running from the top to bottom of the cage. The top of each small wire cage was covered with a tent of duct tape to prevent climbing onto the cage. Each small cage was thoroughly cleaned with 70% alcohol before placement into a home cage. For each test, an unfamiliar, adult male mouse of the same strain as the subject mouse was placed into one of the wire cages (‘stimulus cage’) whereas the other wire cage was left empty. Sibling pairs of mice were tested one after the other, with the same stimulus mouse used for each pair.
At the start of each test, the subject mouse was removed from its holding cage and placed into the home cage at the end opposite the stimulus mouse. The subject mouse then was allowed 10 min to explore the home cage while being videotaped. Measurements of time spent in each half of the cage (stimulus cage half compared with empty cage half) were automatically tallied by the video-tracking software. Using videos, an observer with stopwatches scored time spent in close contact (mouse appeared to be in physical contact) with the stimulus cage and the empty cage.
EPM test.
An elevated plus maze was used to evaluate anxiety-related behavior in a novel environment. The maze was 39 cm high and constructed of clear acrylic glass with a black floor. Each arm was 29.5 cm long and 5.4 cm wide. Sides and ends of the closed arms were 15 cm high. Open arms had a 0.5-cm lip on each side and end. At the start of the test, the subject mouse was placed on the center platform facing a closed arm and allowed to explore the maze for 5 min while being video-recorded. Measurements included time in and number of entries into the open arms and closed arms.
HB test.
An automated holeboard apparatus (Photobeam Activity System—Open Field, San Diego Instruments, San Diego, CA) was used to evaluate exploratory behavior. The holeboard consisted of a square black acrylic glass arena (40.6 cm × 40.6 cm × 38.1 cm deep) marked into 4 equal squares with a 15-mm-diameter hole centered in each. A 16 × 16 photo beam configuration tracked the test mouse's path within the enclosure, with ambulatory movements, rearing, and head dipping recorded. The test mouse was placed in the center of the floor, with the trial automatically initiated after the first beam break. Activity was recorded for 5 min. Photocell beam interruptions for horizontal and vertical activity (rearing) and head-dipping were automatically accumulated into an Excel (Microsoft, Redmond, WA) file every 30 s during the 5-min test.
LM test.
The LM test is a route-learning test that involves the use of egocentric navigation. The maze used in this experiment was made of dark-gray plastic with a red plastic floor in the standard Lashley III configuration2 which rested on a stainless steel table during the testing period. The start box was 8 cm × 9.5 cm × 7 cm. The alleys were 5 cm wide, and the walls were 7 cm high. The openings leading from one alley to the next were 4 cm square and set in 11 cm from the end of each alley, with the total length of the maze being 45 cm. The goal box was 20.5 cm long. A sheet of clear plastic was placed over the maze during testing to prevent the mouse from climbing over the walls.
Mice were housed individually in polypropylene cages (28 × 18 × 13 cm; bedding and nesting material as previously described) modified to allow entrance by the mouse from the LM, as described previously,2 for a minimum of 24 h prior to the start of LM testing. Immediately before transport to the test room, food and water were removed from the home cage.
Each mouse underwent 1 trial daily for 5 consecutive days. To initiate testing, the test mouse was placed in the start box of the maze. Each subject's home cage then was placed at the end of the goal box, with the acrylic door raised so that the mouse could enter it after traversing the maze. The trial was initiated by raising the start box door when the mouse was facing it. A stopwatch was used to record start latency and time to reach the goal box. An error was scored when an animal made a 4-paw entry into a blind alley or retraced to alleys previously traversed. After the animal entered its home cage, the door was lowered, and the home cage was returned to the cart. Maze scoring was conducted using logic functions in an Excel (Microsoft) spreadsheet. Correct entries, forward errors (entries into blind alleys toward the goal box), and backward errors (entries into alleys in the direction of the start box) were accumulated for each daily trial. A learning index ([correct entries / total entries] × 100) was calculated for each trial for each mouse.
Gene expression analysis.
Mice were euthanized by cervical dislocation for the collection of brain tissue for gene expression analysis at 117 to 128 d of age. Samples from the hippocampus and frontal cortex (rostral 1 mm) were collected for analysis of brain-derived neurotropic factor (BDNF), nerve growth factor (NGF), and glucocorticoid receptor (GR). Brain tissue was collected as rapidly as possible by using a chilled dissecting block, and the tissue was placed in RNAlater (Ambion, Austin, TX) for storage until sample processing. RNA was isolated from mouse brain tissue by using the RNeasy Lipid Tissue Mini Kit (Qiagen, Valencia, CA), according to the manufacturer's protocol. A second extraction was done according to the same protocol to purify samples further, and final RNA concentration was assessed by using a NanoDrop spectrophotometer. RT-PCR analysis using TaqMan probes unique to each gene was conducted in an ABI 7300 Sequence Detection System (Applied Biosystems, Foster City, CA) by the Penn State Genomics Core Facility (University Park, PA). Data for each measurement point were analyzed by using the ΔΔCT method from 3 replicate wells for each gene, by using cyclophilin as the control gene.
Experiment 2: CN with unsynchronized births. Breeding animals.
Male and female 129x1/SvJ, DBA/2J and C57BL/6J breeder mice used in experiment 1 were housed together as described for breeding trios for experiment 1. Female mice included both nulliparous and primiparous breeder mice from experiment 1. Male mice were removed from the breeding cages after 5 d, at which time females were assigned randomly to either SN or CN cages. Female mice were housed in polypropylene cages (27 cm × 15 cm × 13 cm) with corncob bedding (Bed-o'Cobs 1/4-in., The Andersons), Enviro-dri (Shepherd Specialty Papers) and stainless steel wire lids under a 12:12-h reversed light:dark schedule. Food (Labdiet 5001, PMI) and water were provided ad libitum. SN female mice were singly housed and CN female mice were housed 3 to a cage. Only cages in which all female mice in the cage gave birth to at least 4 viable pups were used for this experiment. Pups were culled or added from unassigned litters at birth as needed to maintain mixed-sex litter sizes within a range of 5 to 9 (SN) and 15 to 22 (CN) pups per cage. Pups from a single unassigned litter were not added to more than one SN or CN cage.
All CN cages included 3 dams and their litters. Litters within a CN cage were born within a 5-d time period except for one 129x1/SvJ cage in which pups were born within a 7-d period. The average number of pups per CN cage was 18.5 (dam:pup ratio = 1:6.1) and an average number of pups per SN cage of 6.7 (dam:pup ratio = 1:6.7). All pups within SN litters were born within a 24-h period.
At PND 12 to 13 of the youngest litter in the cage, the pups and dams in each CN and SN cage were transferred to a larger cage (44.5 cm × 24 cm × 15 cm) of similar composition and having a stainless steel wire lid and corncob bedding. Nesting material from each cage was transferred with the pups. SN pups were weaned on PND 25. CN pups were weaned on PND 25 of the youngest litter in the cage.
Experimental animals.
For both CN and SN groups, male weanlings were chosen randomly from each cage and housed together as ‘sibling’ (that is, originating from the same CN or SN cage) pairs until the start of LM testing. Sibling pairs were housed as described in experiment 1. Excess weanling mice were used for other research studies or were euthanized.
The SN experimental group consisted of a total of 22 male mice (129x1/SvJ, n = 6; DBA/2J and C57BL/6J, n = 8 each strain) originating from 11 different SN cages. The CN experimental group consisted of a total of 26 male mice (129x1/SvJ, n = 10; DBA/2J and C57BL/6J, n = 8 each strain) originating from 11 CN cages. No more than 2 male mice were used from any one SN cage, and no more than 2 male mice from any one CN cage, except for the 129x1/SvJ strain for which 4 male mice from each of 2 CN cages were used.
Behavioral testing.
Behavioral testing began when the mice were 83 to 88 d old and was performed in the same sequence as for experiment 1. Depending on the individual mouse, the EPM test was performed 4 to 6 d after the SIT test, followed by HB testing 1 d after the EPM test. LM testing began 4 d after the HB test for one male mouse from each experimental pair and 8 d after the HB test for the second male from each experimental pair. For all tests, the testing order was varied by mouse, cage (CN or SN), and strain to reduce the likelihood of order effects. All testing was conducted as described for experiment 1.
Gene expression.
Mice were euthanized by cervical dislocation for collection of brain tissue for gene expression analysis at 124 to 131 d of age. Hippocampus and frontal cortex dissection and gene expression analysis was conducted as described for experiment 1.
Statistical analysis.
In general, data were analyzed via factorial ANOVA (between groups: experiment, 2; treatment, 2; strain, 3; and [when applicable] sex, 2) by using SPSS (IBM, Armonk, NY). Other factors were applied as needed for individual test analyses (see descriptions following). A 2-tailed test was used with a P value less than 0.05 as the level of significance for main effects. Missing data resulted in alterations in degrees of freedom in several analyses. Statistically significant interactions relevant to SN–CN differences within a strain were analyzed further by using the Bonferroni method. If SN–CN effects were found by using individual mouse data, additional analysis with litter means were conducted by using a factorial ANOVA.
SIT testing.
Time spent in each half of the test cage was analyzed initially by using a one-sample binomial test. Hypothetically, if mice showed no preference for either side of the test cage, the expected time in each half would be 50%. Mice were scored regarding whether they spent 50%, greater than 50%, or less than 50% of the test period in the stimulus half of the cage. In addition, percentage time spent in the stimulus half of the cage was calculated and used in ANOVA to determine factorial effects on behavior in the SIT test. In addition, the time spent in close contact with the small wire cages located within each half of the test cage (expressed as a percentage of time in that cage half) was analyzed by ANOVA.
EPM testing.
Because the Anymaze tracking software failed to track the B6 mice in experiment 2 accurately, B6 videos from that experiment were scored manually for time in open and closed arms and analyzed separately. Statistical analyses for experiments 1 and 2 therefore were conducted separately.
HB test.
Repeated-measures ANOVA included interval (10) as a dependent variable to investigate how behavior varied over time during the test period.
Results
Body weight and mortality.
Individual body weight data from CN and SN litters in experiment 1, in which all pups within the cage were born within a 24-h time period, were used to make statistical comparisons regarding PND 1 and 25. There was no significant difference (F1,182 = 0.096, P > 0.05) in body weight (mean ± 1 SD) between CN (n = 143; 1.29 ± 0.14 g) and SN (n = 45; 1.31 ± 0.10 g) groups at birth. There was a main effect of strain on body weight at birth, with D2 pups (n = 61; 1.25 ± 0.13 g) weighing significantly (F2,182 = 5.625, P < 0.05) less than B6 (n = 62; 1.31 ± 0.07 g) and 129 (n = 65; 1.33 ± 0.17) pups. There was no significant interaction between rearing condition and strain at birth (F2,182 = 0.601, P > 0.05).
At weaning, ANOVA of body weight data from experiment 1 revealed a main effect of rearing condition, with CN mice (n = 139; 11.39 ± 1.1 g) weighing significantly (F1,176 = 135.123, P < 0.001) more than SN mice (n = 49; 9.38 ± 1.28 g; Table 1). There was a main effect of strain on weaning weight, with B6 mice (n = 62; 11.21 ± 1.60 g) weighing significantly (F2,176 = 10.708, P < 0.001) more than D2 mice (n = 61; 10.50 ± 1.61 g), although neither of those 2 strains differed significantly from 129 mice (n = 65; 10.89 ± 0.98 g). Although there was no interaction between rearing condition and sex (96 female mice, 92 male mice; F(1,176) = 1.904, P > 0.05), there was an interaction between rearing condition and strain, in which CN B6 mice were significantly (F2,176 = 9.494, P < 0.001) heavier than were CN D2 and CN 129 mice. At the same time, SN D2 mice were significantly lighter than SN 129 mice, but neither of these strains was significantly different from SN B6 mice.
Table 1.
Body weight (mean ± 1 SD) at birth and PND 25 of experiment 1 CN and SN mice
| Weight (g) |
|||||
| Strain | Nesting condition | Birth | PND 25 | PND 25 litter | No. of mice (no. of litters) |
| 129x1/SvJ | CN | 1.32 ± 0.178 | 11.11 ± 0.630a | 11.13 ± 0.247a | 50 (3) |
| SN | 1.36 ± 0.133 | 10.16 ± 1.514 | 9.97 ± 1.483 | 15 (3) | |
| DBA/2J | CN | 1.25 ± 0.143 | 11.14 ± 1.177a | 11.18 ± 0.745a | 46 (3) |
| SN | 1.24 ± 0.051 | 8.53 ± 1.100 | 8.20 ± 1.365 | 15 (4) | |
| C57BL/6J | CN | 1.31 ± 0.073 | 11.99 ± 1.192a | 12.44 ± 1.356a | 43 (3) |
| SN | 1.30 ± 0.070 | 9.43 ± 0.733 | 9.28 ± 0.776 | 19 (3) | |
P < 0.001 between CN and SN mice of the same strain.
The previous weaning body weight analyses treated each mouse as an independent observation and ignored covariation due to litter membership. Reanalyzing the data by using litter means as the unit of analysis confirmed that CN mice weighed more (F1, 18 = 23.25, P < 0.001) than did SN mice at weaning. However, the rearing condition × strain interaction was not upheld (F2,18 = 1.51, P > 0.05).
Experiment 1 showed no significant differences in body weight between the subset of male CN (n = 22; 23.13 ± 0.27 g) and SN mice (n = 21; 23.03 ± 0.37 g) at 62 d of age (F1,37 = 0.06, P > 0.05), although B6 mice (n = 16; 24.00 ± 1.26 g) weighed significantly (F2,37 = 6.81, P < 0.05) more than did D2 (n = 16; 22.41 ± 1.53 g) and 129 (n = 11; 22.72 ± 1.03) mice at that time.
Experiment 1 also showed no significant differences in mortality between CN (n = 192) and SN (n = 69) mice of any strain, with an overall mortality rate (3 strains combined) from birth to weaning of 5% for CN mice and 1% for SN mice that was not statistically different (95% CI, −0.0298 to 0.0804). In experiment 2, the overall mortality rate (3 strains combined) from birth to weaning did not differ between CN (n = 240; mortality, 5%) and SN (n = 94; mortality, 7%) mice (95% CI, −0.0322 to 0.095).
SIT test.
A one-sample binomial test showed that a large majority (85 of 89) of all mice tested spent more than 50% of the test period in the stimulus half of the test cage (P < 0.001) that contained the novel mouse. Taking all animals into consideration showed that mice spent an average of 68.2% of the test period in this half of the cage. In both experiments, rearing condition (CN compared with SN) had no effect on the percentage of time spent in the stimulus half of the test cage (F1,77 = 1.134, P > 0.05).
To assess how mice spent their time within each cage half, analyses of close contact with the small wire cages (a proxy for sniffing) were conducted. Across all groups, when in the stimulus half of the test cage, mice spent 41% of their time in close contact with the cage containing the novel mouse compared with 22% of their time for the analogous measure when in the empty cage half (F1,79 = 152.1, P < 0.001). CN–SN treatment had no effect on the relative time spent in close contact with the small cage containing the novel mouse (F1,77 = 0.913, P > 0.05) or with the empty cage (F1,77 = 0.779, P > 0.05).
EPM test.
There were no significant differences between CN and SN mice in time spent or entries made into closed or open arms in either experiment. In experiment 1, CN mice (n = 22) spent a mean of 144.75 s in (1 SD = 36.61; F1,37 = 0.001, P > 0.05) and made a mean of 12.64 entries into (1 SD = 4.34; F1,37 = 0.5868, P > 0.05) the closed arms, whereas SN mice (n = 21) spent a mean of 144.47 s in (1 SD = 34.25) and made a mean of 13.46 entries into (1 SD = 2.01) the closed arms. In addition, CN mice also spent a mean of 90.95 s in (1 SD = 33.96; F1,37 = 1.2058, P > 0.05) and made a mean of 10.35 entries into (1 SD = 6.33; F1,37 = 1.0332, P > 0.05) the open arms, whereas SN mice spent a mean of 80.06 s in (1 SD = 28.75) and made a mean of 8.66 entries into (1 SD = 3.17) the open arms.
In experiment 2, 129 and D2 CN mice (n = 18) spent a mean of 94.82 s in (1 SD = 44.14; F1,28 = 1.8877, P > 0.05) and made a mean of 4.54 entries into (1 SD = 3.17; F1,28 = 1.1939, P > 0.05) the closed arms, whereas 129 and D2 SN mice (n = 14) spent a mean of 115.65 s in (1 SD = 39.98) and made a mean of 5.78 (1 SD = 2.66) entries into the closed arms. In this experiment, 129 and D2 CN mice also spent a mean of 153.28 s in (1 SD = 54.83; F1,28 = 2.4255, P > 0.05) and made a mean of 6.12 entries into (1 SD = 3.13; F1,28 = 0.3260, P > 0.05) the open arms, whereas 129 and D2 SN mice spent a mean of 121.74 s in (1 SD = 57.67) and made a mean of 5.57 entries into (SD = 1.78) the open arms. CN B6 (n = 8) mice in experiment 2 spent a mean of 182.25 s (1 SD = 56.0; F1,14 = 0.17, P > 0.05) in the closed arms and 104.88 s (1 SD = 55.42; F1,14 = 0.18, P > 0.05) in the open arms compared with SN B6 mice (n = 8), which spent a mean of 192.25 s (1 SD = 40.55) in the closed arms and 116.13 s (1 SD = 50.89) in the open arms.
HB test.
There were no statistically significant differences between CN (n = 47) and SN (n = 43) groups in any measures of the HB test, including measures (mean ± SE) of locomotion (ambulation) in the central zones by CN mice (77.69 ± 2.76 beam breaks; F1,42 = 0.263, P > 0.05) and SN mice (75.65 ± 2.89 beam breaks), locomotion in the peripheral zones by CN mice (14.10 ± 0.91 beam breaks; F1,42 = 1.316, P > 0.05) and SN mice (15.62 ± 0.96 beam breaks), number of rears by CN mice (1.96 ± 0.11 beam breaks; F1,42 = 0.699, P > 0.05) and SN mice (1.83 ± 0.12 beam breaks), and number of head dips by CN mice (1.19 ± 0.09 beam breaks; F1,42 = 0.666, P > 0.05) and SN mice (1.08 ± 0.09 beam breaks).
LM test.
The learning index (mean ± 1 SD) was the principal index used in analyzing the results of this test. There were no statistically significant differences in learning index between CN (n = 48, 59.75 ± 17.26; F1,67 = 2.475, P > 0.05) and SN (n = 43, 57.11 ± 16.85) groups for the 5 trials.
Gene expression.
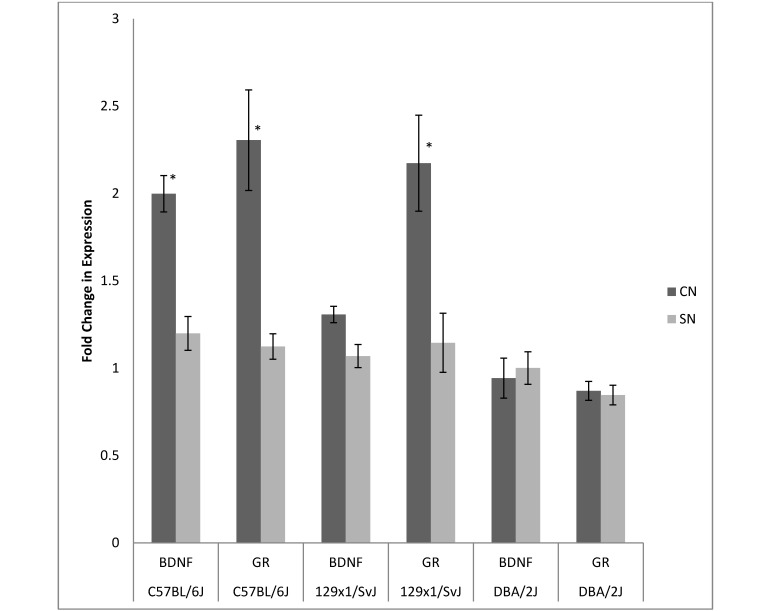
Litter means were used as the unit of analysis for gene expression data. Results illustrate the gene expression level (mean ± 1 SD) relative to the control (cyclophilin) for the gene, strain, and brain region. In experiment 1, BDNF gene expression in the frontal cortex was significantly (F2,14 = 10.81, P < 0.05) higher in CN B6 litters (n = 3, = 2.00 ± 0.21 fold) compared with SN B6 litters (n = 3, = 1.20 ± 0.19; Figure 1). In addition, GR gene expression in the frontal cortex was significantly (F2,14 = 6.25, P < 0.05) higher in CN B6 (2.31 ± 0.58 fold) and CN 129 (2.17 ± 0.55 fold) litters compared with SN B6 (1.12 ± 0.15 fold) and SN 129 (1.14 ± 0.34 fold) litters, respectively. Experiment 1 revealed no significant CN–SN differences in BDNF and NGF expression in the hippocampus or in NGF expression in the frontal cortex. Experiment 2 revealed no significant CN–SN differences in hippocampal or cortical NGF, BDNF, or GR expression, although the extraction yield in SN 129 mice was too low to compare CN–SN treatments.
Figure 1.
Experiment 1. Frontal cortex gene expression in adult mice. The expression of each experimental gene was normalized to that of cyclophilin. *, P < 0.05 between CN and SN mice of the same strain.
Discussion
Without exception, postparturient mice of the DBA/2J, C57BL6/J and 129x1/SvJ strains used in both experiments in this study combined their litters into a common nest when housed with other female mice and their litters. Female mice created a common nest within minutes of their initial placement in the cage and maintained that nest until weaning. All female mice in this study continued to combine their litters into a single nest even after being transferred to larger cages on PND 12 to 13. Our observation of dams in communal cages indicates that parental duties, including nursing, appear to be shared among the dams.
In experiment 1, male and female pups reared in CN cages weighed more at weaning, compared to pups of the same strain but reared in SN cages. This was true despite significant strain variations in weight at birth. Inbred mouse strains are known to show significant differences in reproductive parameters, including pup birth weight.19 Because of the range of birthdates in CN litters, we did not compare the weaning weights of CN and SN mice in experiment 2. Pup mortality did not differ between CN and SN groups in either experiment, consistent with the idea suggested by previous studies26,35,40,41 that, despite the large litter size of the communal group and exposure to multiple mothers, the communal nest is a hospitable environment and favorable to pup development. Although litter sizes were unequal and varied within a predetermined range for each treatment group, the mean dam:pup ratios for CN and SN cages were similar among all 3 strains in both experiments.
Housing multiple dams and litters in CN cages did not adversely affect pup welfare, at least from the standpoint of growth rate and mortality, in the 3 strains tested in our study and resulted in faster growth than that in SN cages. Studies on cooperative breeding in wild rodents, including Mus musculus, have shown that pups reared in communal nests are often heavier than are pups reared by a single female.42 Increased body weight at weaning may lead to not only increased survival rates but also earlier sexual maturity, heavier adult body weight, and greater reproductive success.42 This pattern suggests that communal nesting in laboratory mice is an evolutionarily adaptive behavior that may have beneficial consequences for laboratory mouse welfare. Our results are in agreement with previous studies of laboratory mice,16,26,40,45 in which pups reared in CN cages were heavier at weaning than were SN cohorts. In our current study, body weight differences did not persist into adulthood for male CN and SN mice, as had been shown previously in BALB/c mice.26 However, the possibility exists that the number of adult male mice that we used for body weight comparisons in each of the strains of this study was insufficient to accurately reflect differences, given that only a subset of male mice from experiment 1 was weighed as adults.
Although a definitive explanation for the functional and evolutionary significance of communal nesting in mice remains to be determined,25 it persists as a robust and dominant behavior, even in inbred and genetically manipulated strains of mice.34 Recent changes in floor space recommendations for the housing of mice with litters27 may discourage investigators from rearing multiple litters within the same cage. Concerns about potential overcrowding and deleterious health effects in CN cages are legitimate; nonetheless, the importance of CN in mouse maternal behavior and pup development should not be overlooked and is of concern to those interested in the behavioral welfare of laboratory mice.
A greater growth rate occurred in experiment 1 in CN mice when dams and litters were placed into communal cages after parturition. The social disruption and stress that may have occurred as a result of handling and moving pups and dams soon after parturition did not result in increased mortality or hinder pup growth and development. In contrast, pregnant dams in experiment 2 were placed into communal cages prior to parturition. This practice may be less stressful for the dams and pups than is the process we used in experiment 1.11 The age range of litters in CN cages in experiment 2 (2 to 7 d) was therefore greater than that in experiment 1 (1 to 3 d). Increasing age difference between litters within a communal nest increases dam aggression toward alien mouse pups.41 However, another study3 used litter age ranges as large as 7 d with no reported adverse consequences. Our results showed no significant difference in pup mortality between CN and SN groups in experiment 2.
Litter sizes in both experiments were maintained within a range that resulted in similar dam:pup ratios for CN and SN cages but were not kept rigidly equal or artificially low. In fact, the average size of our litters was higher than those reported by commercial breeders for these strains.28 In addition, we did not manipulate litter sex ratios, although no single-sex litters were used in the current study.
In contrast to pups reared under SN conditions, CN pups grow and develop in a complex social environment, in which each pup is exposed to multiple mothers with varying maternal behavior and to a large number of both sibling and nonsibling cohorts throughout infancy. Historically, communal nesting was used to study the effect of genetic relatedness on female mouse reproductive success.31,35 Recent work has emphasized its use as a model for the effect of early social environment on adult behavior.3,14 CN conditions have been described as a form of social enrichment and contrasted with the relative social isolation of SN. Both social isolation and social enrichment during rodent infant and juvenile periods have been shown to have significant effects on neurobiologic development, with consequent behavioral outcomes that can persist into adulthood.8,29,33
Data resulting from CN rearing have been variable and difficult to reconcile across studies. For example, some studies have concluded that adult mice reared under CN conditions are more fearful or anxious than are SN mice during exploration of an open field as demonstrated by decreased activity (ambulation) and rearing, increased thigmotaxis, and increased latency to initial movement.4,36 Others have found that CN conditions either decrease12,14 or do not affect41,45 anxious behavior as compared with SN conditions. Furthermore, effects specific to individual litter environment or genetics can influence test results if experimental mice are not carefully selected.20 In the current study, we performed behavioral and gene expression testing on a subset of male mice chosen from all available litters in an attempt to reduce litter-specific effects.
The open-field test has been criticized as a contaminated measure of anxiety, in light of the confounding effects of activity and exploration within the test.22 To that end, the EPM test has been advocated as a superior test of anxiety for mice22,44 and is commonly used for this purpose. However, previous studies of CN again found variable results when comparing adult male (outbred) mice reared under CN conditions with mice reared in a SN. One group of researchers4,6 found that CN mice showed greater anxiety-related behavior in the EPM test whereas another11 documented less anxiety in CN compared with SN mice. Our study showed no statistically significant differences in behavior between adult male CN and SN mice in any of the 3 inbred strains during EPM testing in either experiment 1 or 2. Similarly, another study16 did not find any difference in EPM behavior between adult outbred male mice reared in a SN and those reared by using a ‘double mothering’ model, in which 2 lactating mouse mothers raised a single litter.
In our study, exploratory behavior was measured by using the HB test, which has been proposed as a more independent measure of exploration and locomotion than is the open-field test.22 Our HB apparatus consisted of an open arena with 4 equally spaced and sized holes, for which the frequency of head-dipping by the mouse is thought to reflect exploratory behavior whereas distance traveled represents locomotion. In our study, both head dipping and distance traveled within zonal areas of the arena were measured but did not differ between adult male CN and SN mice in either experiment.
Previous CN studies have suggested that differing results in standardized behavioral tests might reflect variation in maternal housing conditions around the time of parturition. In experiment 1 of our study, dams were housed individually until after parturition; dams and their newborn litters were placed in communal cages within 48 h of birth. One author11 proposed that introducing unfamiliar dams with newborn litters into communal cages is stressful and could induce changes in the hormonal composition of the dam's milk that would influence the emotional development of pups. In contrast, placing pregnant dams together prior to parturition is thought to allow for the establishment of a stable social hierarchy before pups are born.11 Accordingly, dams in experiment 2 of our study were placed in communal cages several days prior to parturition. Even so, no significant differences in behavior in the EPM or HB tests were present between adult male CN and SN mice in either experiment.
Previous studies of CN have often focused on its effects on emotionality and exploratory behavior, with only 2 studies reporting on the effects of communal nesting on learning and memory. Neither of those studies found significant differences in learning ability between CN and SN mice in either the water maze17 or zero maze24 test. Similarly, our present study did not show significant differences in learning ability in the LM test between CN and SN mice in any strain tested. Although environmental enrichment (which frequently includes social housing) has been shown to improve learning and memory7,29 in rats and in some mutant mouse strains with impaired cognitive function34 and although social isolation has been reported to decrease learning ability in mice,18,32 the potential effects of social enrichment by itself are less clear and have not been investigated extensively.
Inbred rodents demonstrate reliable strain variations in social behavior, but early life experiences may influence the phenotypic expression of social behavior even as adults.1,15,33,37 Using the SIT test as a means to analyze social behavior, our study found no significant differences between CN and SN mice of any strain in time spent near or in close contact with an unfamiliar mouse confined within the test cage. It should be noted that mouse social interaction tests typically involve placing the mouse in a novel environment for testing. Previous studies4,6 of adult male (outbred) CN mice have indicated that they show greater levels of anxiety in novel situations than do SN mice. Given that rodent social interactions are decreased in a novel environment, especially in male mice,21,38 we conducted SIT testing within the home cage of the experimental mouse. By testing mice within their home cage, the potential confounding effects of fear and anxiety due to an unfamiliar environment likely were minimized. However, testing in the home cage potentially could result in more aggression due to territorial behavior by the experimental male mouse. Previous studies5,17 have shown that outbred CN male mice are more aggressive (for example, increased attack behavior) than are SN male mice in resident intruder tests, which typically include extended periods of isolation housing prior to testing and thus may increase territorial behavior. However, mice in our study were housed in ‘sibling’ pairs prior to the SIT test and may not have been primed to exhibit high territoriality.
Observations in various animal species, including humans, suggest that early life experience, including the quality of maternal care received by infant and juvenile offspring, significantly influences many areas of adult behavior and neurophysiology.9,29 Evidence obtained primarily from rodents demonstrates that varying levels of maternal care, especially tactile stimulation through licking and grooming of pups, influences the development of the brain with functional consequences that may persist throughout the life of the animal. Specifically, increased levels of maternal licking and grooming may result in adult offspring showing diminished hypothalamic–pituitary–adrenal axis responses to stress.9,29 Associated with these outcomes is a corresponding increase in GR expression in the hippocampus and frontal cortex. GR is thought to play an important role in inhibiting forebrain hypothalamic–pituitary–adrenal activity and is especially susceptible to influence from environmental conditions during the first week of life in rodents.39 In our study, adult male C57BL/6J and 129x1/SvJ CN mice in experiment 1 showed increased GR expression in the frontal cortex when compared with SN mice of the same strain. Cortical GR expression did not differ between CN and SN mice in experiment 2, although mRNA extraction yields for SN 129 mice were too low to permit comparison in this strain. However, rather than attribute increased cortical GR expression in experiment 1 strictly to communal rearing, it cannot be ruled out that these changes were influenced by pup exposure to a temporary period of altered maternal behavior during the initial establishment of the CN cage (when dams were combined after giving birth). For example, there may have been a temporary period of maternal inattention, followed by a sharp increase in maternal licking and grooming once the dams returned attention to the pups. Studies have shown that short periods of maternal separation lead to increased licking and grooming behavior by the mother after return to the pups, with resulting increased GR expression in the frontal cortex and hippocampus.23
Neurotrophins are CNS proteins that play important roles in neuronal cell growth and differentiation during early postnatal development. NGF and BDNF are 2 neurotrophins that reportedly are influenced by variations in early life experience in rodents. One group5 demonstrated increased levels of NGF and BDNF gene expression in the brains of adult outbred CN mice compared with SN mice and hypothesized that this difference was the result of the enriched social environment experienced by CN mice, which provided for enhanced brain plasticity and improved social adaptation skills in adults. In the cited study,5 gene expression levels were analyzed in 8-mo-old male mice after 3 wk of isolation housing and a social dominance–submission test. Our study did not reveal significant differences in NGF and BDNF gene expression in the hippocampus between CN and SN groups in any strain, although cortical BDNF was higher in adult male CN C57BL/6J mice compared with adult male SN C57BL/6J mice in experiment 1. Adult male mice in our study were housed singly during LM testing and for several days prior to tissue collection for gene expression analysis. Although it is possible that CN rearing caused the adult C57BL/6J mice to show increased BDNF gene expression later in life, once again, it cannot be ruled out that other preweaning or postweaning experiences influenced BDNF levels. The activity of the BDNF gene in both adult and juvenile rodents is known to be sensitive to environmental changes, with most studies using negative life experiences such as maternal abuse or social defeat, which often lead to decreases in BDNF expression in the FC.39 It is possible that differences between testing age and environmental or social experience prior to analysis may account for differences in results between our current study and others. The effects of relatively subtle positive life experiences in mice, such as an enriched social environment early in life, have been examined infrequently and as such are not definitively established.13,16 Given the complexity of the relationship between genetic background, environmental experiences, and neurobiologic development, it is likely that multiple epigenetic factors play a role in determining the ultimate outcome.14
In conclusion, the current study demonstrated that the offspring of inbred mice can benefit from CN through increased growth rates prior to weaning as compared with those of mice reared in SN. However, compared with SN conditions, CN rearing did not result in significant differences in adult behavior or neurobiology, with specific exceptions. Our results, taken together with contrasting results from previously cited studies and others comparing the effects of environmental enrichment, including social enrichment, on inbred mouse strain behavior,10,32,43,47 suggest that the development of adult behavioral and neurophysiologic phenotypes is heavily influenced by genetically predetermined baseline behavioral characteristics and vulnerability to epigenetic influences. Therefore, subtle effects of variations in periparturient and preweaning social environment may be obscured by individual-and strain-specific trait differences and postweaning environmental influences and probably do not represent a major source of behavioral variability in inbred mice. Nevertheless, communal rearing may, in certain strains, alter adult neurophysiology, such as BDNF and GR gene expression, when compared with that of single-rearing of offspring.
Clearly, communal rearing has the potential to enhance juvenile growth rates and is a significant variable to consider in rodent breeding and production systems. From an evolutionary standpoint, breeding systems that use CN conditions may represent a more species-typical maternal and pup environment than does SN. However, the benefits of CN may be tempered by environmental factors such as husbandry practices and housing systems. For example, increased pup numbers within static microisolation cages could lead to increased intracage humidity or ammonia levels and subsequent detrimental health effects. In keeping with the goal of promoting welfare by allowing mice to perform normal, species-specific behaviors, the provision of CN opportunities must be considered when housing breeding female mice and their litters. Additional research into the behavioral and physiologic aspects of CN is warranted.
Acknowledgments
This research was supported by the American Association for Laboratory Animal Science Grants for Laboratory Science program. We thank Marc Dingman for assistance with RNA extraction, Dr Igor Branchi for counsel on communal nesting behavior, and Dr Jacqueline Crawley for guidance on examining social behavior in mice.
References
- 1.Blizard DA, Adams N. 2002. The Maudsley reactive and nonreactive strains: a new perspective. Behav Genet 32:277–299 [DOI] [PubMed] [Google Scholar]
- 2.Blizard DA, Weinheimer VK, Klein LC, Petrill SA, Cohen R, McClearn GE. 2006. ‘Return to home cage’ as a reward for maze learning in young and old genetically heterogeneous mice. Comp Med 56:196–201 [PubMed] [Google Scholar]
- 3.Branchi I. 2009. The mouse communal nest: Investigating the epigenetic influences of the early social environment on brain and behavior development. Neurosci Biobehav Rev 33:551–559 [DOI] [PubMed] [Google Scholar]
- 4.Branchi I, Alleva E. 2006. Communal nesting, an early social enrichment, increases the adult anxiety-like response and shapes the role of social context in modulating the emotional behavior. Behav Brain Res 172:299–306 [DOI] [PubMed] [Google Scholar]
- 5.Branchi I, D'Andrea I, Fiore M, Di Fausto V, Aloe L, Alleva E. 2006. Early social enrichment shapes social behavior and nerve growth factor and brain-derived neurotrophic factor levels in the adult mouse brain. Biol Psychiatry 60:690–696 [DOI] [PubMed] [Google Scholar]
- 6.Branchi I, D'Andrea I, Sietzema J, Fiore M, Di Fausto V, Aloe L, Alleva E. 2006. Early social enrichment augments adult hippocampal BDNF levels and survival of BrdU-positive cells while increasing anxiety- and ‘depression’-like behavior. J Neurosci Res 83:965–973 [DOI] [PubMed] [Google Scholar]
- 7.Bredy TW, Humpartzoomian RA, Cain DP, Meaney MJ. 2003. Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience 118:571–576 [DOI] [PubMed] [Google Scholar]
- 8.Champagne FA, Curley JP. 2005. How social experiences influence the brain. Curr Opin Neurobiol 15:704–709 [DOI] [PubMed] [Google Scholar]
- 9.Champagne FA, Curley JP. 2009. Epigenetic mechanisms mediating the long-term effects of maternal care on development. Neurosci Biobehav Rev 33:593–600 [DOI] [PubMed] [Google Scholar]
- 10.Chapillon P, Manneché C, Belzung C, Caston J. 1999. Rearing environmental enrichment in 2 inbred strains of mice. 1. Effects on emotional reactivity. Behav Genet 29:41–46 [DOI] [PubMed] [Google Scholar]
- 11.Cirulli F, Berry A, Bonsignore LT, Capone F, D'Andrea I, Aloe L, Branchi I, Alleva E. 2010. Early life influences on emotional reactivity: evidence that social enrichment has greater effects than handling on anxiety-like behaviors, neuroendocrine responses to stress, and central BDNF levels. Neurosci Biobehav Rev 34:808–820 [DOI] [PubMed] [Google Scholar]
- 12.Clausing P, Mothes HK, Opitz B, Kormann S. 1997. Differential effects of communal rearing and preweaning handling on open-field behavior and hot-plate latencies in mice. Behav Brain Res 82:179–184 [DOI] [PubMed] [Google Scholar]
- 13.Coutellier L, Friedrich A-C, Failing K, Würbel H. 2008. Variations in the postnatal maternal environment in mice: effects on maternal behaviour and behavioural and endocrine responses in the adult offspring. Physiol Behav 93:395–407 [DOI] [PubMed] [Google Scholar]
- 14.Curley JP, Davidson S, Bateson P, Champagne FA. 2009. Social enrichment during postnatal development induces transgenerational effects on emotional and reproductive behavior in mice. Front Behav Neurosci 3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curley JP, Rock V, Moynihan AM, Bateson P, Keverne EB, Champagne FA. 2010. Developmental shifts in the behavioral phenotypes of inbred mice: the role of postnatal and juvenile social experiences. Behav Genet 40:220–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Amato FR, Zanettini C, Sgobio C, Sarli C, Carone V, Moles A, Ammassari-Teule M. 2011. Intensification of maternal care by double-mothering boosts cognitive function and hippocampal morphology in the adult offspring. Hippocampus 21:298–308 [DOI] [PubMed] [Google Scholar]
- 17.D'Andrea I, Alleva E, Branchi I. 2007. Communal nesting, an early social enrichment, affects social competences but not learning and memory abilities at adulthood. Behav Brain Res 183:60–66 [DOI] [PubMed] [Google Scholar]
- 18.Dai H, Okuda T, Sakurai E, Kuramasu A, Kato M, Jia F, Xu AJ, Iinuma K, Sato I, Yanai K. 2005. Blockage of histamine H1 receptor attenuates social isolation-induced disruption of prepulse inhibition: a study in H1 receptor gene knockout mice. Psychopharmacology (Berl) 183:285–293 [DOI] [PubMed] [Google Scholar]
- 19.Donahue L. [Internet]. Gestation duration and maternal weight gain in 15 inbred mouse strains. MPD:44405. Mouse phenome database website, The Jackson Laboratory. [Cited 13 September 2013]. Available at: http://phenome.jax.org
- 20.Festing MR. 2006. Design and statistical methods in studies using animal models of development. ILAR J 47:5–14. [DOI] [PubMed]
- 21.File SE. 2001. Factors controlling measures of anxiety and responses to novelty in the mouse. Behav Brain Res 125:151–157 [DOI] [PubMed] [Google Scholar]
- 22.File SE, Hyde JR. 1978. Can social interaction be used to measure anxiety? Br J Pharmacol 62:19–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francis DD, Champagne FA, Liu D, Meaney MJ. 1999. Maternal care, gene expression, and the development of individual differences in stress reactivity. Ann N Y Acad Sci 896:66–84 [DOI] [PubMed] [Google Scholar]
- 24.Gracceva G, Venerosi A, Santucci D, Calamandrei G, Ricceri L. 2009. Early social enrichment affects responsiveness to different social cues in female mice. Behav Brain Res 196:304–309 [DOI] [PubMed] [Google Scholar]
- 25.Hayes LD. 2000. To nest communally or not to nest communally: a review of rodent communal nesting and nursing. Anim Behav 59:677–688 [DOI] [PubMed] [Google Scholar]
- 26.Heiderstadt KM, Blizard DA. 2011. Increased juvenile and adult body weights in BALB/cByJ mice reared in a communal nest. J Am Assoc Lab Anim Sci 50:484–487 [PMC free article] [PubMed] [Google Scholar]
- 27.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): The National Academies Press. [Google Scholar]
- 28.Jackson Laboratory [Internet]. Jax mice database. The Jackson Laboratory. [Cited 13 January 2012]. Available at: http://jaxmice.jax.org/strain/000691.html
- 29.Kaffman A, Meaney MJ. 2007. Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. J Child Psychol Psychiatry 48:224–244 [DOI] [PubMed] [Google Scholar]
- 30.Kirkpatrick BW, Arias JA, Rutledge JJ. 1988. Effects of prenatal and postnatal fraternity size on long-term reproduction in mice. J Anim Sci 66:62–69 [DOI] [PubMed] [Google Scholar]
- 31.König B. 1994. Fitness effects of communal rearing in house mice: the role of relatedness versus familiarity. Anim Behav 48:1449–1457 [Google Scholar]
- 32.Kulesskaya N, Rauvala H, Voikar V. 2011. Evaluation of social and physical enrichment in modulation of behavioural phenotype in C57BL/6J female mice. PLoS ONE 6:e24755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laviola G, Terranova ML. 1998. The developmental psychobiology of behavioural plasticity in mice: the role of social experiences in the family unit. Neurosci Biobehav Rev 23:197–213 [DOI] [PubMed] [Google Scholar]
- 34.Lonetti G, Angelucci A, Morando L, Boggio EM, Giustetto M, Pizzorusso T. 2010. Early environmental enrichment moderates the behavioral and synaptic phenotype of MeCP2 null mice. Biol Psychiatry 67:657–665 [DOI] [PubMed] [Google Scholar]
- 35.Manning CJ, Dewsbury DA, Wakeland EK, Potts WK. 1995. Communal nesting and communal nursing in house mice, Mus musculus domesticus. Anim Behav 50:741–751 [Google Scholar]
- 36.Mothes HK, Opitz B, Werner R, Clausing P. 1996. Effects of prenatal ethanol exposure and early experience on home-cage and open-field activity in mice. Neurotoxicol Teratol 18:59–65 [DOI] [PubMed] [Google Scholar]
- 37.Panksepp JB, Lahvis GP. 2007. Social reward among juvenile mice. Genes Brain Behav 6:661–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Primus RJ, Kellogg CK. 1989. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Dev Psychobiol 22:633–643 [DOI] [PubMed] [Google Scholar]
- 39.Roth TL, Lubin FD, Funk AJ, Sweatt JD. 2009. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry 65:760–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sayler A, Salmon M. 1969. Communal nursing in mice: influence of multiple mothers on the growth of the young. Science 164:1309–1310 [DOI] [PubMed] [Google Scholar]
- 41.Sayler A, Salmon M. 1971. An ethological analysis of communal nursing by the house mouse (Mus musculus). Behaviour 40:62–85 [Google Scholar]
- 42.Solomon NG, Keane B. 2007. Reproductive strategies in female rodents, p 42–56. In: Wolff JO, Sherman PW, editors. Rodent societies: an ecological and evolutionary perspective. Chicago (IL): University of Chicago Press. [Google Scholar]
- 43.Toth LA, Kregel K, Leon L, Musch TI. 2011. Environmental enrichment of laboratory rodents: the answer depends on the question. Comp Med 61:314–321 [PMC free article] [PubMed] [Google Scholar]
- 44.Walf AA, Frye CA. 2007. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2:322–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Werboff J, Steg M, Barnes L. 1970. Communal nursing in mice: strain-specific effects of multiple mothers on growth and behavior. Psychon Sci 19:269–271 [Google Scholar]
- 46.Wilkinson GS, Baker AEM. 1988. Communal nesting among genetically similar house mice. Ethology 77:103–114 [Google Scholar]
- 47.Zhu SW, Yee BK, Nyffeler M, Winblad B, Feldon J, Mohammed AH. 2006. Influence of differential housing on emotional behaviour and neurotrophin levels in mice. Behav Brain Res 169:10–20 [DOI] [PubMed] [Google Scholar]