Abstract
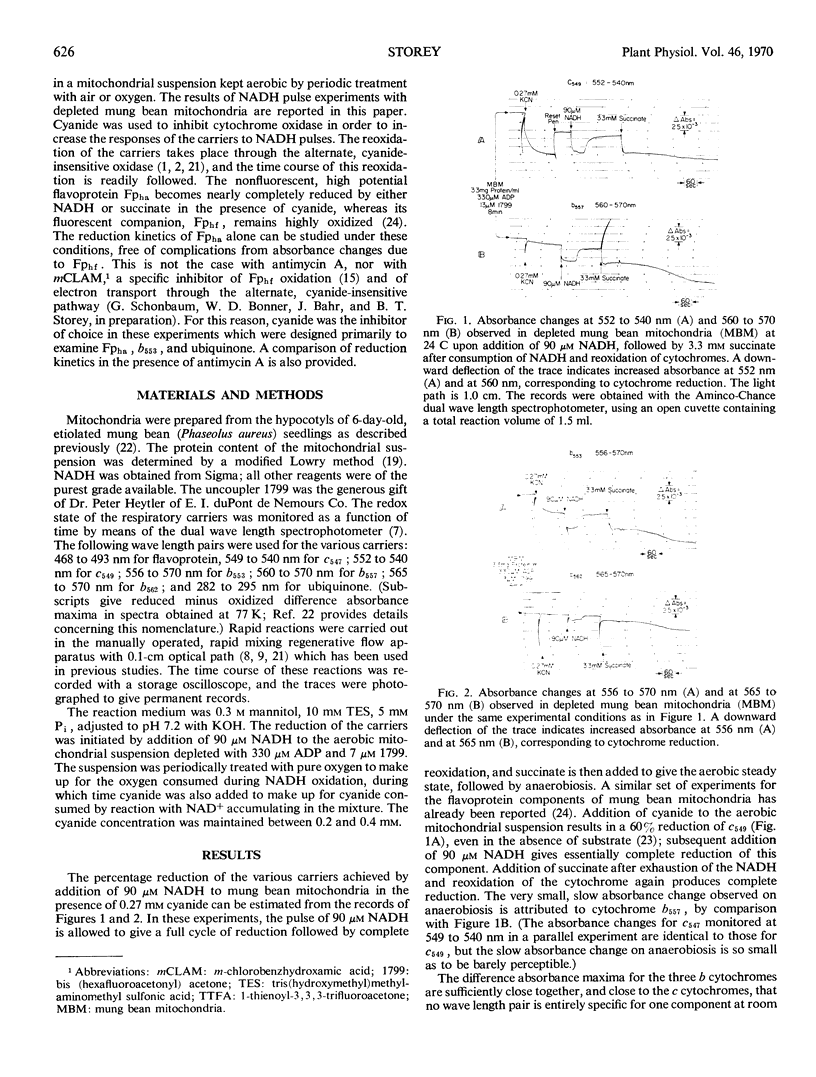
Addition of 90 micromolar reduced nicotinamide adenine dinucleotide (NADH) in the presence of cyanide to a suspension of aerobic mung bean (Phaseolus aureus) mitochondria depleted with ADP and uncoupler gives a cycle of reduction of electron transport carriers followed by reoxidation, as NADH is oxidized to NAD+ through the cyanide-insensitive, alternate oxidase by excess oxygen in the reaction medium. Under these conditions, cytochrome b553 and the nonfluorescent, high potential flavoprotein Fpha of the plant respiratory chain become completely reduced with half-times of 2.5 to 2.8 seconds for both components. Reoxidation of flavoprotein Fpha on exhaustion of NADH is more rapid than that of cytochrome b553. There is a lag of 1.5 seconds after NADH addition before any reduction of ubiquinone can be observed, whereas there is no lag perceptible in the reduction of flavoprotein Fpha and cytochrome b553. The half-time for ubiquinone reduction is 4.5 seconds, and the extent of reduction is 90% or greater. About 30% of cytochrome b557 is reduced under these conditions with a half-time of 10 seconds; both cytochrome b562 and the fluorescent, high potential flavoprotein Fphf show little, if any, reduction. The two cytochromes c in these mitochondria, c547 and c549, are reduced in synchrony with a half-time of 0.8 second. These two components are already 60% reduced in the presence of cyanide but absence of substrate, and they become completely reduced on addition of NADH. These results indicated that reducing equivalents enter the respiratory chain from exogenous NADH at flavoprotein Fpha and are rapidly transported through cytochrome b553 to the cytochromes c; once the latter are completely reduced, reduction of ubiquinone begins. Ubiquinone appears to act as a storage pool for reducing equivalents entering the respiratory chain on the substrate side of coupling site 2. It is suggested that flavoprotein Fpha and cytochrome b553 together may act as the branching point in the plant respiratory chain from which forward electron transport can take place to oxygen through the cytochrome chain via cytochrome oxidase, or to oxygen through the alternate, cyanide-insensitive oxidase via the fluorescent, high potential flavoprotein Fphf.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. D., Jr, Plesnicar M. Electron transport carriers in plant mitochondria. Nature. 1967 May 6;214(5088):616–617. doi: 10.1038/214616a0. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Chance B., Bonner W. D. Kinetics of Cytochrome Oxidation in Skunk Cabbage Mitochondria. Plant Physiol. 1965 Nov;40(6):1198–1204. doi: 10.1104/pp.40.6.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Hackett D. P. The Electron Transfer System of Skunk Cabbage Mitochondria. Plant Physiol. 1959 Jan;34(1):33–49. doi: 10.1104/pp.34.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska M., Storey B. T. The Respiratory Chain of Plant Mitochondria: VII. Kinetics of Flavoprotein Oxidation in Skunk Cabbage Mitochondria. Plant Physiol. 1970 Oct;46(4):618–624. doi: 10.1104/pp.46.4.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGGINS J. Analysis of sequential reactions. Ann N Y Acad Sci. 1963 May 10;108:305–321. doi: 10.1111/j.1749-6632.1963.tb13382.x. [DOI] [PubMed] [Google Scholar]
- Hackett D. P., Haas D. W. Oxidative Phosphorylation and Functional Cytochromes in Skunk Cabbage Mitochondria. Plant Physiol. 1958 Jan;33(1):27–32. doi: 10.1104/pp.33.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. III. Effects of Respiratory Inhibitors. Plant Physiol. 1967 Nov;42(11):1535–1544. doi: 10.1104/pp.42.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The respiratory chain of plant mitochondria. I. Electron transport between succinate and oxygen in skunk cabbage mitochondria. Plant Physiol. 1969 Jan;44(1):115–125. doi: 10.1104/pp.44.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The respiratory chain of plant mitochondria. II. Oxidative phosphorylation in skunk cabbage mitochondria. Plant Physiol. 1969 Jan;44(1):126–134. doi: 10.1104/pp.44.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. Rate of ubiquinone oxidation in electron transport particles reduced by succinate. Arch Biochem Biophys. 1968 Aug;126(2):585–592. doi: 10.1016/0003-9861(68)90445-1. [DOI] [PubMed] [Google Scholar]
- Storey B. T. The Respiratory Chain of Plant Mitochondria. III. Oxidation Rates of the Cytochromes c and b in Mung Bean Mitochondria Reduced With Succinate. Plant Physiol. 1969 Mar;44(3):413–421. doi: 10.1104/pp.44.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The Respiratory Chain of Plant Mitochondria: VI. Flavoprotein Components of the Respiratory Chain of Mung Bean Mitochondria. Plant Physiol. 1970 Jul;46(1):13–20. doi: 10.1104/pp.46.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The respiratory chain of plant mitochondria. IV. Oxidation rates of the respiratory carriers of mung bean mitochondria in the presence of cyanide. Plant Physiol. 1970 Apr;45(4):447–454. doi: 10.1104/pp.45.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban P. F., Klingenberg M. On the redox potentials of ubiquinone and cytochrome b in the respiratory chain. Eur J Biochem. 1969 Jul;9(4):519–525. doi: 10.1111/j.1432-1033.1969.tb00640.x. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Dutton P. L. Energy dependent changes in the oxidation-reduction potential of cytochrome b. Biochem Biophys Res Commun. 1970 Apr 8;39(1):59–64. doi: 10.1016/0006-291x(70)90757-6. [DOI] [PubMed] [Google Scholar]


