Abstract
Polymorphisms of the CYP450 genes that encode for the enzymes that metabolize estrogen are linked to hormone-related cancers. We investigated the impact of two polymorphisms of the CYP1B1 gene previously reported to be associated with hormone-related disorders on estrogen metabolism and bone mineral density (BMD), another hormone-dependent condition, in women from different ethnic backgrounds. Four hundred sixty-eight postmenopausal Caucasian women, 220 from St. Louis, MO, USA (mean age=63.5±0.53 years) and 248 from Palermo, Italy (mean age=72.9±0.44 years) participated in the study. Measurements of urinary estrogen metabolites by enzyme-linked immunoassay, serum estradiol by ultrasensitive radioimmnunoassay, and serum sex hormone-binding globulin by immunoradiometric assay were performed only in the American women, while BMD by dual energy X-ray absorptiometry and genotyping by pyrosequencing were performed in both American and Italian women. Differences in the levels of metabolites, free estradiol index and BMD were analyzed by analysis of covariance. Analysis among the American participants for the Valine432Leucine polymorphism showed that, compared to women with the Val/Val genotype, women with the Leu allele (Val/Leu and Leu/Leu) had significantly higher log-transformed values of total urinary estrogen metabolite (ng/mg-creatinine) levels (1.23±0.04, 1.35±0.02, and 1.34±0.03; p=0.03), and significantly lower BMD (gm/cm2) in the lumbar spine (1.009±0.02, 0.955±0.01 and 0.931±0.02; p=0.03) and the femoral neck (0.748± 0.02, 0.717±0.01 and 0.693±001, p =0.03) for the Val/Val, Val/Leu and Leu/Leu genotypes respectively. There were no significant differences in the urinary metabolites and BMD in the different genotypes for the Alanine119Serine polymorphism among the American women. Meanwhile, a separate analysis among the Italian women revealed no significant differences in BMD among the different genotypes for the two polymorphisms investigated. In conclusion, women with the Leu allele for the CYP1B1 Val432polymorphism have increased estrogen catabolism, as indicated by higher urinary estrogen metabolites, compared to those with Val/Val genotype. This may lead to relative hypoestrogenism and lower BMD in the lumbar spine and femoral neck in these women. Our data suggest that through its effect on the rate of estrogen catabolism, the Val432Leu polymorphism of the CYP1B1 gene may represent as a possible genetic risk factor for osteoporosis in American women.
Keywords: Genetic research, Hormone, Receptors, Osteoporosis, Epidemiology, Menopause
Introduction
Estrogen deficiency remains the most important risk factor for bone loss. However, attempts to correlate bone density with serum levels of estradiol or estrone in postmenopausal women have given conflicting results [1,2]. Such discrepancies may be explained, in part, by the presence of metabolites derived from the catabolism of estradiol and estrone with varying degrees of estrogenic activity [3,4]. Since ovarian activity is minimal after menopause, most circulating estrogen in postmenopausal women is derived from the aromatization of androstenedione to estrone, which can be reversibly oxidized to estradiol [5]. Although other relatively minor oxidative mechanisms exist, circulating estrone is predominantly oxidized through hydroxylation at two primary sites: the 2-hydroxyl pathway which leads to formation of the inactive metabolites, 2-hydroxyestrone (2OHE1) and 2-methoxyestrone (2MeOE1); and the 16α-hydroxy path-way which results in active metabolites as 16α-hydroxyestrone (16αOHE1) and estriol (E3) [4,6–8].
For years, studies have suggested an important role of estrogen metabolism in the pathogenesis of hormone-related cancers, most notably breast cancer [9,10], but little interest has been focused on osteoporosis, another hormone-related disorder. Previously, our group reported that the oxidative metabolism of estrogens may also be an important determinant of postmenopausal bone density [11] and polymorphisms of the cytochrome P450 (CYP450) enzymes that metabolize estrogen may influence bone mineral density (BMD) by their effect on estrogen metabolism [12]. Estrogen is metabolized by the CYP450 group of enzymes namely, CYP1A1, CYP1A2, CYP1B1 and CYP3A4, each encoded by a different gene [13,14]. Since each enzyme has a different 2- and 16α-hydroxylation activity, the overall estrogen metabolic profile of each individual is determined by the relative activity of each of these enzymes. Polymorphisms of the genes that encode for these enzymes have been linked to hormone-related disorders such as breast, prostate, ovarian, and endometrial cancers [15–19]. Lately, our group reported that the Thr461Asn polymorphism in the CYP1A1 gene which had been reported to be associated with differences in the risk for endometrial cancer, was also associated with differences in bone density. Women with the Asn allele had increased estrogen catabolism and low BMD in the proximal femur [12]. In this study, we investigated if polymorphisms in the CYP1B1 gene are also important determinants of bone density and the risk for osteoporosis as it has been reported for certain hormone-related cancers, such as breast cancer [17,18,20].
The objective of this study, therefore, was to evaluate the effects of the Alanine119Serine (rs1056827) and the Valine432Leucine (rs1056836) polymorphisms of the CYP1B1 gene on estrogen metabolism and BMD in Caucasian women from different ethnic origins.
Materials and methods
Experimental subjects
Two Caucasian postmenopausal populations were studied, one from the St. Louis, MO, USA area (N=220) and the other from Palermo, Italy (N=248). Each population included healthy women who were at least one year from the last menstrual period. The American subjects were part of a previously published cross-sectional study [12], conducted on community-dwelling otherwise healthy postmenopausal women, living in the St. Louis, MO, USA, and metropolitan area. All the previous clinical and laboratory data in these women were used in this study. Participants were recruited through flyers, advertisements or direct mailing to physicians and potential patients containing a brief description of the nature of the study and a summary of the inclusion and exclusion criteria. The Italian group, all originated from a limited geographical area in Western Sicily, were recruited from patients referred for bone health assessment at the University of Palermo. These women were part of a larger study investigating the role of genetics on bone mass and cognition. Aside from BMD measurements, all Italian subjects underwent an extensive physical, neuropsychological examination and neuroimaging tests. The results related to the cognitive assessment were not part of this report.
For the most part, the same eligibility criteria were followed in both groups. Subjects who were taking any medication that affect bone metabolism such as estrogen, selective estrogen receptor modulators (SERMS, including raloxifene and tamoxifen), bisphosphonates (alendronate, risedronate, pamidronate, ibandronate, or zoledronate), teriparatide, aromatase inhibitors, GnRH analogs, glucocorticoids (more than 5 mg of prednisone daily or equivalent for more than one month), or phenytoin were excluded from the study. Subjects with diseases or conditions known to interfere with bone metabolism, including hyperthyroidism, osteomalacia, chronic liver disease, renal failure, hypercortisolism, malabsorption, immobilization and alcoholism were excluded. Current tobacco users were also excluded, while past smokers who stopped smoking for at least 6 months prior to enrollment were allowed into the study. Among the American women, additional exclusionary criteria were applied because of our intention to measure urinary metabolites of estrogen. These included the intake of medications known to affect estrogen hydroxylation (phytoestrogens, cimetidine, thyroid hormones, mono-oxygenase inhibitors) and drugs known to affect cytochrome P450 enzyme activity, as was the consumption of more than one serving per day of vegetables containing high levels of phytochemicals known to preferentially enhance 2-hydroxylation of estrogen such as cabbage, cauliflower, Brussels sprouts, broccoli, and kale [21]. The latter exclusion criteria did not apply to the Italian population because estrogen metabolites, whose levels were influenced by the above reported medications and foodstuffs, were not analyzed in this group.
The protocol was approved by the Washington University School of Medicine Institutional Review Board and from the Ethical Committee of the University of Palermo, and informed consent was obtained from each participant.
Clinical, dietary and anthropometric data
Dietary calcium and Vitamin D intake in the American women were estimated from a 7-day dietary record, which was mailed to the participants at least one week prior to the study visit. The record contains a list and serving sizes of common dietary sources of calcium. The participants were asked to record daily intake of these foodstuffs and the average daily calcium intake was determined for 7 days. Information on daily intake of calcium from supplements was obtained at the study visit and added to the daily intake from the diet and both constitute the average daily calcium intake [22]. Diet history in the American women also included intake of vegetables such as cabbage, cauliflower, Brussels sprouts, broccoli, and kale. In the Italian group, calcium intake from both diet and supplements was obtained through an interview at the time of their first visit.
Alcohol intake was expressed as the average number of alcoholic-drink equivalents consumed over a one-week period. A can of beer (336 ml), a glass of wine (112 ml) and 28 ml of a heavy alcoholic beverage were considered one-drink-equivalent. Previous smoking was expressed in pack-years and estimated as the number of 20-cigarette packs smoked per day multiplied by the number of years of smoking. Physical activity was categorized as previously described and scored as follows: low activity (grade 1) mostly sedentary lifestyle, that is, being on feet <50% of the time without performing a regular set of exercise; moderate activity (grade 2), being on feet 50–75% of the time or performing a regular set of exercises, such as jogging, walking, biking, aerobics, for ≥30 min/day and ≥2 days/wk; high activity (grade 3), being on feet >75% of the time or engaging in regular heavy work, sports or exercises for >1 h/day and >4 days/wk [23].
A family history of osteoporosis was coded as positive in the presence of a first degree relative diagnosed with osteoporosis, kyphosis and fragility fractures in the absence of secondary causes. Data on estrogen exposure were assessed through a number of variables as age at menarche, average number of periods per year during the reproductive years, number of years of birth control pill use, total number of pregnancies, number of pregnancies to term, months of lactation, age at menopause and years since menopause (YSM). BMI was calculated as the weight in kilograms divided by the square of the height in meters (kg/m2).
Biochemical data
Both urinary estrogen metabolites and serum free estradiol index were measured only in the American women. Urinary estrogen metabolites were measured on a 24-hour urine specimen using the ESTRAMET™ immunoassay kits (Immuna Care Corp., Bethlehem, PA, USA). The ESTRAMET™ series of test kits are monoclonal antibody-based competitive enzyme immunoassays for estrogen metabolites in microtiter plate format. The antibodies and assays for urinary 2- and 16α-hydroxyestrogen have been described [24]. The monoclonal antibody to 2-hydroxyestrogens recognizes the 2-hydroxy forms of estrone E1, E2, and E3 equivalently. Similarly, the monoclonal antibody to 2-methoxyestrogens recognizes the 2-methoxy forms of estrogen metabolites equivalently and exhibits less than 0.1% cross-reactivity with any other estrogen, including 2-hydroxyestrogens. The monoclonal antibody to E3 exhibited less than 2% cross-reactivity with any other estrogen. All urinary estrogen assays were performed according to methods described previously [11]. Briefly, urine samples were incubated with enzymes that deconjugated estrogen metabolite sulfates and glucuronides to their respective free forms. The amount of estrogen metabolite in the enzymatic hydrolysate was determined by competition between deconjugated estrogen in the hydrolysate and estrogen-labeled alkaline phosphatase for binding to specific monoclonal antibodies attached to the microtiter plate. Greater than 90% of the metabolites in the urine exist as glucuronides and were recovered totally by this method. The inter- and intra-assay coefficients of variability for these enzyme-linked immunoassays were less than 9% and 13% respectively. All urinary metabolites were corrected for 24-hour urinary creatinine (mg/24 h) and expressed in ng/mg creatinine (ng/mg cr).
Serum samples were collected in a non-fasting state. Serum estradiol was measured by ultrasensitive radioimmunoassay technique (Diagnostic Systems Laboratory, Webster, TX.). The inter- and intra-assay coefficients of variability for this assay are <10%. Sex-hormone-binding globulin (SHBG) was measured by immunoradiometric (IRMA) assay (Diagnostic Systems Laboratory, Webster, TX.). The inter- and intra-assay coefficients of variability for serum estradiol and SHBG were <10%. The free estradiol index (FEI) was calculated as the molar ratio of the total estradiol to SHBG [25].
Bone mineral density (BMD)
In the American women, BMD of the lumbar spine and the proximal femur were measured by dual energy X-ray absorptiometry (DEXA) using Hologic QDR 4500 (Hologic Inc., Waltham Ma, U.S.A.). In the Bone Health Program at Washington University where all the bone density tests were performed, the coefficient of variability for this technique using QDR 4500 densitometer is 1.09% for the lumbar spine and 1.2% for the total femur [12]. In the Italian women, BMD of the lumbar spine and the proximal femur were measured by dual energy X-ray absorptiometry (DEXA) using Hologic Prodigy (Hologic Inc., Waltham Ma, U.S.A.).
In both groups, BMD of the lumbar spine was performed using the anteroposterior projection, and was calculated as the average of L1 to L4 vertebrae. The nondominant hip was used for proximal femur scans and values were calculated on the total femur, femoral neck, trochanter and intertrochanteric areas. BMD values were expressed in g/cm2.
The bone areas were obtained from regions of interest, as measured by the DEXA scan, to calculate the BMD of each skeletal site. The hip axis length (HAL), defined as the distance from the base of the greater trochanter to the inner pelvic brim of the acetabulum (mm) along the femoral neck axis was measured only in the American subjects [26]. The dimension was measured from DEXA scans of the proximal left femur using a standard software program provided by the manufacturer (Hologic Inc., Waltham Ma, U.S.A.).
Genotyping for CYP1B1 gene polymorphisms
Genomic DNA was extracted from peripheral leukocytes using the Puregene DNA Isolation Kit (Gentra Systems, Minneapolis, MN) and used as template for genotyping procedures. Genotype characterizations to identify polymorphisms were performed using polymerase chain reaction and suitable DNA fragments were confirmed by gel electrophoresis.
We examined two CYP1B1 polymorphisms, which have been known to be associated with disease, namely the Ala119Ser (rs1056827) in exon 2 and Val432Leu (rs1056836) in exon 3. The PCR reactions were performed using standard protocols with 1 μmol of each primer.
For the Ala119Ser polymorphism PCR reactions started with an initial denaturation at 94 °C for 5 min, followed by 35 cycles of 0.5 min at 94 °C, 1 min at 63 °C, and 1 min at 72 °C, with a final extension of 7 min at 72 °C, using 5′-TAC GGC GAC GTT TTC CAG AT-3′ as the forward and 5′-Biot CGT GAA GAA GTT GCG CAT CA-3′ as reverse primers. For the Val432Leu polymorphism PCR conditions were the following: an initial denaturation at 94 °C for 5 min, followed by 35 cycles of 0.5 min at 94 °C, 1 min at 63 °C, and 1 min at 72 °C, with a final extension of 7 min at 72 °C using 5′-TGT CCT GGC CTT CCT TTA TG-3′ as the forward and 5′-Biot TCC TTG TCC AAG AAT CGA GC-3′ as the reverse primers.
For both polymorphisms the genotype analysis was performed directly by Pyrosequencing (Biotage, Uppsala, Sweden) using the following sequencing primers: 5′-CAC GGA AGG AGG CGA-3′ for the Ala119Ser and a 5′-GTC TGT GAA TCA TGA CC-3′ for the Val432Leu.
Statistical analysis
Results are expressed as means±SE. Each urinary metabolite was regressed with BMD (g/cm2) in each of the skeletal sites analyzed to determine the effect of each urinary metabolite on BMD as previously described [11]. This type of analysis was done only in the American women who had biochemical data available. Values for urinary metabolites were not normally distributed and, therefore, were log transformed. The log-transformed values were then used in the analysis of covariance comparing metabolite values among the different genotypes adjusted for covariates previously reported to influence urine metabolite levels as age, YSM, BMI, and smoking history [12]. BMD (g/cm2) values among the genotypes for each CYP1B1 polymorphism were compared separately for the American and the Italian women by analysis of covariance adjusted for age, BMI, YSM, and smoking history. T-scores, Z-scores, bone areas, HAL and serum free estradiol index were compared by one-way analysis of variance. The clinical features of the different genotypes were compared using one-way analysis of variance for continuous variables (e.g. age, BMI) and using chi-square test for categorical variables (e.g. family history of osteoporosis, percentage of past smokers and percentage of patients with osteoporosis) as appropriate. Data were managed using Excel 2000 (Microsoft Corp., Redmond, WA, U.S.A.) and were analyzed using Statgraphic Plus 5.0 (Manugistic, Inc., Rockville, MD). The significance level was set at P<0.05. Analysis for Hardy–Weinberg equilibrium was carried out using the Polymorphism and Haplotype Analysis Suite (http://krunch.med.yale.edu/hwsim/).
Results
The association between Ala119Ser and Val432Leu polymorphisms of the CYP1B1 with estrogen metabolism and BMD were evaluated in 220 Caucasian postmenopausal women from St Louis, MO, USA and with BMD in 248 postmenopausal Caucasian women from Palermo, Italy. Among the American women, 266 volunteered to participate. Because of the well-known effect of ethnicity on estrogen metabolism, 14 women of Asian ancestry and 10 African-American women were not included in the analysis. Twenty-two other women were excluded from participation due to the following reasons: 5 were smokers, 4 were taking hormones, 4 were taking a selective estrogen receptor modulator, 3 were on bisphosphonates, 1 on calictonin nasal spray, 1 on parathyroid hormone injections, 2 refused to proceed with the study after signing the consent form, 1 could not provide a blood specimen and 1 was taking a supplement containing phytoestrogens. Because of the geographic location of Sicily, the Italian study volunteers were one hundred percent Caucasians. Since we did not intend to analyze for urinary estrogen metabolites in these women, none were excluded because of current use of medications or substances that alter estrogen metabolism. Moreover, none of the Italian patients were on medications that might interfere with BMD while 7 were excluded because of current tobacco use.
Table 1 shows the clinical features of the American and Italian women participants. The American women were younger in their chronological and menopausal age and have higher calcium intake than the Italians. There were also higher numbers of past smokers and subjects with positive family history for osteoporosis in the American women. However, age-adjusted BMD (Z-scores) was higher in Americans and there were fewer American women with osteoporosis (i.e. T-score of ≤ −2.5) compared to the Italians.
Table 1.
Clinical features of the American and Italian participants
| Clinical features | American N=220 | Italian N=248 |
|---|---|---|
| Age | 63.5±0.53 | 72.9±0.51 |
| BMI | 28.3±0.37 | 27.9±0.27 |
| YSM | 13.9±0.7 | 25.4±0.7 |
| Calcium | 1144.5±32.6 | 540.9±31.8 |
| History of smoking | ||
| Percent past smokers | 42.5% | 10.6% |
| Total past smoking (pack-years) | 8.59±13.4 | 1.4±5.8 |
| Positive family history of osteoporosis | 48.4% | 21.8% |
| BMD (Z-scores) | ||
| Spine | 0.80±0.1 | 0.22±0.1 |
| Total femur | 0.52±0.07 | 0.01±0.07 |
| Femoral neck | 0.27±0.07 | −0.24± 0.07 |
| Trochanter | 0.53±0.07 | −0.02±0.07 |
| Intertrochanter | 0.857±0.05 | 0.53±0.05 |
| % of patients with osteoporosis | ||
| Spine T score <−2.5 | 13.1% | 31.2% |
| Femoral neck T score <−2.5 | 9.9% | 33.7% |
Biochemical data were available only in the American women. One hundred seventy three had urinary estrogen metabolite measurements and 141 had serum free estradiol index (FEI). As previously reported [12], simple correlation analysis revealed weak inverse correlations between levels of urinary E3 and BMD of the total hip and intertrochanter. Total urinary metabolites (2OHE1 + 2MeOE1 + 16αOHE1 +E3), likewise, showed weak inverse correlations with BMD of the total hip, femoral neck, and intertrochanter, while no significant correlations observed between the spine BMD with any of the metabolites, and for 2MeOE1,16αOHE1, and 2OHE1/16αOHE1 ratio, with any skeletal site analyzed [12].
Comparing biochemical phenotypes for the Val432Leu of the CYP1B1 gene revealed that American women carrying the Leu allele (Val/Leu and Leu/Leu) had higher urinary estrogen metabolites the differences of which were significant for 2MeOE1 and total metabolites (2OHE1 +2MeOE1 +16αOHE1 +E3) compared to the Val/Val genotype (Table 2). There were no significant differences in the ratios between the inactive and the active metabolites (2OHE1/16αOHE1 or 2MeOE1/16αOHE1) (Table 2) among the genotypes.
Table 2.
Urinary metabolites of the different CYP1B1 Val432Leu genotypes in the American population
| Urine metabolites (log-transformed, ng/mg cr) | VAL/VAL N=34 | VAL/LEU N=90 | LEU/LEU N=49 | *P |
|---|---|---|---|---|
| 2OHE1 | 0.72±0.05 | 0.85±0.03 | 0.82±0.04 | 0.07 |
| 2MeOE1 | 0.49±0.04 | 0.61±0.02 | 0.61±0.04 | 0.03 |
| 16αOHE1 | 0.50±0.04 | 0.59±0.03 | 0.54±0.04 | 0.22 |
| E3 | 0.68±0.04 | 0.78±0.03 | 0.79±0.04 | 0.24 |
| 2OHE1/16αOHE1 | 0.20±0.04 | 0.24±0.03 | 0.29±0.04 | 0.35 |
| 2OHE1 + 2MeOE1 + 16αOHE1 +E3 | 1.23±0.04 | 1.35±0.02 | 1.34±0.03 | 0.03 |
| FEI | 0.57±0.06 | 0.45±0.03 | 0.47±0.04 | 0.16 |
P by ANOVA adjusted for age, BMI, YSM, and history of smoking.
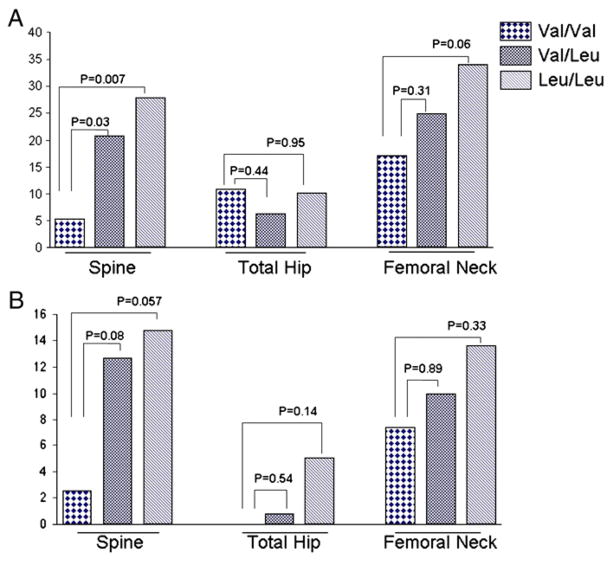
Analysis of the BMD data (g/cm2) adjusted for age, BMI, YSM and history of smoking in the American women showed that those carrying the Val/Leu and Leu/Leu genotypes had significantly lower BMD at the lumbar spine and femoral neck compared to those with Val/Val genotype. A trend for lower BMD in the other femoral sites was also observed but the differences were not statistically significant (Table 3). T-scores (BMD expressed as standard deviations from the mean of young adults) were also significantly lower in the spine, femoral neck and femoral trochanter in those carrying the Leu allele, while Z-scores (standard deviations from the mean of gender and age-matched individuals) were also lower in the spine and femoral neck but the differences did not reach statistical significance. There were no significant differences in bone size or bone area and in HAL among the genotypes. There were significantly more subjects with BMD T-scores of −2.0 or less, and more women with osteoporosis (T-score of −2.5 or less) in the spine in women with the Leu allele compared to those without the allele (Fig. 1), but the difference was barely significant. A similar trend was observed in the femoral neck. Fracture data were not available in our participants.
Table 3.
Skeletal features of the different CYP1B1 Val432Leu genotypes in the American population
| BMD (g/cm2) | VAL/VAL N=42 | VAL/LEU N=113 | LEU/LEU N=61 | *P |
|---|---|---|---|---|
| Spine | 1.009±0.02 | 0.957±0.01 | 0.932± 0.02 | 0.03 |
| Total femur | 0.878±0.02 | 0.868±0.01 | 0.848±0.01 | 0.39 |
| Femoral neck | 0.748±0.01 | 0.718±0 01 | 0 .693±0 01 | 0.03 |
| Trochanter | 0.674±0.01 | 0.653±0.01 | 0.639±0.01 | 0.22 |
| Intertrochanter | 1.039±0.02 | 1.034±0.01 | 1.024±0.01 | 0.94 |
| T-scores | P | |||
| Spine | −0.43±0.20 | −0.96±0.12 | −1.3±0.17 | 0.02 |
| Total femur | −0.37±0.15 | −0.71±0.10 | −0.79±0.14 | 0.10 |
| Femoral neck | −0.89±0.14 | −1.29±0.09 | −1.47±0.12 | 0.008 |
| Trochanter | −0.21±01.5 | −0.59±0.09 | −0.69±0.13 | 0.04 |
| Intertrochanter | −0.27±0.16 | −0.46±0.10 | −0.51±0.14 | 0.48 |
| Z-scores | P | |||
| Spine | 1.23±0.22 | 0.75±0.13 | 0.56±0.18 | 0.05 |
| Total hip | 0.69±0.17 | 0.46±0.10 | 0.47±0.14 | 0.51 |
| Femoral neck | 0.57±0.15 | 0.23±0.09 | 0.09±0.13 | 0.05 |
| Trochanter | 0.80±0.17 | 0.47±0.10 | 0.43±0.14 | 0.17 |
| Intertrochanter | 0.59±0.17 | 0.48±0.10 | 0.55±0.14 | 0.83 |
| Area (cm2) | P | |||
| Spine | 56.46±0.83 | 57.62±0.52 | 56.98±0.71 | 0.47 |
| Total femur | 33.30±0.44 | 34.00±0.28 | 33.80±0.38 | 0.39 |
| Femoral neck | 5.01±0.05 | 5.05±0.03 | 5.05±0.05 | 0.21 |
| Trochanter | 10.54±0.21 | 10.90±0.13 | 11.07±0.18 | 0.15 |
| Intertrochanter | 17.74±0.34 | 18.05±0.22 | 17.67±0.30 | 0.55 |
| Hip axis length | P | |||
| (mm) | 102.7±1.09 | 102.8±0.65 | 101.6±0.88 | 0.55 |
All analysis by ANOVA,
P adjusted for age, BMI, YSM and history of smoking.
Fig. 1.
Proportion of patients with T-scores of −2.0 and less (panel A), and those with osteoporosis, i.e. T-score of −2.5 or less (panel B), among the different genotypes for the Val432Leu polymorphism of the CYP1B1. Group comparisons by T-test.
A comparison of the clinical features in the American subjects according to Val432Leu polymorphisms showed significant differences in age among the genotypes (Table 4) but no significant differences were found for other clinical features as YSM, BMI, calcium intake, alcohol use, past smoking, family history of osteoporosis and activity score. There were no significant associations observed for the CYP1B1 Ala119Ser with estrogen metabolites or BMD in the American women. There were also no significant differences in BMD phenotypes for either the Ala119Ser or Val432Leu polymorphisms among the Italian women (data not shown).
Table 4.
Clinical features of the different CYP1B1 Val432Leu genotypes in the American population
| Clinical features | VAL/VAL N=42 | VAL/LEU N=113 | LEU/LEU N=61 | *P |
|---|---|---|---|---|
| Age | 61.0±1.24 | 63.1±0.76 | 65.9±1.03 | 0.02 |
| BMI | 29.4±0.85 | 27.6±0.52 | 28.2±0.71 | 0.2 |
| YSM | 12.0±1.7 | 13.5±1.1 | 16.3±1.4 | 0.1 |
| Positive family history of osteoporosis | 53.6% | 47.7% | 48.3% | 0.80 |
| History of smoking | ||||
| Percent past smokers | 50% | 39.%% | 43.3% | 0.51 |
| Total past smoking (pack-years) | 5.75±2.7 | 7.6±1.6 | 12.3±2.2 | |
| Calcium intake | 1066.6±99.5 | 1138.4±60.3 | 1215.6±84.1 | 0.1 |
| Alcohol intake (oz-Eq/wk) | 1.32±0.40 | 1.24±0.24 | 1.38±0.34 | 0.92 |
| Activity score | 2.2±0.10 | 2.3±0.06 | 2.1±0.08 | 0.38 |
P by ANOVA for continuous variables and χ2 for categorical variables.
The genotype frequencies of these polymorphisms in the American women were as follows: 19.4% (42/216) had Val/Val, 52.3% (113/216) had Val/Leu and 28.3% (61/216) had Leu/Leu genotypes in the Val432Leu polymorphism, while 54.5% (120/220) had Ala/Ala, 39.1% (86/220) had Ala/Ser and 6.4% (14/220) had Ser/Ser genotypes in the Ala119Ser polymorphism. The frequencies of both polymorphisms in the American women are in agreement with the Hardy–Weinberg equilibrium. The genotype frequencies for these polymorphisms in the Italian women were: 16.2% (40/248) had Val/Val, 56.4% (140/248) had Val/Leu and 27.4% (68/248) had Leu/Leu genotypes in the Val432Leu polymorphism, while 21% (44/210) had Ala/Ala, 66.2% (139/210) had Ala/Ser and 12.8% (27/210) had Ser/Ser genotypes in the Ala119Ser polymorphism. Contrary to the findings in the American women, the genotype frequencies for both polymorphisms in the Italian women were not in Hardy–Weinberg equilibrium.
Discussion
Our results indicate that the Val432Leu polymorphism of the CYP1B1 gene may represent as one of the determinants of post-menopausal BMD through its effect on estrogen metabolism in certain racial groups, in this particular study, American women. Those carrying the Leu allele for this polymorphism had significantly increased levels of total estrogen metabolites suggesting an increase in estrogen catabolism, and significantly lower bone density at the spine and the femoral neck. The differences in BMD were not accounted for by differences in bone size or length. In agreement with our previous report on the C4887A polymorphism of the CYP1A1 gene [12], our current results provide additional evidence that polymorphisms in the CYP450 genes may influence postmenopausal BMD and the future risk for osteoporosis because of their effects on estrogen metabolism. In the absence of fracture data, the influence of this polymorphism on fracture risk remains unknown. Several factors have been identified to contribute to the risk for fracture other than BMD [27], but BMD has traditionally been used as an objective method for predicting risk for osteoporotic fractures [28]. In our study, a greater proportion of women carrying the Leu allele have low T-scores. These women, therefore, may represent a subgroup who may benefit from aggressive bone loss prevention measures, most especially at the time of menopause, before a fracture has occurred.
Polymorphisms in the CYP450 enzyme genes that metabolize estrogen have been reported to result in variable enzymatic activity leading to differences in the risk for hormone-related disorders [15,17,18,20]. For instance, the Val/Val genotype at position 432 carries an odds ratio of 3.8 for developing ovarian cancer in a study of women with multiethnic background [18], whereas the Leu/Leu variant is associated with a risk of 2.3 for breast cancer among Chinese women [20]. Among Japanese women, the Ala→Ser substitution in codon 119 is associated with increased risk of breast and lung cancers, while Val→Leu substitution in codon 432 has no relation to either condition [17].
Results from in-vitro experiments have shown that the catalytic efficiencies of certain CYP1B1 variants far exceed that of the wild type [29]. In our study, the increase in estrogen metabolites in the American women carrying the Leu allele for the Val432Leu polymorphism compared to those without the allele is most likely a result of an enhanced enzyme activity resulting in a higher rate of estrogen catabolism. As a consequence, a relative hypoestrogenic state may occur in these patients perhaps leading to a lower BMD in the lumbar spine and femoral neck. Whereas the above studies on the association between polymorphisms in the CYP1B1 gene and hormone-related disorders failed to measure estrogen metabolites in their subjects [17,20], our findings provided proof to the concept that certain variants of the CYP1B1 gene have altered enzyme activity as initially suggested [29]. By increasing the rate of estrogen catabolism, the presence of the Leu allele at codon 432 in the CYP1B1 gene, represents as another potential genetic risk factor for low BMD and osteoporosis.
Consistent with the notion that the risk factors for osteoporosis are opposite that of breast cancer, women with the Val/Val genotype, who have relatively slower rate of estrogen catabolism, were reported to be at a higher risk for breast cancer. A recent meta-analysis which pooled the data from 9 genetic association studies showed that Caucasian women with the Val/Val and Val/Leu genotypes together have an odds ratio 1.5 for developing breast cancer. This relationship, however, was not observed in Asians and African-Americans [30].
The role of ethnicity in explaining the variable phenotypes in population studies has been widely-reported for a variety of diseases including osteoporosis [31,32]. It is possible that reported association in certain genetic studies is primarily due to ethnicity rather that actual genetic effect. Although all of the American and the Italian women in this study are considered Caucasians, important differences in terms of ethnic origins should be taken into account which may explain the difference in the results obtained between the 2 ethnic groups. The Caucasian women from the St. Louis area are mainly of German, French, Italian, Jewish and Anglo-Saxon descents, while Sicily is a mixture of Latin, Greek, Spanish and Arabic influences. The deviation from the Hardy–Weinberg equilibrium in genotype distribution among the Italians is not surprising, considering a similar observation has already been reported in another genetic study on the people living in the same geographical area [33]. These subjects who lived in an island in the Mediterranean Sea might have come from a homogenous genetic pool having undergone low immigration and expansion and could be geographical isolates. Meanwhile, the American population is admixture of individuals from several ethnic backgrounds resulting in population stratification. Population stratification has been regarded as a potential problem for association studies, primarily in case-control studies, where the association observed could be due to the underlying structure of the population under investigation and not a disease associated locus [34]. On the other hand, an analysis of several cancer genetic case-control studies in non-Hispanic Caucasian women in the US showed a very minor effect of population stratification, on the associations found [35]. In our study, the concordant relationship between the alteration in estrogen metabolism and BMD further argues against the possibility of a spurious relationship between Val432Leu polymorphism and BMD.
While the demographic characteristics of both ethnic groups are different, all of our analyses were adjusted for age, BMI, YSM and history of smoking to eliminate any potential bias. For example, the association between Leu/Leu genotypes and lower BMD at the lumbar spine and femoral neck remained significant even after adjustment for age, despite the older age among carriers of the Leu allele. It is possible that we were not able to find a difference in BMD in the Italian women for this polymorphism as majority of these women are over 65 years old and are several years past menopause. A pooled analysis on Caucasian patients with breast cancer showing a higher risk for the disease in those carrying the Val/Val or Val/Leu genotypes is limited to women between 45 and 59 years of age [30]. Unfortunately, the limited number of patients in our study does not allow us to have adequate statistical power to test the effect of this polymorphism in different age groups and postmenopausal age. Potentially, this polymorphism may be more relevant mostly during the menopausal transition and early menopause where residual ovarian production of estrogen may still exist and becomes less important with advancing age. Because of the well-known effect of tobacco use on BMD, history of smoking was also used as a covariate in our analysis. Our results revealed that the significant inter-genotype differences in BMD among Americans persist even after adjustment for previous tobacco use. The lower number of past smokers in the Italian women could be cultural in nature as women smokers in this generation of women were deemed undesirable in Sicily. The fewer number of women with family history of osteoporosis in the Italian group may reflect an ascertainment bias, as concern for osteoporosis may not be high in this particular region of Italy.
The Ala119Ser polymorphism which was found to alter the risk for endometrial [36] and breast cancers [17] in Japanese women, doesn’t seem to have any effect on estrogen and bone metabolism in our study in either the American or the Italian women. The fact that we have found no significant role for this polymorphism on estrogen metabolism, implies, that although this polymorphism has been associated with hormone-related disorders, the underlying mechanism in the generation of these conditions may not be a derangement in estrogen metabolism. Since CYP1B1 also metabolizes common environmental substances, exposure to carcinogenic metabolites resulting from degradation of these compounds may have contributed an important part in the genesis of these cancers alone or in conjunction with this polymorphism [37,38].
Our study has some limitations. Firstly, our participants were self-selected and consequently our findings may be biased to a subset of participants who may have had a special interest in this particular study by virtue of family history of osteoporosis. Secondly, we have a relatively limited sample size and this may have contributed to our failure to detect significant differences in FEI. Nevertheless, we were able to show significant differences in urinary metabolites and BMD in the spine and femoral neck in the current sample size, which would imply that a larger sample size might not be necessary. In fact, posthoc power calculations indicated that our sample size had sufficient power (0.99) with a sufficiently small probability of type 1 error (α<0.05) to detect an 8% difference in femoral neck BMD between carriers of the Val/Val and Leu/Leu genotypes and a power of 0.8 to detect a 4% difference between the Val/Val and Val/Leu genotypes, in both instances, assuming equal variances of 0.06. Finally, the lack of a significant difference in FEI levels in this study could also be due to technical reasons. Although an ultrasensitive ELISA kit was used to analyze serum E2 levels, it is possible that results would have been different if a more sensitive and more accurate method of assay, such as mass spectrometry, was used [39].
In summary, our results show that carriers of the Leu allele for the Val432Leu polymorphism of the CYP1B1 gene have accelerated estrogen catabolism as indicated by significantly higher levels of urinary metabolites relative to those without the allele. This alteration resulted in lower BMD in the spine and femoral neck. Thus, the presence of the Leu allele at codon 432 of the CYP1B1 gene may represent as a possible genetic risk factor for osteoporosis and women with this allele could be potential candidates for aggressive measures to prevent rapid bone loss at the time of menopause. Since our findings are limited to BMD measurements, we cannot make any projection on the fracture risk in women with the Leu allele relative to those without it. Considering the multitude of factors that contribute to influence the risk for fractures in addition to BMD, it would be relevant to know if this difference in BMD translates into differences in fracture rates and would be a topic for future investigation.
Acknowledgments
This work has been supported by NIH grants R03 AR049401 (RAV), and K12 HD01459 (Building Interdisciplinary Research Careers in Women’s Health).
Footnotes
Presented in part at the 26th Annual Meeting of the American Society for Bone and Mineral Research, September 19–22, 2004, Seattle, Washington.
References
- 1.Slemenda C, Hui SL, Longcope C, Johnston CC. Sex steroids and bone mass. A study of changes about the time of menopause. J Clin Invest. 1987;80(5):1261–9. doi: 10.1172/JCI113201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett-Connor E, Mueller JE, von Muhlen DG, Laughlin GA, Schneider DL, Sartoris DJ. Low levels of estradiol are associated with vertebral fractures in older men, but not women: the Rancho Bernardo study. J Clin Endocrinol Metab. 2000;85 (1):219–23. doi: 10.1210/jcem.85.1.6327. [DOI] [PubMed] [Google Scholar]
- 3.Adlercreutz H, Gorbach SL, Goldin BR, Woods MN, Dwyer JT, Hamalainen E. Estrogen metabolism and excretion in Oriental and Caucasian women. J Natl Cancer Inst. 1994;86(14):1076–82. doi: 10.1093/jnci/86.14.1076. [DOI] [PubMed] [Google Scholar]
- 4.Lippert TH, Seeger H, Mueck AO. The impact of endogenous estradiol metabolites on carcinogenesis. Steroids. 2000;65(7):357–69. doi: 10.1016/s0039-128x(00)00101-x. [DOI] [PubMed] [Google Scholar]
- 5.Grodin JM, Siiteri PK, MacDonald PC. Source of estrogen production in postmenopausal women. J Clin Endocrinol Metab. 1973;36(2):207–14. doi: 10.1210/jcem-36-2-207. [DOI] [PubMed] [Google Scholar]
- 6.Fishman J, Bradlow HL, Gallagher TF. Oxidative metabolism of estradiol. J Biol Chem. 1960;235:3104–7. [PubMed] [Google Scholar]
- 7.Bradlow HL, Telang NT, Sepkovic DW, Osborne MP. 2-hydroxyestrone: the ‘good’ estrogen. J Endocrinol. 1996;150 (Suppl):S259–65. [PubMed] [Google Scholar]
- 8.Westerlind KC, Gibson KJ, Malone P, Evans GL, Turner RT. Differential effects of estrogen metabolites on bone and reproductive tissues of ovariectomized rats. J Bone Miner Res. 1998;13(6):1023–31. doi: 10.1359/jbmr.1998.13.6.1023. [DOI] [PubMed] [Google Scholar]
- 9.Muti P, Bradlow HL, Micheli A, et al. Estrogen metabolism and risk of breast cancer: a prospective study of the 2:16alpha-hydroxyestrone ratio in premenopausal and postmenopausal women. Epidemiology. 2000;11(6):635–40. doi: 10.1097/00001648-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Osborne MP, Bradlow HL, Wong GY, Telang NT. Upregulation of estradiol C16 alpha-hydroxylation in human breast tissue: a potential biomarker of breast cancer risk. J Natl Cancer Inst. 1993;85(23):1917–20. doi: 10.1093/jnci/85.23.1917. [DOI] [PubMed] [Google Scholar]
- 11.Leelawattana R, Ziambaras K, Roodman-Weiss J, et al. The oxidative metabolism of estradiol conditions postmenopausal bone density and bone loss. J Bone Miner Res. 2000;15(12):2513–20. doi: 10.1359/jbmr.2000.15.12.2513. [DOI] [PubMed] [Google Scholar]
- 12.Napoli N, Villareal DT, Mumm S, et al. Effect of CYP1A1 gene polymorphisms on estrogen metabolism and bone density. J Bone Miner Res. 2005;20(2):232–9. doi: 10.1359/JBMR.041110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martucci CP, Fishman J. P450 enzymes of estrogen metabolism. Pharmacol Ther. 1993;57(2–3):237–57. doi: 10.1016/0163-7258(93)90057-k. [DOI] [PubMed] [Google Scholar]
- 14.Badawi AF, Cavalieri EL, Rogan EG. Role of human cytochrome P450 1A1, 1A2, 1B1, and 3A4 in the 2-, 4-, and 16alpha-hydroxylation of 17beta-estradiol. Metabolism. 2001;50(9):1001–3. doi: 10.1053/meta.2001.25592. [DOI] [PubMed] [Google Scholar]
- 15.Taioli E, Bradlow HL, Garbers SV, et al. Role of estradiol metabolism and CYP1A1 polymorphisms in breast cancer risk. Cancer Detect Prev. 1999;23(3):232–7. doi: 10.1046/j.1525-1500.1999.09912.x. [DOI] [PubMed] [Google Scholar]
- 16.Esteller M, Garcia A, Martinez-Palones JM, Xercavins J, Reventos J. Germ line polymorphisms in cytochrome-P450 1A1 (C4887 CYP1A1) and methylenetetrahydrofolate reductase (MTHFR) genes and endometrial cancer susceptibility. Carcinogenesis. 1997;18(12):2307–11. doi: 10.1093/carcin/18.12.2307. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe J, Shimada T, Gillam EM, et al. Association of CYP1B1 genetic polymorphism with incidence to breast and lung cancer. Pharmacogenetics. 2000;10(1):25–33. doi: 10.1097/00008571-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Goodman MT, McDuffie K, Kolonel LN, et al. Case-control study of ovarian cancer and polymorphisms in genes involved in catecholestrogen formation and metabolism. Cancer Epidemiol Biomarkers Prev. 2001;10(3):209–16. [PubMed] [Google Scholar]
- 19.Krajinovic M, Ghadirian P, Richer C, et al. Genetic susceptibility to breast cancer in French-Canadians: role of carcinogen-metabolizing enzymes and gene-environment interactions. Int J Cancer. 2001;92(2):220–5. doi: 10.1002/1097-0215(200102)9999:9999<::aid-ijc1184>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 20.Zheng W, Xie DW, Jin F, et al. Genetic polymorphism of cytochrome P450-1B1 and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2000;9(2):147–50. [PubMed] [Google Scholar]
- 21.Michnovicz JJ, Adlercreutz H, Bradlow HL. Changes in levels of urinary estrogen metabolites after oral indole-3-carbinol treatment in humans. J Natl Cancer Inst. 1997;89(10):718–23. doi: 10.1093/jnci/89.10.718. [DOI] [PubMed] [Google Scholar]
- 22.Napoli N, Thompson J, Civitelli R, rmamento-Villareal RC. Effects of dietary calcium compared with calcium supplements on estrogen metabolism and bone mineral density. Am J Clin Nutr. 2007;85(5):1428–33. doi: 10.1093/ajcn/85.5.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armamento-Villareal R, Villareal DT, Avioli LV, Civitelli R. Estrogen status and heredity are major determinants of premenopausal bone mass. J Clin Invest. 1992;90(6):2464–71. doi: 10.1172/JCI116138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klug TL, Bradlow HL, Sepkovic DW. Monoclonal antibody-based enzyme immunoassay for simultaneous quantitation of 2- and 16 alpha-hydroxyestrone in urine. Steroids. 1994;59(11):648–55. doi: 10.1016/0039-128x(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 25.Szulc P, Hofbauer LC, Heufelder AE, Roth S, Delmas PD. Osteoprotegerin serum levels in men: correlation with age, estrogen, and testosterone status. J Clin Endocrinol Metab. 2001;86(7):3162–5. doi: 10.1210/jcem.86.7.7657. [DOI] [PubMed] [Google Scholar]
- 26.Clark P, Tesoriero LJ, Morton DJ, et al. Hip axis length variation: its correlation with anthropometric measurements in women from three ethnic groups. Osteoporos Int. 2008 doi: 10.1007/s00198-008-0572-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dawson-Hughes B. A revised clinician’s guide to the prevention and treatment of osteoporosis. J Clin Endocrinol Metab. 2008 Jul;93(7):2463–5. doi: 10.1210/jc.2008-0926. Electronic Publication 2008 Jun 10. [DOI] [PubMed] [Google Scholar]
- 28.Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fractures. The study of Osteoporotic Fractures Research Group. Lancet. 1993;341(8837):72–5. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 29.Hanna IH, Dawling S, Roodi N, Guengerich FP, Parl FF. Cytochrome P450 1B1 (CYP1B1) pharmacogenetics: association of polymorphisms with functional differences in estrogen hydroxylation activity. Cancer Res. 2000;60(13):3440–4. [PubMed] [Google Scholar]
- 30.Paracchini V, Raimondi S, Gram IT, et al. Meta- and pooled analyses of the cytochrome P-450 1B1 Val432Leu polymorphism and breast cancer: a HuGE-GSEC review. Am J Epidemiol. 2007;165(2):115–25. doi: 10.1093/aje/kwj365. [DOI] [PubMed] [Google Scholar]
- 31.Gong G, Haynatzki G. Association between bone mineral density and candidate genes in different ethnic populations and its implications. Calcif Tissue Int. 2003;72 (2):113–23. doi: 10.1007/s00223-002-1005-x. [DOI] [PubMed] [Google Scholar]
- 32.Lei SF, Deng FY, Liu XH, et al. Polymorphisms of four bone mineral density candidate genes in Chinese populations and comparison with other populations of different ethnicity. J Bone Miner Metab. 2003;21(1):34–42. doi: 10.1007/s007740300006. [DOI] [PubMed] [Google Scholar]
- 33.Falchetti A, Sferrazza C, Cepollaro C, et al. FokI polymorphism of the vitamin D receptor gene correlates with parameters of bone mass and turnover in a female population of the Italian island of Lampedusa. Calcif Tissue Int. 2007;80(1):15–20. doi: 10.1007/s00223-005-0295-1. [DOI] [PubMed] [Google Scholar]
- 34.Freedman ML, Reich D, Penney KL, et al. Assessing the impact of population stratification on genetic association studies. Nat Genet. 2004;36(4):388–93. doi: 10.1038/ng1333. [DOI] [PubMed] [Google Scholar]
- 35.Wacholder S, Rothman N, Caporaso N. Population stratification in epidemiologic studies of common genetic variants and cancer: quantification of bias. J Natl Cancer Inst. 2000;92(14):1151–8. doi: 10.1093/jnci/92.14.1151. [DOI] [PubMed] [Google Scholar]
- 36.Sasaki M, Tanaka Y, Kaneuchi M, Sakuragi N, Dahiya R. CYP1B1 gene polymorphisms have higher risk for endometrial cancer, and positive correlations with estrogen receptor alpha and estrogen receptor beta expressions. Cancer Res. 2003;63(14):3913–8. [PubMed] [Google Scholar]
- 37.Spink BC, Pang S, Pentecost BT, Spink DC. Induction of cytochrome P450 1B1 in MDA-MB-231 human breast cancer cells by non-ortho-substituted polychlorinated biphenyls. Toxicol In Vitro. 2002;16(6):695–704. doi: 10.1016/s0887-2333(02)00091-7. [DOI] [PubMed] [Google Scholar]
- 38.Nock NL, Tang D, Rundle A, et al. Associations between smoking, polymorphisms in polycyclic aromatic hydrocarbon (PAH) metabolism and conjugation genes and PAH-DNA adducts in prostate tumors differ by race. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1236–45. doi: 10.1158/1055-9965.EPI-06-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santen RJ, Lee JS, Wang S, et al. Potential role of ultra-sensitive estradiol assays in estimating the risk of breast cancer and fractures. Steroids. 2008;73(13):1318–21. doi: 10.1016/j.steroids.2008.05.008. [DOI] [PubMed] [Google Scholar]