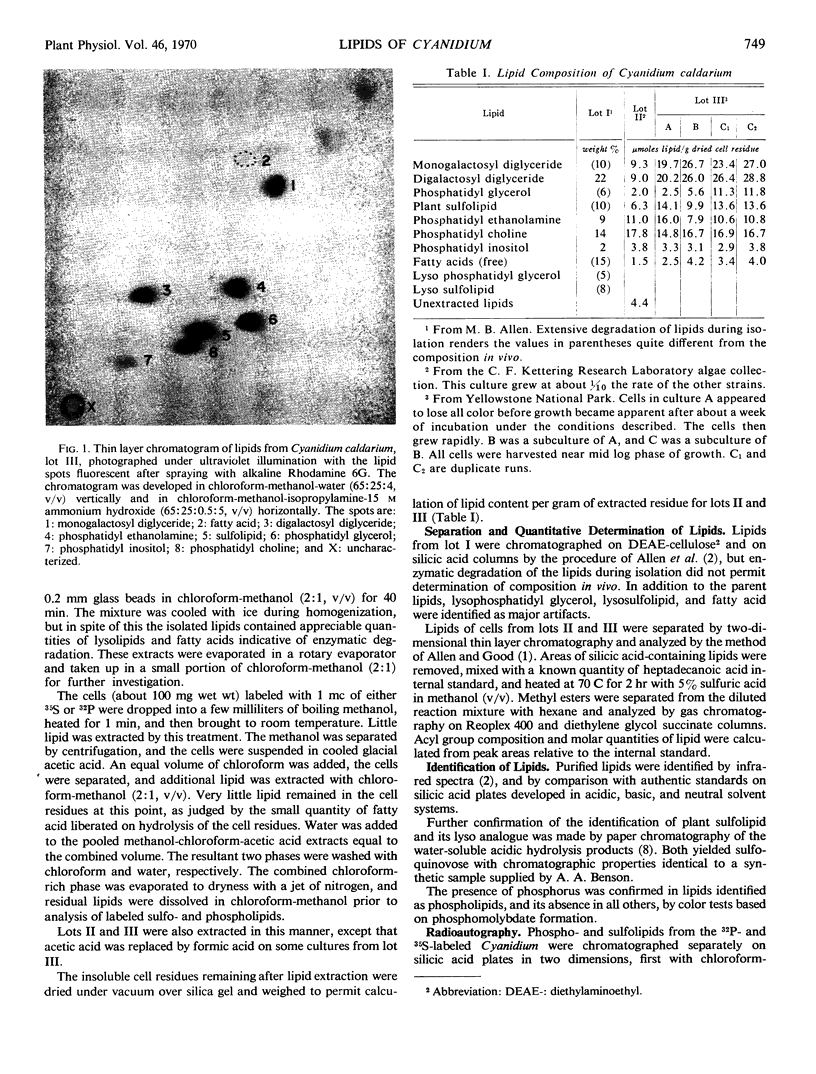
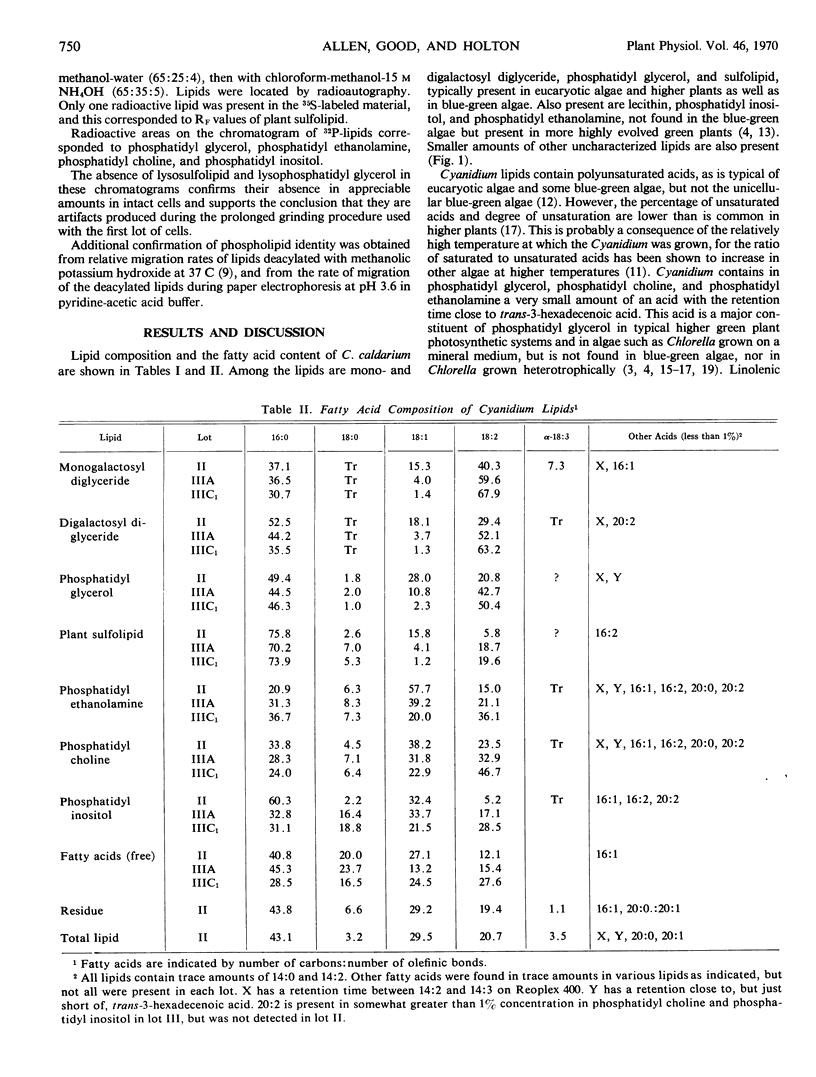
Abstract
The major lipids in Cyanidium caldarium Geitler are monogalactosyl diglyceride, digalactosyl diglyceride, plant sulfolipid, lecithin, phosphatidyl glycerol, phosphatidyl inositol, and phosphatidyl ethanolamine. Fatty acid composition varies appreciably among the lipids, but the major ones are palmitic acid, oleic acid, linoleic acid, and moderate amounts of stearic acid. Trace amounts of other acids in the C14 to C20 range were also present. Moderate amounts of linolenic acid were found in two strains, but not in a third. The proportion of saturated acid is relatively high in all lipids ranging from about a third in monogalactosyl diglyceride to three-fourths in sulfolipid. This may be a result of the high growth temperature. Lipases forming lysosulfolipid, and lysophosphatidyl glycerol are active in ruptured cells; galactolipid is degraded with loss of both acyl residues. Thus the lipid and fatty acid composition of Cyanidium more closely resembles that of green algae than that of the blue-green algae, although there are differences of possible phylogenetic interest.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN M. B. Studies with Cyanidium caldarium, an anomalously pigmented chlorophyte. Arch Mikrobiol. 1959;32(3):270–277. doi: 10.1007/BF00409348. [DOI] [PubMed] [Google Scholar]
- Allen C. F., Good P., Davis H. F., Fowler S. D. Plant and chloroplast lipids. I. Separation and composition of major spinach lipids. Biochem Biophys Res Commun. 1964 Apr 22;15(5):424–430. doi: 10.1016/0006-291x(64)90479-6. [DOI] [PubMed] [Google Scholar]
- Ascione R., Southwick W., Fresco J. R. Laboratory culturing of a thermophilic alga at high temperature. Science. 1966 Aug 12;153(3737):752–755. doi: 10.1126/science.153.3737.752. [DOI] [PubMed] [Google Scholar]
- BENSON A. A., MARUO B. Piant phospholipids. I. Identification of the phosphatidyl glycerols. Biochim Biophys Acta. 1958 Jan;27(1):189–195. doi: 10.1016/0006-3002(58)90308-1. [DOI] [PubMed] [Google Scholar]
- Holton R. W., Blecker H. H., Stevens T. S. Fatty acids in blue-green algae: possible relation to phylogenetic position. Science. 1968 May 3;160(3827):545–547. doi: 10.1126/science.160.3827.545. [DOI] [PubMed] [Google Scholar]
- KLENK E., KNIPPRATH W., EBERHAGEN D., KOOF H. P. UBER DIE UNGESAETTIGTEN FETTSAEUREN DER FETTSTOFFE VON SUESSWASSER- UND MEERESALGEN. Hoppe Seylers Z Physiol Chem. 1963;334:44–59. doi: 10.1515/bchm2.1963.334.1.44. [DOI] [PubMed] [Google Scholar]
- Klein R. M., Cronquist A. A consideration of the evolutionary and taxonomic significance of some biochemical, micromorphology, and physiological characters in the thallophytes. Q Rev Biol. 1967 Jun;42(2):105–296. [PubMed] [Google Scholar]
- RICHTER G. The lack of diphosphofructose aldolase in two photosynthetic organisms: Anacystis nidulans and Rhodopsudomonas spheroides. Biochim Biophys Acta. 1961 Apr 15;48:606–608. doi: 10.1016/0006-3002(61)90065-8. [DOI] [PubMed] [Google Scholar]