Abstract
PURPOSE
Radiotherapy is thought to produce clinical responses in cancer patients, through not only direct toxicity to cancer cells and supporting tumor stroma cells, but also through activation of immunological effectors. More recently, radiotherapy (RT) has potentiated local and systemic effects of cancer immunotherapy (IT). However, combination regimens that maximize immunologic and clinical efficacy remain undefined.
METHODS and MATERIALS
We evaluated the impact of local RT on adenoviral-mediated vaccination against the colorectal cancer antigen GUCY2C (Ad5-GUCY2C) in a murine subcutaneous tumor model using mouse CT26 colon cancer cells (CT26-GUCY2C). Immune responses were assessed by ELISpot and clinical responses were assessed by tumor size and incidence.
RESULTS
The specific sequence of tumor-directed RT preceding Ad5-GUCY2C IT transformed inactive therapeutic Ad5-GUCY2C vaccination into a curative vaccine. GUCY2C-specific T cell responses were amplified (p<0.05), tumor eradication maximized (p<0.01), and tumor volumes minimized (p<0.001) in mice whose tumors were irradiated prior to, compared to following, Ad5-GUCY2C vaccination. The immunologic and antitumor efficacy of Ad5-GUCY2C was amplified comparably by unfractionated (8 Gy × 1), or biologically equivalent doses of fractionated (3.5 Gy × 3), RT. Antitumor effects of sequential RT and IT (RT-IT) depended on expression of GUCY2C by tumor cells and the adenoviral vaccine vector, and tumor volumes were inversely related to the magnitude of GUCY2C-specific T cell responses. Moreover, mice cured of CT26-GUCY2C tumors by RT-IT exhibited long-lasting antigen-dependent protection, resisting tumors formed by GUCY2C-expressing 4T1 breast cancer cells inoculated 50 days after CT26 cells.
CONCLUSIONS
Optimal sequencing of RT and IT amplifies antigen-specific local and systemic immune responses, revealing novel acute and long-term therapeutic antitumor protection. These observations underscore the importance of modality sequence optimization prior to initiating clinical trials of RT and IT to maximize immune and antitumor responses.
Keywords: GUCY2C, vaccine, radiation therapy, immunotherapy, colorectal cancer
Introduction
Radiotherapy (RT) plays a central role in the management of most malignancies. Historically, clinical responses following RT were attributed to radiation-induced mitotic catastrophe and apoptosis within cancer cells and to destruction of the tumor microenvironment [1,2]. Beyond these toxicities, the therapeutic efficacy of RT may reflect, in part, immunologic mechanisms. The efficacy of RT in murine fibrosarcoma is impaired in mice that lack a normal T cell repertoire [3]. Similarly, patients with compromised immune systems exhibit markedly higher rates of local failures following RT compared to matched immunocompetent patients [4]. Further, tumor-directed RT in patients produces systemic immune responses associated with regression of non-irradiated metastases [5,6]. Moreover, prospective clinical trials revealed that tumordirected RT adjuvanated systemic responses to immunotherapy (IT) [7,8].
In contrast to the established benefit of RT, the clinical efficacy of cancer vaccines generally has been unremarkable, particularly in patients with substantial volumes of disease. For example, while colorectal cancer patients with minimal residual disease enjoy modest improvements in disease-free (HR = 0.76, p = 0.03) and overall survival (HR = 0.76, p = 0.007) following vaccine therapy, clinical response rates in patients with advanced disease only approaches 1–2% [9]. This inefficacy reflects inhibitory immunologic mechanisms, which co-evolve during tumorigenesis, and quantitative tumor burden, which becomes immunologically insurmountable [10,11].
An emerging paradigm at the intersection of radiation-induced immunologic mechanisms and immune-based therapies suggests that RT can be systematically exploited to amplify local and systemic IT responses that overcome challenges in eradicating established tumors [8,12]. However, effective combinations of RT and IT, and their precise sequencing to maximize immune responses and tumor eradication have not been defined [13–15]. Here, we combined an adenoviral-based vaccine against the colorectal cancer antigen guanylyl cyclase C (GUCY2C) with different schedules of tumor-directed RT to augment local and systemic GUCY2C-specific immune responses, which produced acute and long-term antitumor protection.
Methods and Materials
Mice and immunizations
Balb/c mice were obtained from the NCI Animal Production Program (Frederick, MD). Animal protocols were approved by our Institutional Animal Care and Use Committee. Vaccines included a second-generation adenovirus (Ad5) vector expressing the extracellular domain of mouse GUCY2C fused to the S1 CD4+ T helper epitope (Ad5-GUCY2C) [16] and an Ad5-Her2 negative control vector. Mice received 1×108 IFU of adenovirus by IM injection of the anterior tibialis.
Tumor models
GUCY2C-deficient CT26-WT cells were from ATCC® (CRL-2638). Generation of mouse CT26-GUCY2C colorectal cancer cells was previously described [16]. 4T1 mouse metastatic breast cancer cells (ATCC® CRL-2539) were transduced with pMSCV-Puro (Clontech) expressing a truncated GUCY2C-construct (GUCY2C1–461). Subcutaneous tumors were established by injection of 5×105 cells in the flanks or in the left hind leg as indicated. Tumor volumes were calculated by measuring three orthogonal diameters and calculating volumes using: 4/3π × r1 × r2 × r3.
Radiotherapy
Mice were anesthetized and irradiated using X-rays generated by a PanTak, 310 kVe X-ray machine. Each mouse was confined in a lead casing with its tumor-bearing left hind leg extended through an opening on the side to allow local tumor irradiation. Biologically equivalent doses (BED) of radiation, calculated using an α\β of 10 and the formula BED = [(nd) + (nd2 ÷ α\β)], were administered as a single 8 Gy fraction (BED = 14.4 Gy) or as three fractions of 3.5 Gy delivered over one week (BED = 14.2 Gy).
ELISpot
IFNγ ELISpot assays were described previously [16]. Briefly, splenocytes (1×106) were stimulated with 10 µg/mL of peptide [GUCY2C254–262 [17] or adenovirus DBP412–420 [18]] for 24 hours prior to spot development.
Statistics
Statistical differences between groups were analyzed with ANOVA and correlations between T cell responses and tumor volumes were analyzed with Pearson’s Coefficient using GraphPad Prism (GraphPad Software). A p-value of <0.05 was considered statistically significant.
Results
Ad5-GUCY2C IT or 8 Gy RT, alone, have limited effecacy against CT26-GUCY2C tumors
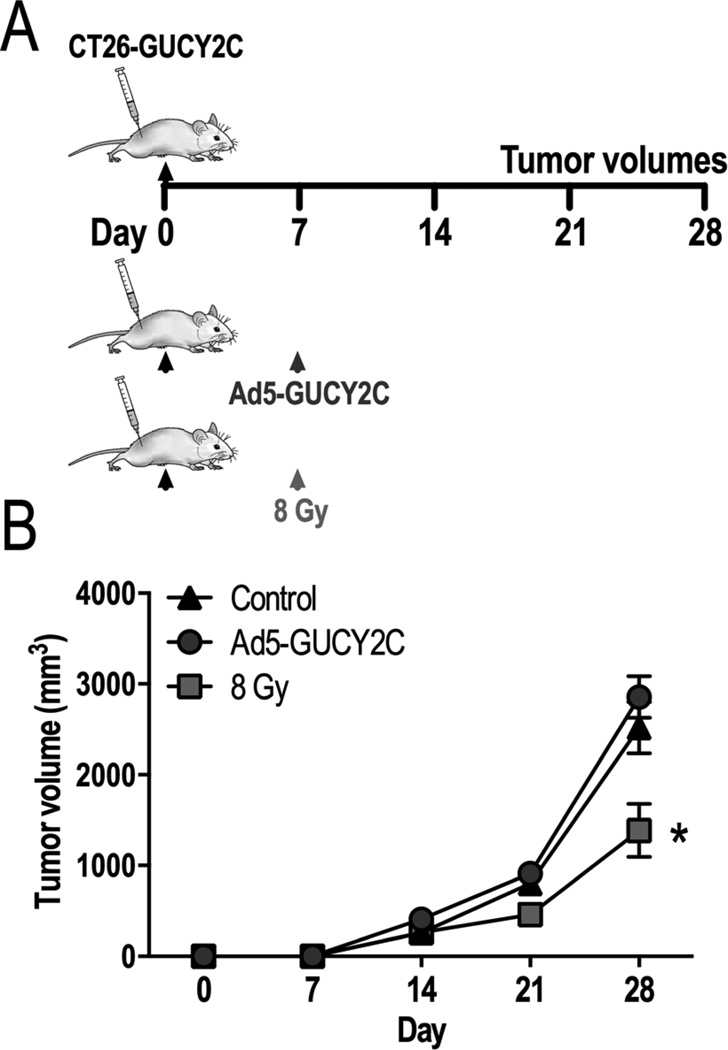
Balb/c mice bearing established GUCY2C-expressing CT26 (CT26-GUCY2C) subcutaneous leg tumors were immunized with Ad5-GUCY2C, or irradiated with 8 Gy 7 days after implantation (Fig. 1A). All inoculated mice developed subcutaneous tumors (incidence=100%). By day 28, the volumes of tumors from mice vaccinated with Ad5-GUCY2C (2,856.4 mm3 ± 229.2) were similar, while those from irradiated mice were smaller (1,385.4 mm3 ± 291.4 [8 Gy]; p <0.05), than tumor volumes from untreated control mice (2,519.6 mm3 ± 283.6; Fig. 1B).
Figure 1. Individual Ad5-GUCY2C and 8 Gy modalities are therapeutically ineffective.
(A) Mice challenged with CT26-GUCY2C cells were observed (n=10), vaccinated with Ad5-GUCY2C (n=10), or irradiated with 8 Gy (n=5) on day 7. (B) All mice developed tumors. Vaccinated mice exhibited growth rates similar to control. Tumors in irradiated mice were significantly smaller than controls.
Radiotherapy prior to Ad5-GUCY2C amplified GUCY2C-specific T cell responses creating novel antitumor efficacy
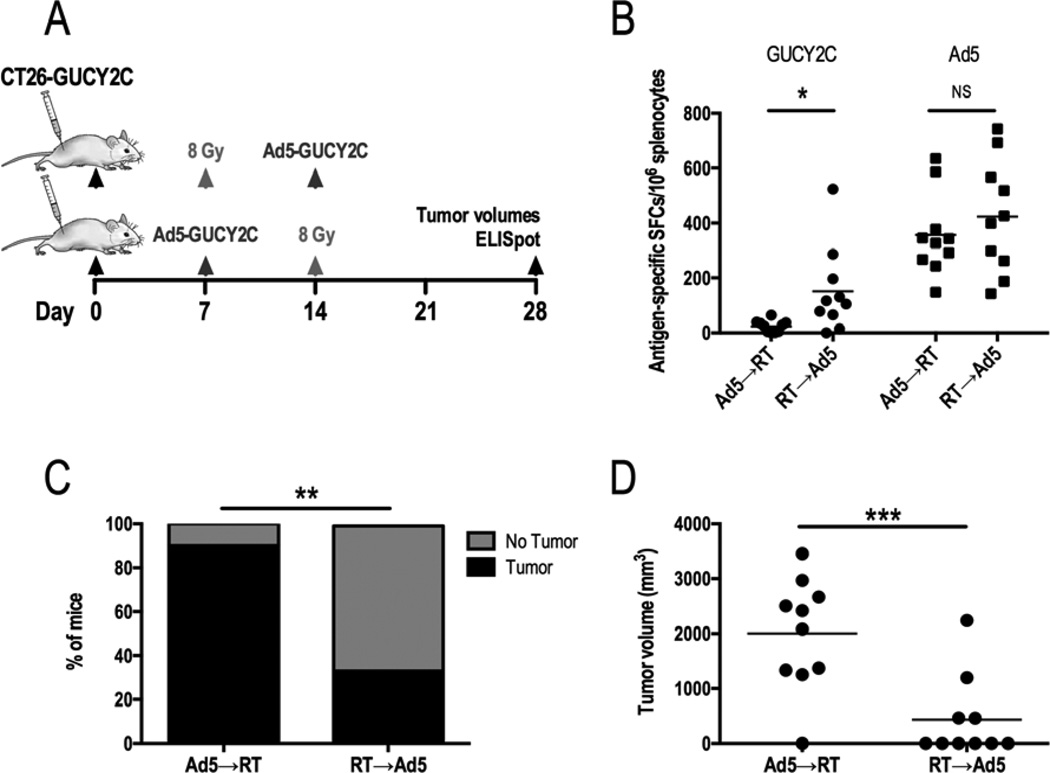
Balb/c mice bearing CT26-GUCY2C subcutaneous leg tumors were treated with Ad5-GUCY2C immunization on day 7 after tumor inoculation, followed by 8 Gy 7 days later (Ad5-GUCY2C→RT; Fig. 2A). Alternatively, tumor-bearing mice were treated with 8 Gy on day 7 after tumor inoculation, followed by Ad5-GUCY2C immunization 7 days later (RT→Ad5-GUCY2C; Fig. 2A). On day 28 after tumor inoculation, T cell responses to GUCY2C were amplified about 6-fold in the RT→Ad5-GUCY2C group compared to Ad5-GUCY2C→RT (151.7 ± 49.09 vs 23.9 ± 6.7 spots; p<0.05; Fig. 2B). In contrast, Ad5-specific T cell responses were similar between treatment groups (356.3 ± 47.2 vs 423.7 ± 65.1 spots; p=NS; Fig. 2B). Importantly, amplified immunologic responses produced by combining RT and IT created novel antitumor efficacy, eradicating some tumors (Fig. 2C). Importantly, tumor eradication by RT→Ad5-GUCY2C was augmented 6-fold compared to Ad5-GUCY2C→RT (60% vs 10% disease-free; p<0.01; Fig. 2C). Moreover, tumor volumes for RT→Ad5-GUCY2C were reduced about 5-fold compared to Ad5-GUCY2C→RT (436 mm3 ± 234.9 vs 2,006.0 mm3 ± 321.9; p <0.001; Fig. 2C).
Figure 2. Radiotherapy prior to Ad5-GUCY2C amplifies GUCY2C-specific T cells and reduces tumor growth.
(A) Mice challenged with CT26-GUCY2C cells (day 0) were treated with Ad5-GUCY2C or 8 Gy on day 7 and then treated with the opposing modality on day 14, generating two cohorts: Ad5→RT (n=10) and RT→Ad5 (n=10). Tumor volumes and GUCY2C-specific T cell responses were quantified on day 28. (B) T cell responses to GUCY2C were increased in the RT→Ad5 cohort compared to Ad5→RT. There was no difference in Ad5-specific T cell responses. Tumor cure rates (C) were increased and volumes (D) were decreased following RT→Ad5 compared to Ad5→RT.
RT amplification of IT responses is independent of fractionation
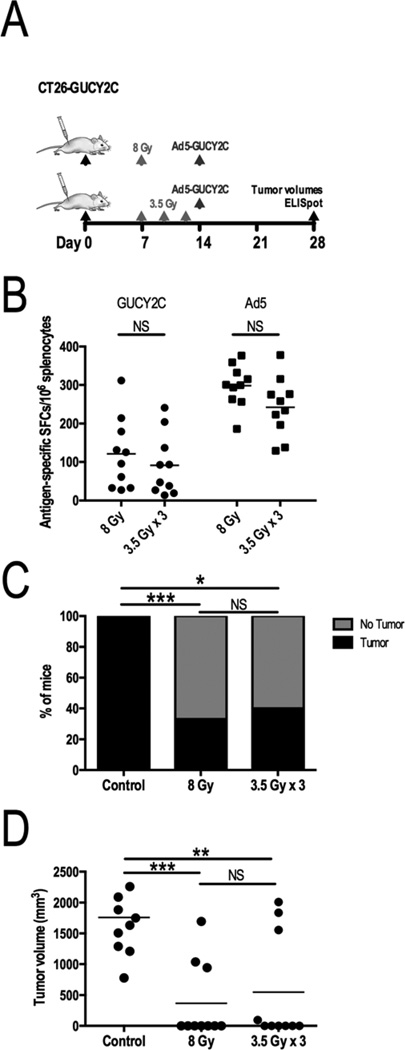
To determine if RT amplification of Ad5-GUCY2C vaccination was impacted by RT fractionation, cohorts were evaluated using a single dose of 8 Gy or a fractionated schedule of 3.5 Gy × 3 that had similar predicted biologic effects. Indeed, 8 Gy and 3.5 Gy × 3 regimens comparably amplified GUCY2C-specific T cell responses (121.0 ± 29.3 vs 91.2 ± 25.2 spots; p=NS; Fig. 3A) and similarly improved tumor eradication (67% vs 60%; p=NS, Fig. 3B) and volumes (367.4 mm3 ± 196.8 vs 549.5 mm3 ± 275.2; p=NS; Fig. 3C).
Figure 3. Unfractionated or fractionated RT preceding Ad5-GUCY2C produces similar immunologic and antitumor responses.
(A) Mice challenged with CT26-GUCY2C cells (day 0) were observed (n=10), treated with 8 Gy on day 7 (n=10), or treated with 3.5 Gy on days 7, 10, and 13 (n=10). Irradiated mice received Ad5-GUCY2C on day 14. GUCY2C-specific and Ad5-specific T cell responses (B), tumor cure rates (C), and volumes (D) were similar between unfractionated and fractionated RT treatment groups.
RT amplification of IT immunologic and tumor responses is GUCY2C-specific
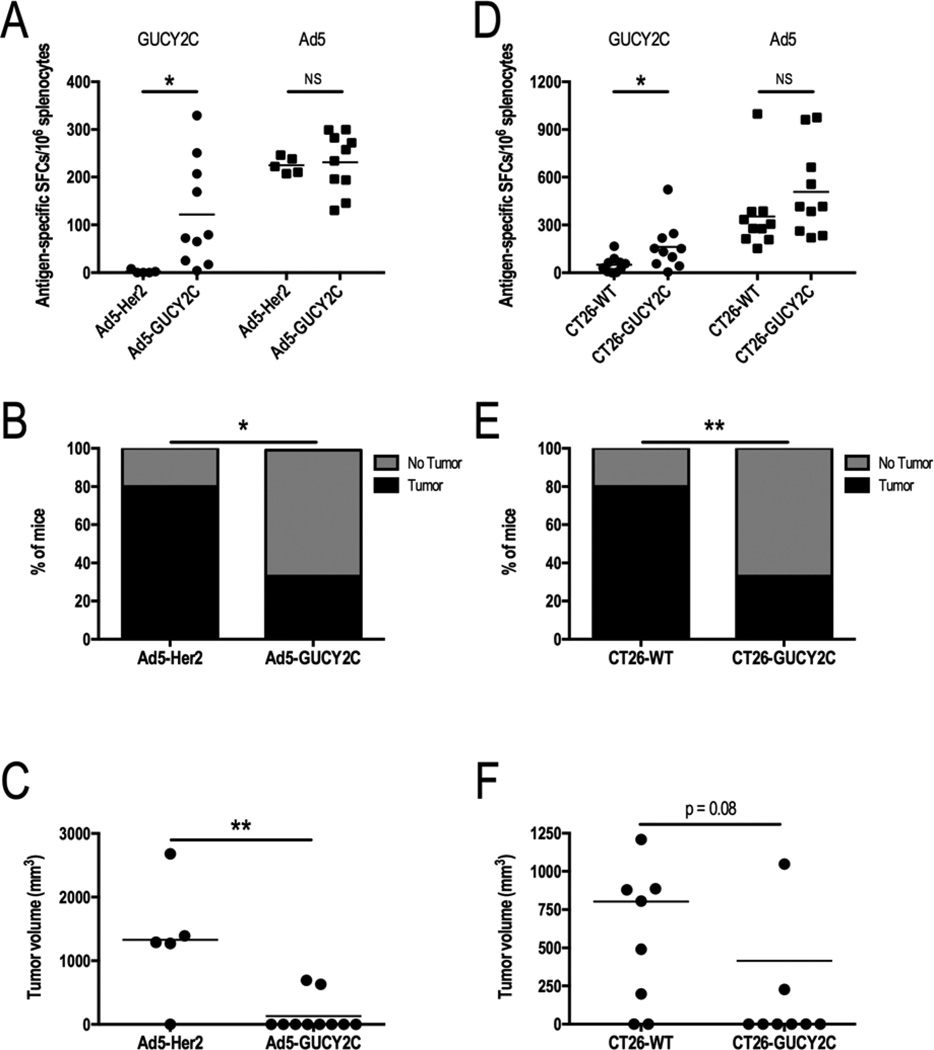
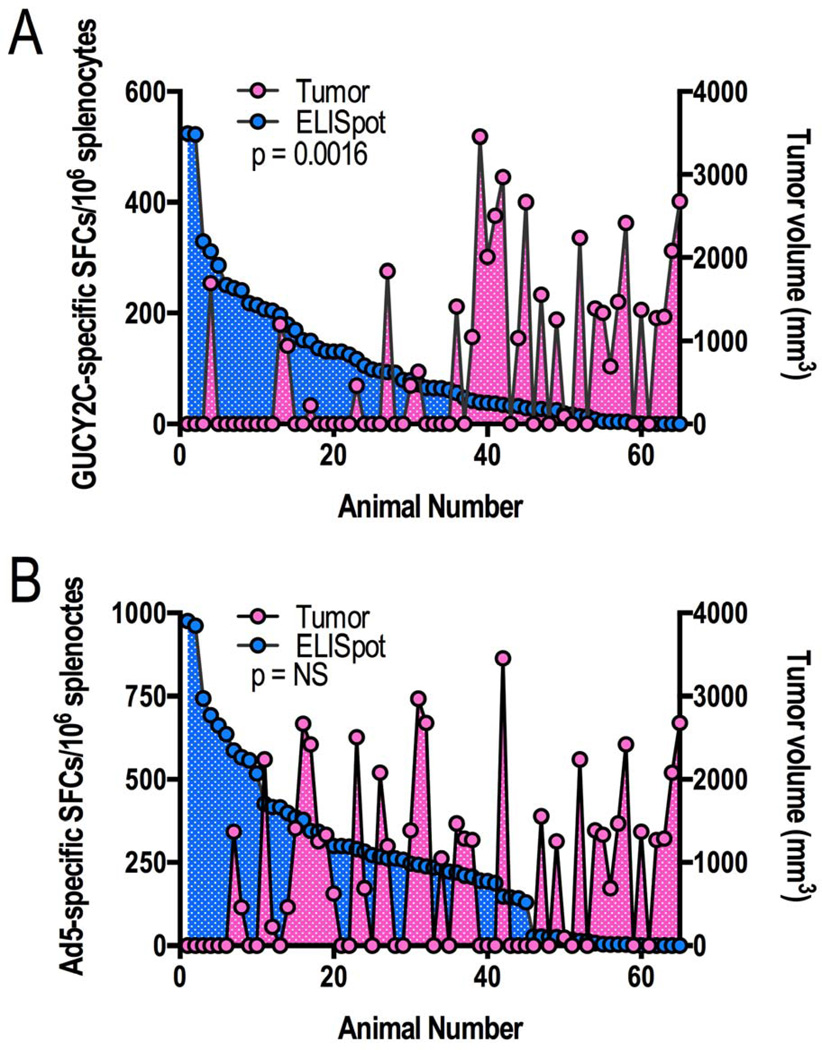
Mice bearing CT26-GUCY2C tumors received 8 Gy radiation to the tumor on day 7 following tumor inoculation, and vaccination 7 days later with either Ad5-Her2 (control) or Ad5-GUCY2C. On day 28 following tumor inoculation, GUCY2C-specific T cell responses were amplified (121.8 ± 35.1 vs 2.1 ± 1.6 spots; p<0.05), while Ad5-specific T cell responses were comparable (231.1 ± 19.6 vs 224.7 ± 7.7 spots; p=NS), in mice vaccinated with Ad5-GUCY2C compared to Ad5-Her2 (Fig. 4A). Similarly, tumor eradication (70% vs 20%; p<0.05; Fig. 4B) and volumes (1,324.8 mm3 ± 424.2 vs 131.9 mm3 ± 88.0; p<0.01; Fig. 4C) were improved in mice vaccinated with Ad5-GUCY2C compared to Ad5-Her2. Conversely, mice bearing GUCY2C-deficient (CT26-WT) or GUCY2C-expressing (CT26-GUCY2C) tumors received 8 Gy radiation to the tumor on day 7 following tumor inoculation, and received vaccination 7 days later with Ad5-GUCY2C. On day 28 following tumor inoculation, GUCY2C-specific T cell responses were amplified (161.7 ± 46.6 vs 50.3 ± 15.9 spots; p<0.05), while Ad5-specific T cell responses were comparable (353.4 ± 75.5 vs 508.6 ± 88.3 spots; p=NS), in mice bearing CT26-GUCY2C, compared to those bearing CT26-WT, tumors (Fig. 4D). Amplification of T cell responses in mice inoculated with CT26-GUCY2C, compared to CT26-WT, cells reflects adjuvanation of immune responses by tumor cells undergoing radiation-induced immunogenic cell death. Similarly, tumor eradication (60% vs 20%; p < 0.01; Fig. 4E) and volumes (415.5 mm3 ± 199.2 vs 802.3 mm3 ± 210.3; p = 0.08; Fig. 4F) in mice bearing CT26-GUCY2C tumors were improved compared to those bearing CT26-WT tumors. Moreover, analysis of all CT26-GUCY2C-bearing mice across experimental regimens in Fig. 2–4 revealed an inverse relationship between GUCY2C (p=0.0016; Fig. 5A), but not Ad5 (p=NS; Fig. 5B), -specific T cell responses and tumor volume.
Figure 4. GUCY2C is required for RT-amplified therapeutic vaccination.
(A–C) Mice challenged with CT26-GUCY2C cells (day 0) were irradiated with 8 Gy on day 7 followed by Ad5-Her2 (n=5) or Ad5-GUCY2C (n=10) on day 14, and immune and antitumor responses quantified on day 28. (A) GUCY2C-specific, but not Ad5-specific, T cell responses were amplified in mice vaccinated with Ad5-GUCY2C compared to Ad5-Her2 vaccine. Tumor cure rates were increased (B) and volumes were decreased (C) in mice vaccinated with Ad5-GUCY2C, compared to Ad5-Her2. (D–F) Mice challenged with CT26-WT (n=10) or CT26-GUCY2C (n=10) cells (day 0) were irradiated with 8 Gy on day 7, vaccinated with Ad5-GUCY2C on day 14, and immune and antitumor responses quantified on day 28. (D) GUCY2C-specific, but not Ad5-specific, T cell responses were amplified in mice challenged with CT26-GUCY2C cells compared to mice challenged with CT26-WT cells. Tumor cure rates were increased (E) and volumes were decreased (F) in mice challenged with CT26-GUCY2C, compared to CT26-WT, cells.
Figure 5. Tumor volumes correlate with GUCY2C, but not Ad5, -specific T cell responses.
Antigen-specific immune responses by individual mice were rank-ordered for GUCY2C (A) or Ad5 (B) from animals across all experiments employing CT26-GUCY2C tumors, regardless of treatment regimen. There was a significant association between GUCY2C-specific (A), but not Ad5-specific (B), T cell responses and tumor volumes.
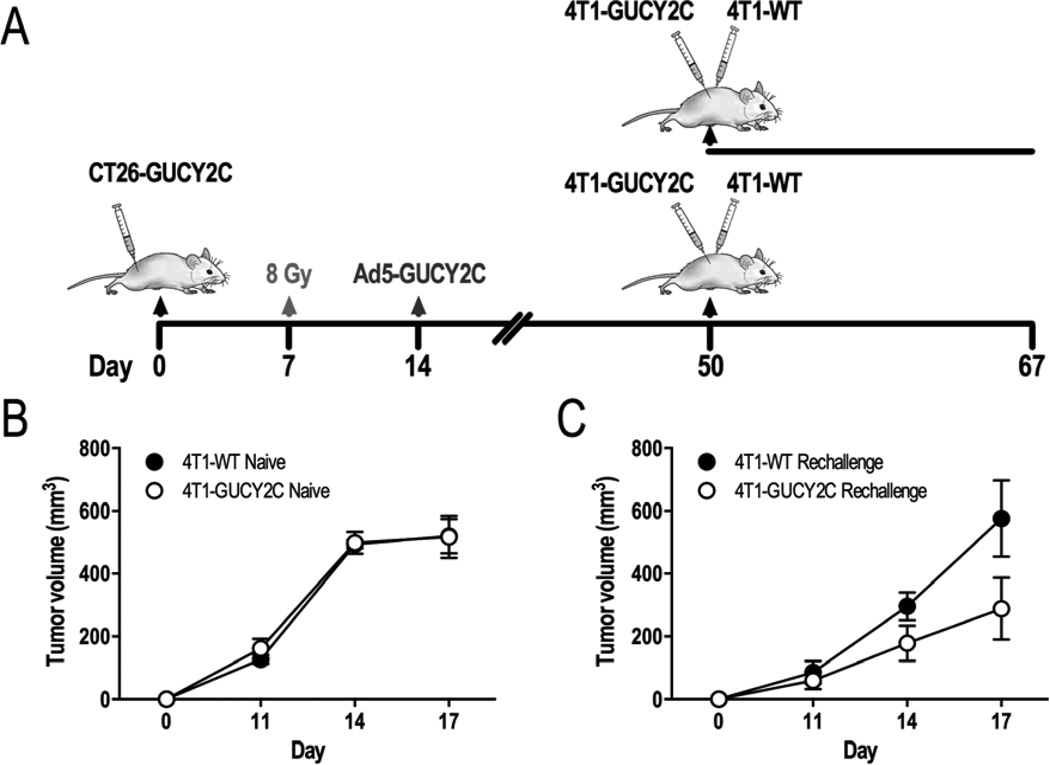
Sequential RT-IT induces long-term antitumor protection
Naïve mice, or those cured of subcutaneous CT26-GUCY2C leg tumors by RT→Ad5-GUCY2C, were inoculated in the left flank with the Balb/c metastatic breast cancer cells (4T1-WT) and in the right flank with 4T1 cells expressing GUCY2C (4T1-GUCY2C) (Fig. 6A). On day 17 after tumor inoculation, 4T1-WT and 4T1-GUCY2C tumor volumes were identical in naïve mice (519.9 mm3 ± 54.6 vs 517.3 mm3 ± 66.9; p=NS; Fig. 6B). However, 4T1-GUCY2C tumor volumes were reduced, compared to 4T1-WT tumors, in mice previously cured of CT26-GUCY2C tumors (575.7 mm3 ± 121.2 vs 288.6 mm3 ± 98.39; p<0.001; Fig. 6C).
Figure 6. Mice cured of CT26-GUCY2C tumors by sequential RT-IT exhibit GUCY2C-dependent long-term antitumor protection.
(A) Mice were challenged with CT26-GUCY2C cells and cured with 8 Gy followed by therapeutic Ad5-GUCY2C vaccination. Fifty days following initial tumor challenge, cured and naïve mice were challenged with 4T1-WT in the left flank and 4T1-GUCY2C in the right flank and tumor volumes were measured longitudinally. (B) 4T1-WT and 4T1-GUCY2C tumor growth was equivalent in naïve mice. (C) In contrast, 4T1-GUCY2C tumor growth was inhibited, compared to 4T1-WT tumors, in mice previously cured of CT26-GUCY2C tumors.
Discussion
RT is thought to induce immunogenic cancer cell death that, in part, activates tumor antigen-specific immune responses [19]. Combining RT with immunotherapies amplifies local and systemic antitumor activity beyond that of either modality alone [8,12]. Although molecular mechanisms underlying the synergy of combination RT and IT have been described [20], optimal sequencing of these treatments remains to be defined [13–15]. To our knowledge, this is the first study evaluating the impact of sequencing of combination RT and IT on immunologic responses and tumor indices. Here, we demonstrate that immunologic responses to the colorectal cancer antigen GUCY2C are amplified, and associated with the creation of novel therapeutic tumor responses by irradiating tumors before, rather than after, vaccination.
In addition to the optimal scheduling of RT and IT, the most effective RT dose and fractionation remains to be defined. Here, a dose of 8 Gy was selected because it caused a transient tumor growth delay that potentially could be amplified by an immune response. In that context, moderate RT doses (~8 Gy) optimize tumor control and immunity [21]. Although large fraction sizes delivered to bone metastases or small visceral lesions is considered safe in clinical practice, 8 Gy delivered to large intra-abdominal or intra-thoracic lesions, such as those encountered in patients with GI malignancies, may produce unacceptable side effects, since long term toxicity increases with fraction size. Here, we evaluated whether fractionated RT with a similar biologically-equivalent dose, and potentially a more favorable side effect profile, could produce antitumor and immune responses that were comparable to a large single fraction of radiotherapy. Indeed, 3.5 Gy × 3 produced similar increases in GUCY2C-specific T cell responses and improvement in tumor eradication and volumes, compared to a single 8 Gy dose (Fig. 3). Thus, the temporal relationship between RT and IT may be more important than RT dose or fractionation. Indeed, clinical responses in a recent phase I trial evaluating three stereotactic RT dose cohorts prior to interleukin-2 administration in patients with metastatic melanoma or renal cell carcinoma did not support an RT dose response [7].
Ideally, tumor antigens would be expressed specifically by neoplastic tissues, thereby limiting on-target, off-site effects of immunotherapeutic responses on normal tissues. A variation on this theme exploits the structural and functional compartmentalization of central and mucosal immune systems by employing vaccines against antigens that are normally confined to intestinal epithelial cells and their derivative malignancies that would be considered foreign following systemic dissemination [22]. GUYC2C is normally expressed by enterocytes lining the small and large intestines [23–25] and is retained by nearly all colorectal tumors and their associated metastases [26–28], underscoring its utility as a marker for colorectal cancer staging [29] and making it an attractive vaccine target [22]. Thus, the dependence on GUCY2C expression for achieving synergy between RT and Ad5-GUCY2C in our therapeutic model was evaluated (Fig. 4). Indeed, in mice challenged with cancer cells or receiving vaccine in which GUCY2C was absent, GUYC2C-specific T cell responses and antitumor efficacy were reduced compared to mice challenged with cancer cells and vaccinated with adenovirus expressing GUCY2C. Thus, GUCY2C antigen expression by tumors and the vaccine is critical to produce maximum T cell and antitumor responses. The highly selective expression of GUCY2C within intestinal epithelia [23,24,30] and the near universal over-expression of GUCY2C by intestinal malignancies, combined with the safety and efficacy of GUCY2C-targeted immunotherapies [16,17,31,32], make GUCY2C an ideal target for combined RT-IT therapy in patients.
The abscopal effect, which occurs when RT induces immune-mediated regression of tumors beyond the irradiation field, has emerged as a key focus of intense study in model systems and patients [5,7,8]. Abscopal regression depends on the ability to generate local immunologic responses that translate systemically to tumors anatomically separated from, but coincident in time with, the irradiated tumor. While bridging this spatial continuum to treat coincident metastases, the ability of the abscopal effect to create memory responses that endure temporally to provide long-term antitumor protection over time has not yet been defined. Here, mice previously cured of subcutaneous CT26-GUYC2C tumors by RT→Ad5-GUCY2C were challenged 50 days after the first cancer cell inoculation with an unrelated (breast) tumor cell line engineered to express GUCY2C (4T1-GUCY2C). Mice cured of CT26-GUCY2C tumors by sequential RT-IT resisted 4T1-GUCY2C, but not 4T1-WT (control), tumor growth. These observations suggest that sequential RT-IT induces long-term GUCY2C-specific immune memory, supporting the use of RT-IT in the curative setting to provide systemic surveillance against metastatic recurrences.
Sequential RT-IT approaches ultimately will require surrogate biomarkers of efficacy that predict clinical responses, and recent guidelines focus on detection and quantification of antigen-specific T cells [33]. In that context, T cell responses to HPV E6 and E7 antigens were associated with a complete resolution of vulvar intraepithelial neoplasia in patients vaccinated with those proteins [34]. However, it remains unknown if minimum T cell responses are required to produce clinical effects. Here, quantification of T cell responses in individual tumor-bearing mice across all treatment regimens enabled evaluation of the relationship between T cell and tumor responses. Indeed, GUCY2C, but not Ad5, -specific T cell responses strongly correlated with tumor responses (Fig. 5). In these analyses, independence of Ad5-specific T cell and tumor responses confirms that the observed relationship reflects treatment-induced tumor responses, rather than variations in immunocompetency of individual tumor-bearing animals. Moreover, these analyses suggest T cell response thresholds are required for tumor responses. Thus, the majority (73%) of mice that produced less than 60 GUCY2C-specific T cells/106 splenocytes experienced progressive disease. In contrast, the majority (77%) of mice that exceeded 60 GUCY2C-specific T cells/106 splenocytes were cured of their tumors. If confirmed in patients, T cell response thresholds as a surrogate marker for clinical responses to sequential RT-IT could accelerate the development of new therapeutic paradigms, offering patients an opportunity to receive additional treatments before disease progression.
While RT-IT produced favorable murine outcomes, correlating with immunological responses, limitations in translating animal studies to clinical practice suggest that clinical comparisons of RT-IT regimens are required to maximize patient outcomes. Spontaneously metastatic tumors are rare in mice [35], limiting animal studies to orthotopic or ectopic models of metastasis. Xenogeneic systems are not feasible for these studies, because intact immunity is required to produce immunological responses following RT-IT. Avatars - mice expressing human GUCY2C, containing a human immune system and human colorectal cancer xenografts - would be ideal for testing GUCY2C immunotherapy [36], but these systems are not yet developed.
The present study reveals a relationship between the sequence of RT and IT and immunologic and antitumor efficacy. Unfractionated or fractionated RT prior to IT with a single dose of an adenoviral GUCY2C-based vaccine amplifies antigen-specific T cell responses creating novel antigen-dependent antitumor responses. Generally, these observations suggest the importance of careful assessment of sequencing modalities in clinical trials integrating RT and IT. More specifically, these studies highlight the translational potential for sequential RT-IT employing GUCY2C as an antigen target. Indeed, an ongoing phase I clinical trial is evaluating the immunogenicity of Ad5-GUCY2C in patients with colon cancer. Pending positive outcomes in that study, the results presented here can be directly translated to patients with esophageal, gastric and rectal cancer, which express GUCY2C as part of their pathophysiology [26,37] and which frequently include tumor-directed RT as standard of care [38–40].
SUMMARY.
The temporal relationship between radiotherapy and therapeutic vaccination was evaluated in an ectopic syngeneic mouse colorectal cancer model. Local tumor irradiation before therapeutic vaccination was more effective than the reverse. Importantly, mice cured of their tumors possessed long-lasting protection against tumor recurrence (re-challenge), demonstrating an antigen-specific memory response. These results define an optimal combination treatment regimen employing irradiation and therapeutic vaccination producing effective and long-lasting immunity that may be utilized in designing future clinical trials.
Acknowledgments
Financial Support: These studies were supported by grants from the National Institutes of Health (RC1 CA146033, P30 CA56036, R01 CA170533), the Pennsylvania Department of Health (SAP #4100059197, SAP #4100051723), and Targeted Diagnostic and Therapeutics Inc. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. A.E.S. is the recipient of the Measey Foundation Fellowship. M.S.M is the recipient of a Ruth L. Kirschstein National Research Service Award from NIH (F31CA171672). S.A.W. is the Samuel MV Hamilton Professor of Thomas Jefferson University.
Disclosures: S.A.W. is the Chair of the Data Safety Monitoring Board for the C-Cure Trial™ sponsored by Cardio Biosciences, and the Chair (uncompensated) of the Scientific Advisory Board of Targeted Diagnostics & Therapeutics, Inc. which provided research funding that, in part, supported this work and has a license to commercialize inventions related to this work.
Abbreviations
- BED
biologically equivalent doses
- GUCY2C
guanylyl cyclase C
- Gy
Gray
- IFU
infectious units
- IM
intramuscular
- IT
immunotherapy
- RT
radiotherapy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eriksson D, Stigbrand T. Radiation-induced cell death mechanisms. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2010;31:363–372. doi: 10.1007/s13277-010-0042-8. [DOI] [PubMed] [Google Scholar]
- 2.Chi KH, Wang YS, Kao SJ. Improving radioresponse through modification of the tumor immunological microenvironment. Cancer biotherapy & radiopharmaceuticals. 2012;27:6–11. doi: 10.1089/cbr.2011.1048. [DOI] [PubMed] [Google Scholar]
- 3.Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. Journal of the National Cancer Institute. 1979;63:1229–1235. [PubMed] [Google Scholar]
- 4.Oehler-Janne C, Seifert B, Lutolf UM, et al. Local tumor control and toxicity in hiv-associated anal carcinoma treated with radiotherapy in the era of antiretroviral therapy. Radiat Oncol. 2006;1:29. doi: 10.1186/1748-717X-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stamell EF, Wolchok JD, Gnjatic S, et al. The abscopal effect associated with a systemic anti-melanoma immune response. International journal of radiation oncology, biology, physics. 2013;85:293–295. doi: 10.1016/j.ijrobp.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. The New England journal of medicine. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seung SK, Curti BD, Crittenden M, et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2--tumor and immunological responses. Science translational medicine. 2012;4:137ra174. doi: 10.1126/scitranslmed.3003649. [DOI] [PubMed] [Google Scholar]
- 8.Verbrugge I, Hagekyriakou J, Sharp LL, et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 2012;72:3163–3174. doi: 10.1158/0008-5472.CAN-12-0210. [DOI] [PubMed] [Google Scholar]
- 9.Rao B, Han M, Wang L, et al. Clinical outcomes of active specific immunotherapy in advanced colorectal cancer and suspected minimal residual colorectal cancer: A meta-analysis and system review. J Transl Med. 2011;9:17. doi: 10.1186/1479-5876-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wing K, Onishi Y, Prieto-Martin P, et al. Ctla-4 control over foxp3+ regulatory t cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 11.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodge JW, Sharp HJ, Gameiro SR. Abscopal regression of antigen disparate tumors by antigen cascade after systemic tumor vaccination in combination with local tumor radiation. Cancer biotherapy & radiopharmaceuticals. 2012;27:12–22. doi: 10.1089/cbr.2012.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frey B, Rubner Y, Wunderlich R, et al. Induction of abscopal anti-tumor immunity and immunogenic tumor cell death by ionizing irradiation - implications for cancer therapies. Current medicinal chemistry. 2012;19:1751–1764. doi: 10.2174/092986712800099811. [DOI] [PubMed] [Google Scholar]
- 14.Frey B, Rubner Y, Kulzer L, et al. Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol Immunother. 2013 doi: 10.1007/s00262-013-1474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Multhoff G, Rodel F, Pockley AG, et al. Frontiers research topic: Radiation-induced effects and the immune system. Frontiers in oncology. 2013;3:55. doi: 10.3389/fonc.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snook AE, Stafford BJ, Li P, et al. Guanylyl cyclase c-induced immunotherapeutic responses opposing tumor metastases without autoimmunity. J Natl Cancer Inst. 2008;100:950–961. doi: 10.1093/jnci/djn178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snook AE, Magee MS, Marszalowicz GP, et al. Epitope-targeted cytotoxic t cells mediate lineage-specific antitumor efficacy induced by the cancer mucosa antigen gucy2c. Cancer immunology, immunotherapy : CII. 2012;61:713–723. doi: 10.1007/s00262-011-1133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKelvey T, Tang A, Bett AJ, et al. T-cell response to adenovirus hexon and DNA-binding protein in mice. Gene Ther. 2004;11:791–796. doi: 10.1038/sj.gt.3302232. [DOI] [PubMed] [Google Scholar]
- 19.Kwilas AR, Donahue RN, Bernstein MB, et al. In the field: Exploiting the untapped potential of immunogenic modulation by radiation in combination with immunotherapy for the treatment of cancer. Frontiers in oncology. 2012;2:104. doi: 10.3389/fonc.2012.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: A paradigm shift. J Natl Cancer Inst. 2013 doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaue D, Ratikan JA, Iwamoto KS, et al. Maximizing tumor immunity with fractionated radiation. International journal of radiation oncology, biology, physics. 2012;83:1306–1310. doi: 10.1016/j.ijrobp.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snook AE, Eisenlohr LC, Rothstein JL, et al. Cancer mucosa antigens as a novel immunotherapeutic class of tumor-associated antigen. Clin Pharmacol Ther. 2007;82:734–739. doi: 10.1038/sj.clpt.6100369. [DOI] [PubMed] [Google Scholar]
- 23.Cagir B, Gelmann A, Park J, et al. Guanylyl cyclase c messenger rna is a biomarker for recurrent stage ii colorectal cancer. Ann Intern Med. 1999;131:805–812. doi: 10.7326/0003-4819-131-11-199912070-00002. [DOI] [PubMed] [Google Scholar]
- 24.Carrithers SL, Barber MT, Biswas S, et al. Guanylyl cyclase c is a selective marker for metastatic colorectal tumors in human extraintestinal tissues. Proc Natl Acad Sci U S A. 1996;93:14827–14832. doi: 10.1073/pnas.93.25.14827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrithers SL, Parkinson SJ, Goldstein SD, et al. Escherichia coli heat-stable enterotoxin receptors. A novel marker for colorectal tumors. Dis Colon Rectum. 1996;39:171–181. doi: 10.1007/BF02068072. [DOI] [PubMed] [Google Scholar]
- 26.Birbe R, Palazzo JP, Walters R, et al. Guanylyl cyclase c is a marker of intestinal metaplasia, dysplasia, and adenocarcinoma of the gastrointestinal tract. Hum Pathol. 2005;36:170–179. doi: 10.1016/j.humpath.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Schulz S, Hyslop T, Haaf J, et al. A validated quantitative assay to detect occult micrometastases by reverse transcriptase-polymerase chain reaction of guanylyl cyclase c in patients with colorectal cancer. Clin Cancer Res. 2006;12:4545–4552. doi: 10.1158/1078-0432.CCR-06-0865. [DOI] [PubMed] [Google Scholar]
- 28.Witek ME, Nielsen K, Walters R, et al. The putative tumor suppressor cdx2 is overexpressed by human colorectal adenocarcinomas. Clin Cancer Res. 2005;11:8549–8556. doi: 10.1158/1078-0432.CCR-05-1624. [DOI] [PubMed] [Google Scholar]
- 29.Waldman SA, Hyslop T, Schulz S, et al. Association of gucy2c expression in lymph nodes with time to recurrence and disease-free survival in pn0 colorectal cancer. Jama. 2009;301:745–752. doi: 10.1001/jama.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrithers SL, Parkinson SJ, Goldstein S, et al. Escherichia coli heat-stable toxin receptors in human colonic tumors. Gastroenterology. 1994;107:1653–1661. doi: 10.1016/0016-5085(94)90804-4. [DOI] [PubMed] [Google Scholar]
- 31.Snook AE, Li P, Stafford BJ, et al. Lineage-specific t-cell responses to cancer mucosa antigen oppose systemic metastases without mucosal inflammatory disease. Cancer Res. 2009;69:3537–3544. doi: 10.1158/0008-5472.CAN-08-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snook AE, Huang L, Schulz S, et al. Cytokine adjuvanation of therapeutic antitumor immunity targeted to cancer mucosa antigens. Clinical and Translational Science. 2008;1:263–264. doi: 10.1111/j.1752-8062.2008.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butterfield LH, Palucka AK, Britten CM, et al. Recommendations from the isbtc-sitc/fda/nci workshop on immunotherapy biomarkers. Clin Cancer Res. 2011;17:3064–3076. doi: 10.1158/1078-0432.CCR-10-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kenter GG, Welters MJ, Valentijn AR, et al. Vaccination against hpv-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 35.Suman S, Jr, AJF Datta K. Animal models of colorectal cancer in chemoprevention and therapeutics development. In: Ettarh R, editor. Colorectal cancer – from prevention to patient care: InTech. 2012. [Google Scholar]
- 36.Koo GC, Hasan A, O'Reilly RJ. Use of humanized severe combined immunodeficient mice for human vaccine development. Expert Rev Vaccines. 2009;8:113–120. doi: 10.1586/14760584.8.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park J, Schulz S, Haaf J, et al. Ectopic expression of guanylyl cyclase c in adenocarcinomas of the esophagus and stomach. Cancer Epidemiol Biomarkers Prev. 2002;11:739–744. [PubMed] [Google Scholar]
- 38.Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310–2317. doi: 10.1200/JCO.2005.00.034. [DOI] [PubMed] [Google Scholar]
- 39.Stahl M, Walz MK, Stuschke M, et al. Phase iii comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27:851–856. doi: 10.1200/JCO.2008.17.0506. [DOI] [PubMed] [Google Scholar]
- 40.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]