Abstract
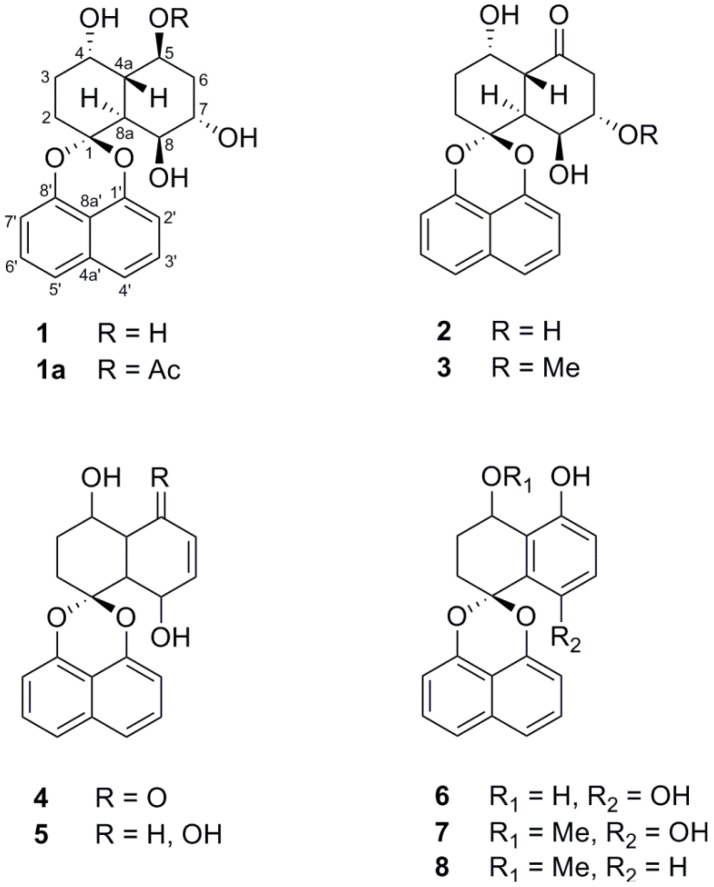
Three new spirobisnaphthalenes (1–3) were isolated from the mangrove-derived fungus Rhytidhysteron sp., together with five known derivatives (4–8). The structures of the compounds were established on the basis of extensive spectroscopic data, and the relative configurations of their stereogenic carbons were determined by a single-crystal X-ray crystallographic analysis. Compounds 3–5 displayed cytotoxicity against both cancer cell lines, MCF-7 and CaSki, while 2 was active only on CaSKi cells.
Keywords: endophytic fungus, Rhytidhysteron sp., spirobisnaphthalene, cytotoxicity
1. Introduction
Spirobisnaphthalenes, a series of compounds consisting of two naphthalene-derived C10 units bridged through a spiroketal linkage, have been mainly isolated from fungi [1,2,3,4,5]. This class of compounds is of great interest as potential leads for medicinal chemistry, since they have interesting structures and a variety of biological activities such as antibacterial, antifungal, anticancer, and antileishmanial activities [4,5,6,7].
Endophytic fungi are known as a prolific source for the discovery of structurally interesting and biologically active metabolites [8,9,10,11]. Among plant-derived fungi, those associated with the trees growing up in mangrove areas have received much attention from medicinal chemists owing to the unique ecosystem [12]. In our continued investigation into new bioactive compounds from Thai mangrove-derived fungi, we describe the isolation and structure elucidation of three new spirobisnaphthalenes, rhytidones A–C (1‒3), together with five known derivatives from an endophytic Rhytidhysteron sp. fungus. In addition, all isolated compounds were evaluated for their cytotoxic activities against human cancer cell lines.
2. Results and Discussion
The Rhytidhysteron sp. fungus was cultured in malt extract broth (MEB) under static conditions for 21 days. The EtOAc crude extract of the culture broth was successively subjected to Sephadex LH-20 and silica gel column chromatography to afford three new spironaphthalenes, rhytidones A–C (1–3), and five known analogues including MK3018 (4), palmarumycin CR1 (5), CJ-12,372 (6), 4-O-methyl-CJ-12,372 (7) and 4-O-methyl-CJ-12,371 (8) as shown in Figure 1. The structures of the known compounds were determined by comparison of their NMR spectroscopic data with those in the literature [13,14,15,16].
Figure 1.
Structures of compounds 1‒8 isolated from fungus Rhytidhysteron sp.
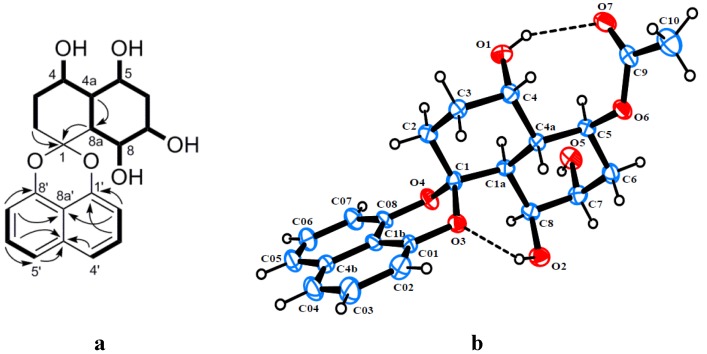
Rhytidone A (1) was obtained as a light brown powder and its molecular formula was established as C20H22O6 from HRESIMS at m/z 381.1319 [M + Na]+ (calcd 381.1314), implying 10 degrees of unsaturation. Detailed analysis of the 1H, 13C and HSQC NMR data revealed the presence of six methine carbons (four oxygenated), three methylene carbons, one doubly oxygenated quaternary carbon (δC 104.4) and 10 aromatic carbons. In the 1H-1H COSY spectrum, homonuclear coupling correlations of H-2′/H-3′ and H-3′/H-4′, as well as correlations of H-5′/H-6′ and H-6′/H-7′ indicated the presence of two isolated three-proton spin systems corresponding to the C-2′–C-4′ and C-5′–C-7′ subunits of 1, which displayed the ortho coupling constant with J values of 7.2, 7.6 and 8.0 Hz. The HMBC correlations of H-2′/C-1′, H-2′/C-8a′, H-3′/C-4a′, H-6′/C-4a′, H-7′/C-8′ and H-7′/C-8a′ led to the attachment of both subunits at C-4a′ and C-8a′, suggesting the presence of a naphthalene moiety. In addition, the chemical shifts of the nonprotonated carbons C-1′ and C-8′ at δC 147.4 and 145.9, respectively, were indicative of a 1,8-dioxynaphthalene moiety. Based on the above evidence, compound 1 was recognized as a member of the spirobisnaphthalene, characteristic of a 1,8-dioxynaphthalene linked with the second half of the molecule via a spiroketal carbon. The remaining part of the molecule was mainly elucidated by analysis of 1H-1H COSY and HMBC data. The COSY correlations confirmed the presence of the new extended spin system corresponding to the C-2–C-8 subunit (including C-4a and C-8a) (Figure 2a). HMBC correlations of H-2 and H-8a with C-1 led to the attachment of the spiroketal bridge carbon to the last subunit at C-2 and C-8a. Moreover, four exchangeable protons, observed at δH 4.41, 4.81, 3.85, 4.22, were assigned to OH-4, OH-5, OH-7 and OH-8, respectively, by their COSY correlations with their vicinal protons (Figure 2a). The relative configuration of 1 was determined through single-crystal X-ray diffraction analysis of its 5-acetate derivative (1a), obtained from acetylation of 1 with acetic anhydride in the presence of a catalytic amount of DMAP. The perspective ORTEP plot of 1a is shown in Figure 2b.
Figure 2.
(a) Key HMBC and 1H-1H COSY correlations of rhytidone A (1); (b) ORTEP diagram of the 5-acetate derivative of 1 (1a).
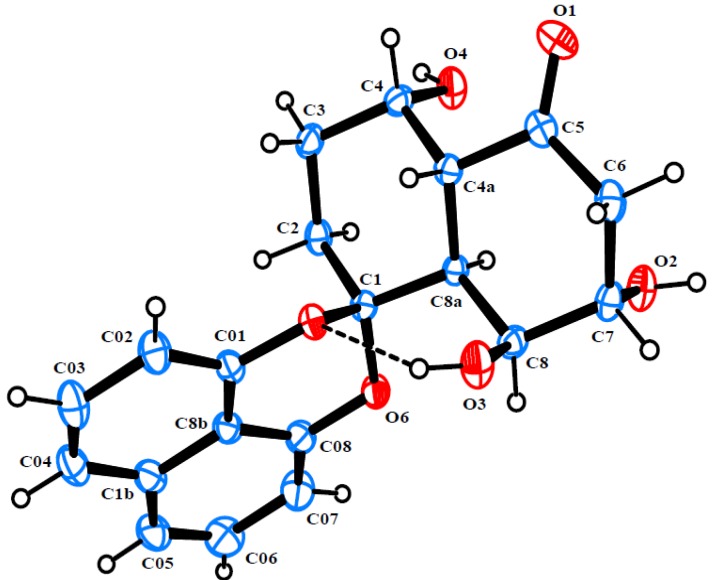
Rhytidone B (2) was isolated as colorless crystals. Its HRESIMS spectrum exhibited a pseudo-molecular ion at m/z 379.1153 ([M + Na]+, calcd 379.1158), consistent with the molecular formula C20H20O6. The NMR data of 2 also displayed characteristic signals associated with a spirobisnaphthalene, including a 1,8-dioxynaphthalene moiety and a spiroketal bridge carbon. Moreover, its NMR data (Table 1) were similar to those of 1, except for the replacement of one oxygenated methine carbon in 1 by a new ketone carbon (δC 212.2). The –CH2(2)-CH2(3)-CH(4)OH-CH(4a)-CH(8a)-CH(8)OH-CH(7)-OH-CH2(6)– subunit was established by analysis of 1H-1H COSY. HMBC correlations from H-4a and H2-6 to the ketone carbon clearly indicated the location of the ketone carbonyl at C-5. Ultimately, the structure and relative configuration of 2 was clarified by single-crystal X-ray diffraction analysis (Figure 3).
Table 1.
NMR spectroscopic data of compounds 1–3.
| No. | 1 a | 2 b | 3 b | |||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | - | 104.4 | - | 104.4 | - | 104.4 |
| 2 | 1.73, m | 25.6 | 1.95, m | 25.6 | 1.94, m | 25.6 |
| 3 | 1.53, m | 28.2 | 1.75, m 1.66, m |
26.5 | 1.72, m 1.62, m |
26.6 |
| 4 | 4.16, br s | 61.2 | 4.54, br s | 62.9 | 4.52, br s | 62.9 |
| 4a | 1.97, ddd (12.8, 10.0, 2.4) | 42.9 | 3.31, d (13.6) | 49.0 | 3.27, dd (13.2, 1.6) | 48.9 |
| 5 | 3.70, br s | 68.8 | - | 212.2 | - | 211.8 |
| 6 | 1.75, m | 35.7 | 3.13, br s 2.47, dd (14.4, 2.8) |
44.2 | 3.05, m 2.60, dd (14.4, 2.0) |
41.2 |
| 7 | 4.21, br s | 66.9 | 4.43, t (3.2) | 71.3 | 3.89, m | 80.1 |
| 8 | 3.93, m | 63.3 | 4.77, d (3.2) | 67.4 | 4.88, br d (3.6) | 65.1 |
| 8a | 2.44, dd (12.8, 1.6) | 38.6 | 3.17, br s | 41.5 | 3.03, m | 41.9 |
| 1' | - | 146.5 | - | 147.3 | - | - |
| 2' | 6.96, d (7.2) | 109.4 | 6.94, d (7.2) | 109.8 | 6.96, d (7.6) | 109.8 |
| 3' | 7.45, t (7.6) | 127.6 | 7.43, t (8.0) | 127.8 | 7.44, t (7.6) | 127.7 |
| 4' | 7.50, d (8.0) | 119.7 | 7.53, d (8.4) | 121.5 | 7.48, d (8.4) | 120.5 |
| 4a' | - | 133.6 | - | 134.2 | - | 134.2 |
| 5' | 7.52, d (8.0) | 120.1 | 7.49, d (8.4) | 120.5 | 7.53, d (8.4) | 121.4 |
| 6' | 7.45, t (7.6) | 127.5 | 7.43, t (8.0) | 127.1 | 7.42, t (7.6) | 127.1 |
| 7' | 6.94, d (7.2) | 108.8 | 6.95, d (7.2) | 109.6 | 6.93, d (7.6) | 109.6 |
| 8' | - | 147.6 | - | 145.9 | - | 145.9 |
| 8a' | - | 113.3 | - | 113.8 | - | 113.9 |
| 4-OH | 3.70, br s | - | - | - | - | - |
| 5-OH | 4.81, d (2.8) | - | - | - | - | - |
| 7-OH | 3.85, d (2.4) | - | - | - | - | - |
| 8-OH | 4.21, br s | - | 3.77, s | - | 3.71, s | - |
| 7-OMe | - | - | - | - | 3.43, s | 56.9 |
a Measured in DMSO-d6; b measured in CDCl3.
Figure 3.
ORTEP diagram of rhytidone B (2).
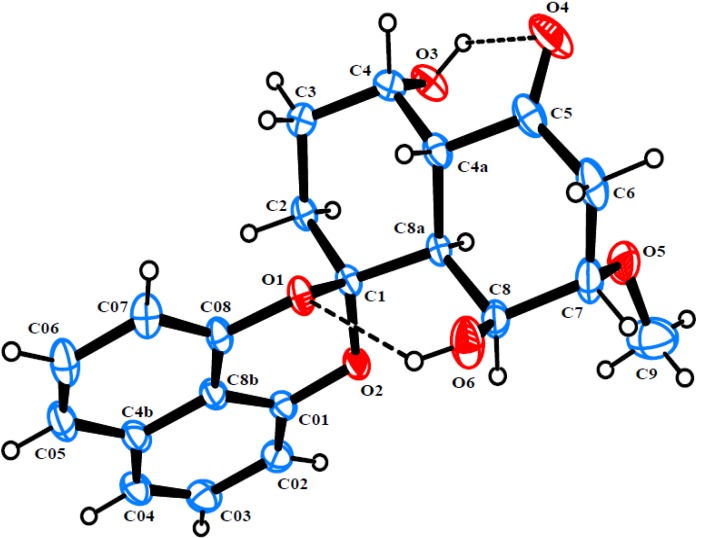
Rhytidone C (3), obtained as colorless crystals, gave the molecular formula C21H22O6, as established by HRESIMS (m/z 393.1315 ([M + Na]+, calcd. 393.1314). The NMR data of 3 (Table 1) were very similar to those of 2, except for the presence of an additional methoxy group (δH 3.43 s, δC 56.9). Strong HMBC correlation from methoxyl protons to C-7 at δC 80.1 indicated the attachment of the methoxyl group at C-7. Compound 3 was found to have the same configuration as in 2, which was also determined by single-crystal X-ray diffraction analysis (Figure 4).
Figure 4.
ORTEP diagram of rhytidone C (3).
Cytotoxic activity of isolated compounds against human breast cancer (MCF-7) and cervical carcinoma (CaSki) cell lines was evaluated by the MTT method (Table 2) [17]. Compounds 3‒5 showed moderate activity against both cell lines with IC50 values between 14.47 and 25.59 μM, while compound 2 was active only for CaSki with IC50 value of 22.81 μM. It should be noted that compounds 6‒8, possessing an additional aromatic ring in the structure, did not exhibit any significant activity against the cell lines tested (IC50 > 10 μg/mL).
Table 2.
Cytotoxicity of compounds 2–5 against human breast and cervical cancer cell lines.
| Compound | IC50 (μM) | |
|---|---|---|
| MCF-7 | CaSki | |
| 2 | − a | 22.81 ± 1.33 |
| 3 | 17.30 ± 2.11 | 24.44 ± 0.22 |
| 4 | 20.10 ± 1.52 | 25.59 ± 1.70 |
| 5 | 14.47 ± 0.51 | 21.95 ± 2.56 |
| Doxorubicin | 0.06 ± 0.01 | 0.20 ± 0.02 |
a IC50 > 10 μg/mL.
More details are available at the Supplementary Information.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were measured on a Perkin-Elmer 341 polarimeter. UV spectra were recorded on a Shimadzu UV-160 UV-visible spectrometer (Shimadzu, Kyoto, Japan). NMR spectra were acquired on a Varian Mercury-400 Plus NMR spectrometer (Varian, CA, USA) with TMS as internal standard. HRESIMS was carried out on a micrOTOF-Q II ESI mass spectrometer (Bruker, Bremen, Germany). Single-crystal X-ray diffraction analysis was performed on a Bruker APEX II diffractometer (Bruker, Bremen, Germany).
3.2. Fungal Material and Fermentation
The fungus AS21B used in the present study was isolated from leaves of Azima sarmentosa, collected from the mangrove forest in Samutsakhon province, Thailand in July 2008. The fungus was identified as a Rhytidhysteron sp. based on the ITS sequences, and was deposited at Department of Chemistry, Faculty of Science, Chulalongkorn Universtiy. The strain AS21B was grown on potato dextrose agar (PDA) plate at room temperature for 7 days. Five pieces (5 × 5 mm2) of mycelial agar plugs were inoculated into 1 L Erlenmeyer flasks (×50) containing 200 mL of malt extract broth (MEB). The cultivation was kept at room temperature for 21 days under static conditions.
3.3. Extraction and Isolation
The mycelia were separated off from the broth by filtration. The filtrate was extracted with an equal amount of EtOAc for 3 times. The EtOAc solution was evaporated under reduced pressure to afford a crude extract (7.0 g). The extract was subjected to a Sephadex LH20 column and eluted with MeOH to give six fractions (F1–F6). Subsequently, fraction 5 was fractionated by silica gel (SiO2) column chromatography eluted by a gradient of MeOH/CH2Cl2 from 1:99 to 1:9 to yield nine subfractions. The fraction F5.3 was purified by SiO2 column chromatography (a gradient of EtOAc/hexane from 2:8 to 1:1) to give 3 (20.1 mg) and 4 (47.8 mg). Subfraction F5.4 was rechromatographed on SiO2 with a 1:1 mixture of EtOAc/hexane to obtain 2 (13.5 mg). Fraction F5.6 was applied to SiO2 column chromatography eluted with MeOH/CH2Cl2 (1:19) to afford 5 (15.2 mg). Fraction F5.7 was divided into four fractions by column chromatography on Sephadex LH20 (MeOH), then F5.7.4 was further purified by SiO2 column chromatography with MeOH/CH2Cl2 (1:9) to give 1 (83 mg). Fraction F6 was subjected to a Sephadex LH20 column eluted with MeOH to yield five subfractions. F6.4 was recrystallized with MeOH to provide 7 (32.6 mg). Subfraction F6.5 was rechromatographed over a SiO2 column to give three fractions. The fraction F6.5.3 was further purified by SiO2 column chromatography eluted with EtOAc/hexane (1:3) to yield 6 (12.8 mg) and 8 (8.2 mg).
Rhytidone A (1): light brown powder; [α]25D +21 (c 0.10, MeOH); 1H and 13C NMR, see Table 1; HRESIMS m/z 381.1319 [M + Na]+ (calcd for C20H22O6Na, 381.1314).
Rhytidone B (2): colorless crystals; [α]25D +15 (c 0.10, MeOH); 1H and 13C NMR, see Table 1; HRESIMS m/z 379.1153 [M + Na]+ (calcd for C20H20O6Na, 379.1158).
Rhytidone C (3): colorless crystals; [α]25D +18 (c 0.10, MeOH); 1H and 13C NMR, see Table 1; HRESIMS m/z 393.1315 [M + Na]+ (calcd for C21H22O6Na, 393.1314).
Preparation of 5-O-Acetyl-rhytidone A (1a). A mixture of compound 1 (10 mg), acetic anhydride (0.1 mL) and a catalytic amount of DMAP in CH2Cl2 (2 mL) was stirred at room temperature for 30 min. Then, water (5 mL) was added to the reaction mixture, and extracted with EtOAc (3 × 5 mL). The combined organic layer was washed with brine, dried over MgSO4, and evaporated after filtration. The residue was purified by SiO2 column chromatography (MeOH/CH2Cl2, 2:98) to yield 1a (7.2 mg, 64%) as colorless crystals: 1H NMR (CDCl3, 400 MHz) δ 7.51 (1H, d, J = 8.4 Hz, H-5′), 7.47 (1H, d, J = 8.4 Hz, H-4′), 7.44 (1H, t, J = 7.2 Hz, H-6′), 7.39 (1H, t, J = 7.2 Hz, H-3′), 6.93 (1H, d, J = 8.4 Hz, H-2′), 6.90 6.90 (1H, d, J = 8.4 Hz, H-7′), 5.34 (1H, ddd, J = 5.2, 10.0, 16.8 Hz, H-5), 4.56 (1H, br s, H-8), 4.13 (1H, br m, H-7), 3.88 (1H, br s, 4-OH), 3.70 (1H, s, 8-OH), 3.58 (1H, br s, H-4), 2.76 (1H, dd, J = 1.6, 12.8 Hz, H-8a), 2.43 (1H, ddd, J = 2.0, 12.8, 16.8 Hz, H-4a), 2.27 (1H, ddd, J = 2.4, 12.8, 14.4 Hz, H-6a), 2.15 (3H, s, 5-OAc), 2.00 (1H, m, H-6b), 1.97 (2H, m, H-2), 1.73 (2H, m, H-2); 13C NMR (CDCl3, 100 MHz) δ 172.7 (5-OCOCH3), 147.5 (C-8′), 146.0 (C-1′), 134.2 (C-4a′), 127.7 (C-6′), 127.0 (C-3′), 121.3 (C-5′), 120.3 (C-4′), 113.9 (C-8a′), 109.8 (C-2′), 109.5 (C-7′), 104.7 (C-1), 69.4 (C-5), 69.2 (C-7), 67.6 (C-8), 62.6 (C-4), 41.3 (C-4a), 39.0 (C-8a), 32.3 (C-6), 27.2 (C-3), 25.6 (C-2), 21.2 (5-OCOCH3).
X-ray Crystallographic Analysis of compounds 1a, 2 and 3. All single crystal X-ray diffraction data were collected at 296 K on a Bruker APEX II diffractometer with Mo Kα radiation (λ = 0.71073 Å). The structures were solved by direct methods using SHELXS-97 and refined full-matrix least squares on all F2 data using SHELXL97 to final R values [18,19]. All hydrogen atoms were added at calculated positions and refined using a rigid model. Crystallographic data for 1a, 2 and 3 have been deposited with the Cambridge Crystallographic Data Centre (Cambridge, UK) [20].
Crystal data of 1a: colorless crystal; C22H24O7, Mr = 400.41, monoclinic, a = 12.220(2) Å, b = 5.7498(9) Å, c = 13.925(3) Å, space group P21, Z = 2 and V = 956.2(3) Å3, μ(Mo Kα) = 0.10 mm−1, and F(000) = 424. Crystal dimensions: 0.42 × 0.27 × 0.25 mm. Independent reflections: 1492 (Rint = 0.063). The final R1 values were 0.039, wR2 = 0.113 (I > 2σ(I)). CCDC number: 977804.
Crystal data of 2: colorless crystal; C20H20O6, Mr = 356.36, orthorhombic, a = 7.6970(6) Å, b = 8.4012(8) Å, c = 25.845(2) Å, space group P212121, Z = 4 and V = 1617.3(2) Å3, μ(Mo Kα) = 0.11 mm−1, and F(000) = 752. Crystal dimensions: 0.40 × 0.22 × 0.20 mm. Independent reflections: 3200 (Rint= 0.025). The final R1 values were 0.037, wR2 = 0.110 (I > 2σ(I)). CCDC number: 977805.
Crystal data of 3: colorless crystal; C21H22O6, Mr = 370.39, monoclinic, a = 23.9484(6) Å, b = 5.6338(1) Å, c = 15.5486(4) Å, space group P212121, Z = 4 and V = 1783.37(7) Å3, μ(Mo Kα) = 0.10 mm−1, and F(000) = 784. Crystal dimensions: 0.36 × 0.34 × 0.24 mm. Independent reflections: 3133 (Rint = 0.016). The final R1 values were 0.037, wR2 = 0.119 (I > 2σ(I)). CCDC number: 898711.
3.4. Cytotoxicity Assay
Cytotoxicity of all compounds was assayed with a modification of the MTT (3-[4,5-dimethylthiazol-2-yl-2,5-diphenyltetrazolium] bromide) colorimetric method. Cytotoxicity assays were performed according to previously described procedures [16]. The following human cancer cell lines were used in the assay: human breast cancer (MCF-7) and cervical carcinoma (CaSki) cell lines. Doxorubicin was used as the reference compound.
4. Conclusions
The chemical investigation of the EtOAc extract of the endophytic fungus Rhytidhysteron sp. has led to the isolation and characterization of three spirobisnaphthalenes, along with five known derivatives. All isolated compounds (1‒8) were evaluated for their cytotoxicity against breast and cervical cancer cells. Compounds 3‒5 exhibited cytotoxicity against both cell lines, whereas compound 2 was active only for cervical carcinoma cells.
Acknowledgments
This work was supported by the Thailand Research Fund (RSA5580023), and the Ratchadaphiseksomphot Endowment Fund of Chulalongkorn University (RES560530208-AS). The authors also gratefully thank Miss Sujitra Hantanong, Program in Biotechnology, Faculty of Science, Chulalongkorn University, for fungal cultivation.
Supplementary Files
Supplementary Information (PDF, 1501 KB)
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Jiao P., Swenson D.C., Gloer J.B., Campbell J., Shearer C.A. Decaspirones A‒E, bioactive spirodioxynaphthalenes from the freshwater aquatic fungus Decaisnella thyridioides. J. Nat. Prod. 2006;69:1667–1671. doi: 10.1021/np060385k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu H., Guo H., Li E., Zhou Y., Che Y. Decaspirones F‒I, bioactive secondary metabolites from the saprophytic fungus Helicoma viridis. J. Nat. Prod. 2006;69:1672–1675. doi: 10.1021/np0603830. [DOI] [PubMed] [Google Scholar]
- 3.Macías-Rubalcava M.L., Hernández-Bautista B.E., Jiménez-Estrada M., González M.C., Glenn A.E., Hanlin R.T., Hernández-Ortega S., Saucedo-García A., Muria-González J.M., Anaya A.L. Naphthaquinone spiroketal with allelochemical activity from the newly discovered endophytic fungus Edenia gomezpompae. Phytochemistry. 2008;69:1185–1196. doi: 10.1016/j.phytochem.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Matínez-Luis S., Della-Togna G., Coley P.D., Kursar T.A., Gerwick W.H., Cubilla-Rios L. Antileishmanial constituents of the Panamanian endophytic fungus Edenia sp. J. Nat. Prod. 2008;71:2011–2014. doi: 10.1021/np800472q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van der Sar S.A., Blunt J.W., Munro M.H.G. Spiro-Mamakone A, a unique relative of the spironaphthalene class of compounds. Org. Lett. 2006;8:2059–2061. doi: 10.1021/ol060434k. [DOI] [PubMed] [Google Scholar]
- 6.Chen X., Shi Q., Lin G., Guo S., Yang J. Spirobisnaphthalene analogues from the endophytic fungus Preussia sp. J. Nat. Prod. 2009;72:1712–1715. doi: 10.1021/np900302w. [DOI] [PubMed] [Google Scholar]
- 7.Cai Y.-S., Guo Y.-W., Krohn K. Structure, bioactivities, biosynthetic relationships and chemical synthesis of the spirodioxynaphthalenes. Nat. Prod. Rep. 2010;27:1840–1870. doi: 10.1039/c0np00031k. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H.W., Song Y.C., Tan R.X. Biology and chemistry of endophytes. Nat. Prod. Res. 2006;23:753–771. doi: 10.1039/b609472b. [DOI] [PubMed] [Google Scholar]
- 9.Gunatilaka A.A.L. Natural products from plant-associated microorganisms: Distribution, structural diversity, bioactivity and implications of their occurrence. J. Nat. Prod. 2006;69:509–526. doi: 10.1021/np058128n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strobel G., Diasy B., Castillo U., Harper J. Natural products form endophytic microorganisms. J. Nat. Prod. 2004;67:257–268. doi: 10.1021/np030397v. [DOI] [PubMed] [Google Scholar]
- 11.Strobel G.A. Endophytes as sources of bioactive products. Microbes Infect. 2003;5:535–544. doi: 10.1016/s1286-4579(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 12.Debbab A., Aly A.H., Proksch P. Mangrove derived fungal endophytes—A chemical and biological perception. Fungal Divers. 2013;61:1–27. doi: 10.1007/s13225-013-0243-8. [DOI] [Google Scholar]
- 13.Sakemi S., Inagaki T., Kaneda K., Hirai H., Iwata E., Sakakibara T., Yamauchi Y., Norcia M., Wondrack L.M., Sutcliffe J.A., Kojima N. CJ-12,371 and CJ-12,372, two novel DNA gyrase inhibitors, fermentation, isolation, structural elucidation and biological activities. J. Antibiot. 1995;48:134–142. doi: 10.7164/antibiotics.48.134. [DOI] [PubMed] [Google Scholar]
- 14.Wipf P., Lynch S.M., Birmingham A., Tamayo G., Jiménez A., Campos N., Powis G. Natural product based inhibitors of the thioredoxin-thioredoxin reductase system. Org. Biomol. Chem. 2004;2:1651–1658. doi: 10.1039/b402431a. [DOI] [PubMed] [Google Scholar]
- 15.Ragot J.P., Steeneck C., Alcaraz M.-L., Taylor R.J.K. The synthesis of 1,8-dihydroxynaphthalene-derived natural products: Palmarymycin CP1, Palmarymycin CP2, Palmarymycin CP11, CJ-12,371, deoxypreussomerin A and novel analogues. J. Chem. Soc. Perkin Trans. 1. 1999:1073–1082. doi: 10.1039/A901076I. [DOI] [Google Scholar]
- 16.Ohishi H., Chiba N., Mikawa T., Sakaki T., Miyaji S., Sezaki M. Novel Antibiotic MK3018 substance— Useful as antimicrobial agent. 01294686. Jpn. Pat. 1989 Nov 28;
- 17.Chokpaiboon S., Sommit D., Teerawatananond T., Muangsin N., Bunyapaiboonsri T., Pudhom K. Cytotoxic nor-chamigrane and chamigrane endoperoxides from a basidiomycetous fungus. J. Nat. Prod. 2010;73:1005–1007. doi: 10.1021/np100103j. [DOI] [PubMed] [Google Scholar]
- 18.Sheldrick G.M. SHELXS-97, Program for Crystal Structure Solution. University of Göttingen; Göttingen, Germany: 1997. [Google Scholar]
- 19.Sheldrick G.M. SHELXS-97, Program for Crystal Structure Refinement. University of Göttingen; Göttingen, Germany: 1997. [Google Scholar]
- 20.Cambridge Crystallographic Data Centre. [(accessed on 24 February 2014)]. Available online: http://www.ccdc.cam.ac.uk/data_request/cif.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information (PDF, 1501 KB)