Abstract
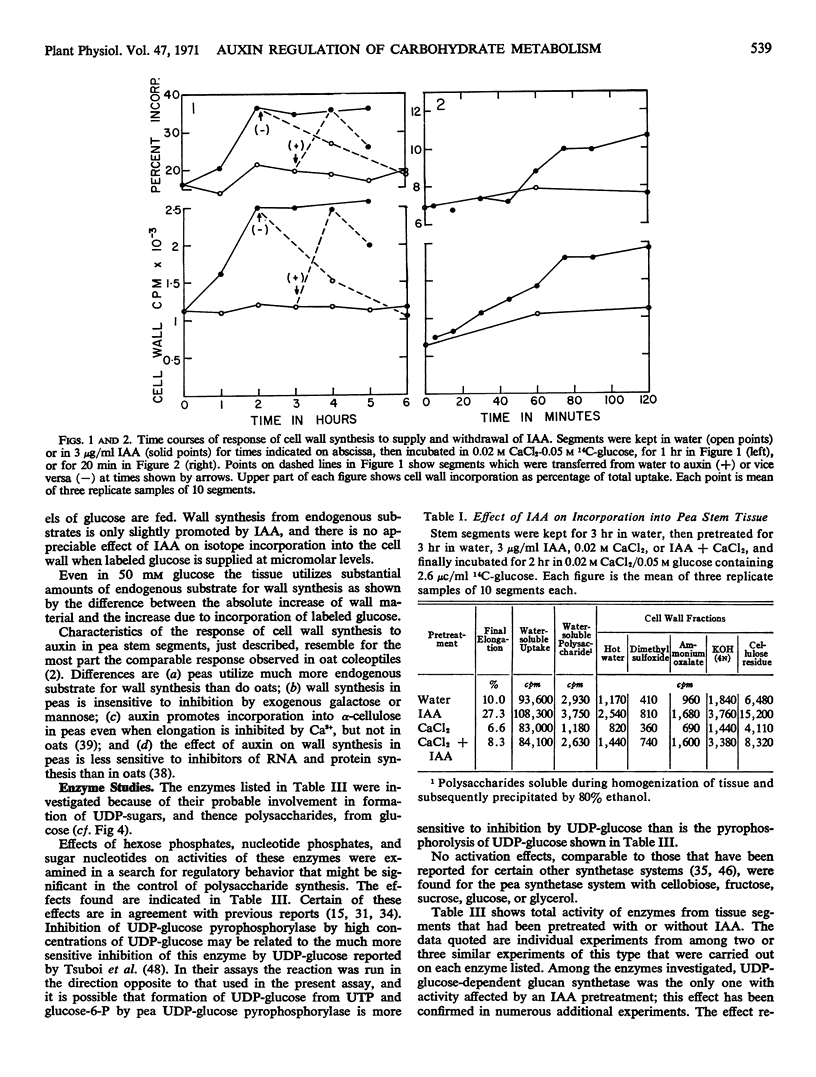
Promotion of cell wall synthesis (from glucose) in pea (Pisum sativum) stem segments by indoleacetic acid (IAA) develops over a period of 1 to 2 hours and is comprised of a promotion of glucose uptake plus a promotion of the utilization of absorbed glucose. The effect of IAA resembles, in these and other respects, its effect on cell wall synthesis in oat coleoptile segments, but the pea system differs in not being inhibited by galactose or mannose, in involving considerably more isotope dilution by endogenous substrates, and in certain other respects.
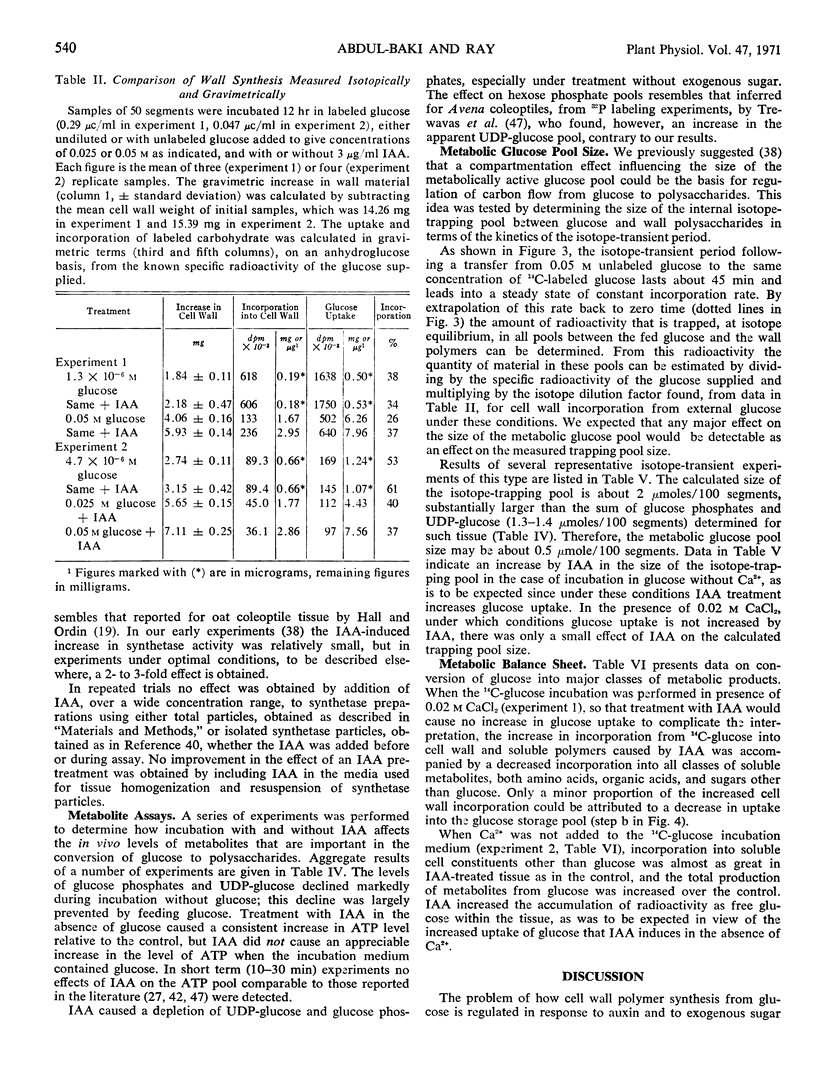
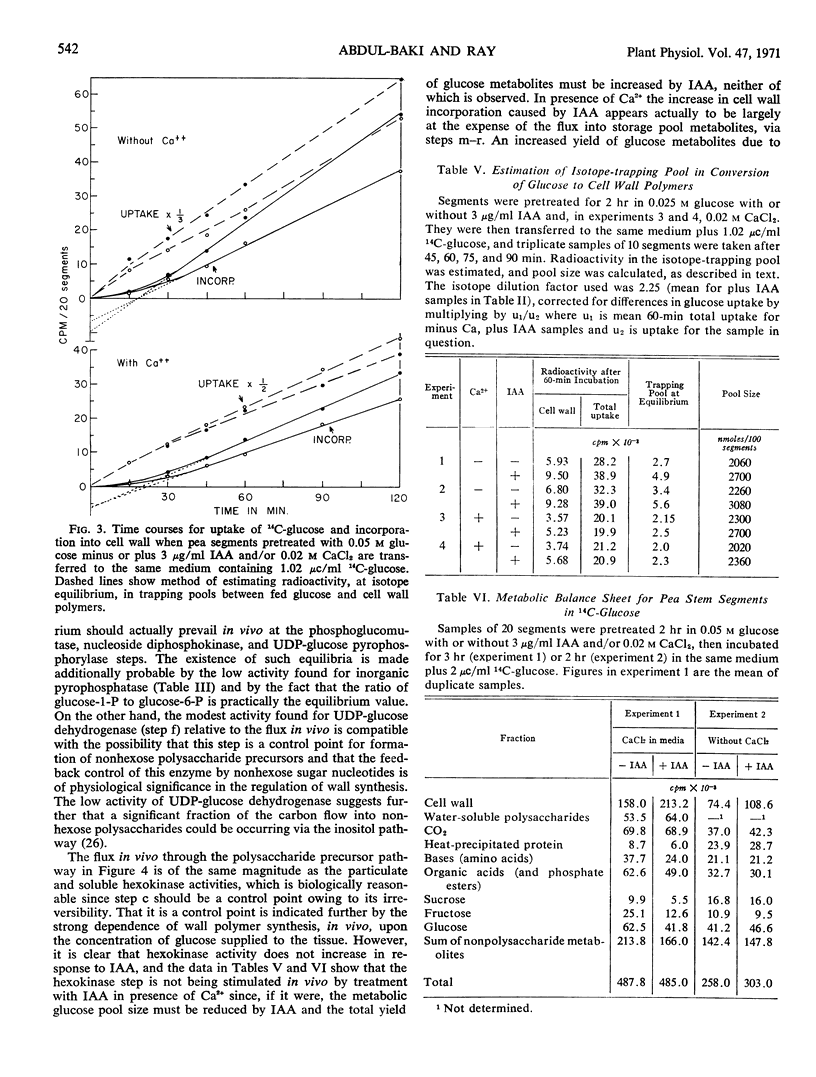
Effector influences upon and total activities of the following enzymes obtained from etiolated pea stem segments pretreated with or without IAA were examined: phosphoglucomutase, uridine diphosphate glucose (UDP-glucose) pyrophosphorylase, nucleoside diphosphokinase, UDP-glucose dehydrogenase, inorganic pyrophosphatase, hexokinase (particulate and soluble), and UDP-glucose-β-1,4-glucan-glucosyl transferase (β-glucan synthetase). The first three enzymes mentioned exhibit high activity relative to the flux in vivo, do not appear to show physiologically significant effector responses, and are concluded not to be control points. UDP-glucose dehydrogenase activity is regulated by UDP-xylose. Hexokinase is a potential control point but does not exhibit regulatory effects related to the IAA response. β-Glucan synthetase is the only one of these enzymes with activity which is increased by treatment of tissue with IAA, and this may be responsible for the effect of IAA on wall synthesis.
Assays of metabolite pools support the conclusion that stimulation of polysaccharide synthesis by IAA is due partly to changes in hexokinase reaction rate resulting from an increase in metabolic glucose pool size caused by increased glucose uptake, and partly to increased activity at the polysaccharide synthetase level.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARBER H. A., ELBEIN A. D., HASSID W. Z. THE SYNTHESIS OF CELLULOSE BY ENZYME SYSTEMS FROM HIGHER PLANTS. J Biol Chem. 1964 Dec;239:4056–4061. [PubMed] [Google Scholar]
- BERG P., JOKLIK W. K. Enzymatic phosphorylation of nucleoside diphosphates. J Biol Chem. 1954 Oct;210(2):657–672. [PubMed] [Google Scholar]
- Baker D. B., Ray P. M. Direct and Indirect Effects of Auxin on Cell Wall Synthesis in Oat Coleoptile Tissue. Plant Physiol. 1965 Mar;40(2):345–352. doi: 10.1104/pp.40.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamini E., Bombara G., Pellegrino C. The effect of testosterone on glycogen metabolism in rat levator ani muscle. Biochim Biophys Acta. 1969 Apr 1;177(2):220–234. doi: 10.1016/0304-4165(69)90131-7. [DOI] [PubMed] [Google Scholar]
- Bonner J. Studies on the Growth Hormone of Plants: V. The Relation of Cell Elongation to Cell Wall Formation. Proc Natl Acad Sci U S A. 1934 Jun;20(6):393–397. doi: 10.1073/pnas.20.6.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J. W., Rall T. W., Larner J. The influence of insulin and epinephrine on adenosine 3',5'-phosphate and glycogen transferase in muscle. Biochim Biophys Acta. 1969 Apr 1;177(2):213–219. doi: 10.1016/0304-4165(69)90130-5. [DOI] [PubMed] [Google Scholar]
- Dickinson D. B., Preiss J. ADP glucose pyrophosphorylase from maize endosperm. Arch Biochem Biophys. 1969 Mar;130(1):119–128. doi: 10.1016/0003-9861(69)90017-4. [DOI] [PubMed] [Google Scholar]
- Furlong C. E., Preiss J. Biosynthesis of bacterial glycogen. VII. Purification and properties of the adenosine diphosphoglucose pyrophosphorylase of Rhodospirillium rubrum. J Biol Chem. 1969 May 25;244(10):2539–2548. [PubMed] [Google Scholar]
- GINSBURG V. Purification of uridinediphosphate glucose pyrophosphorylase from mung bean seedlings. J Biol Chem. 1958 May;232(1):55–61. [PubMed] [Google Scholar]
- Garces E., Cleland W. W. Kinetic studies of yeast nucleoside diphosphate kinase. Biochemistry. 1969 Feb;8(2):633–640. doi: 10.1021/bi00830a026. [DOI] [PubMed] [Google Scholar]
- Goldberg N. D., O'Toole A. G. The properties of glycogen synthetase and regulation of glycogen biosynthesis in rat brain. J Biol Chem. 1969 Jun 10;244(11):3053–3061. [PubMed] [Google Scholar]
- HEPPEL L. A., HILMOE R. J. Purification of yeast inorganic pyrophosphatase. J Biol Chem. 1951 Sep;192(1):87–94. [PubMed] [Google Scholar]
- HORNBROOK K. R., BURCH H. B., LOWRY O. H. CHANGES IN SUBSTRATE LEVELS IN LIVER DURING GLYCOGEN SYNTHESIS INDUCED BY LACTATE AND HYDROCORTISONE. Biochem Biophys Res Commun. 1965 Jan 18;18:206–211. doi: 10.1016/0006-291x(65)90741-2. [DOI] [PubMed] [Google Scholar]
- KIRKLAND R. J., TURNER J. F. Nucleoside diphosphokinase of pea seeds. Biochem J. 1959 Aug;72:716–720. doi: 10.1042/bj0720716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewus F. Metabolism of inositol in higher plants. Ann N Y Acad Sci. 1969 Oct 17;165(2):577–598. [PubMed] [Google Scholar]
- MUNCH-PETERSEN A., KALCKAR H. M., CUTOLO E., SMITH E. E. Uridyl transferases and the formation of uridine triphosphate; enzymic production of uridine triphosphate: uridine diphosphoglucose pyrophosphorolysis. Nature. 1953 Dec 5;172(4388):1036–1037. doi: 10.1038/1721036a0. [DOI] [PubMed] [Google Scholar]
- Mersmann H. J., Segal H. L. Glucocorticoid control of the liver glycogen synthetase-activating system. J Biol Chem. 1969 Apr 10;244(7):1701–1704. [PubMed] [Google Scholar]
- NEUFELD E. F., HALL C. W. INHIBITION OF UDP-D-GLUCOSE DEHYDROGENASE BY UDP-D-XYLOSE: A POSSIBLE REGULATORY MECHANISM. Biochem Biophys Res Commun. 1965 May 3;19:456–461. doi: 10.1016/0006-291x(65)90146-4. [DOI] [PubMed] [Google Scholar]
- Newell P. C., Ellingson J. S., Sussman M. Synchrony of enzyme accumulation in a population of differentiating slime mold cells. Biochim Biophys Acta. 1969 May 6;177(3):610–614. doi: 10.1016/0304-4165(69)90326-2. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Robinson J., Taylor K. A radiochemical enzymatic activity assay for glycerol kinase and hexokinase. Biochim Biophys Acta. 1967 Mar 15;132(2):338–346. doi: 10.1016/0005-2744(67)90153-2. [DOI] [PubMed] [Google Scholar]
- OLIVER I. T. Inhibitor studies on uridine diphosphoglucose pyrophosphorylase. Biochim Biophys Acta. 1961 Sep 2;52:75–81. doi: 10.1016/0006-3002(61)90905-2. [DOI] [PubMed] [Google Scholar]
- Ordin L., Hall M. A. Cellulose synthesis in higher plants from UDP glucose. Plant Physiol. 1968 Mar;43(3):473–476. doi: 10.1104/pp.43.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piras R., Staneloni R. In vivo regulation of rat muscle glycogen synthetase activity. Biochemistry. 1969 May;8(5):2153–2160. doi: 10.1021/bi00833a056. [DOI] [PubMed] [Google Scholar]
- Ray P. M., Baker D. B. The Effect of Auxin on Synthesis of Oat Coleoptile Cell Wall Constituents. Plant Physiol. 1965 Mar;40(2):353–360. doi: 10.1104/pp.40.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Shininger T. L., Ray M. M. ISOLATION OF beta-GLUCAN SYNTHETASE PARTICLES FROM PLANT CELLS AND IDENTIFICATION WITH GOLGI MEMBRANES. Proc Natl Acad Sci U S A. 1969 Oct;64(2):605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman L. B., Cabib E. Regulation of glycogen synthesis in the intact yeast cell. Biochemistry. 1969 Aug;8(8):3332–3341. doi: 10.1021/bi00836a030. [DOI] [PubMed] [Google Scholar]
- STROMINGER J. L., MAPSON L. W. Uridine diphosphoglucose dehydrogenase of pea seedlings. Biochem J. 1957 Aug;66(4):567–572. doi: 10.1042/bj0660567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring A., Rowan K. S. Phosphorus metabolism and auxin action. Nature. 1966 Jun 11;210(5041):1166–1167. doi: 10.1038/2101166a0. [DOI] [PubMed] [Google Scholar]
- Trewavas A. J., Johnston I. R., Crook E. M. The effects of some auxins on the levels of phosphate esters in Avena sativa coleoptile sections. Biochim Biophys Acta. 1967 Mar 22;136(2):301–311. doi: 10.1016/0304-4165(67)90076-1. [DOI] [PubMed] [Google Scholar]
- Tsuboi K. K., Fukunaga K., Petricciani J. C. Purification and specific kinetic properties of erythrocyte uridine diphosphate glucose pyrophosphorylase. J Biol Chem. 1969 Feb 10;244(3):1008–1015. [PubMed] [Google Scholar]
- Zetsche K. Regulation der UDPG-Pyrophosphorylase-Aktivität in Acetabularia I. Morphogenese und UDPG-Pyrophosphorylase-Synthese in kernhaltigen und kernlosen Zellen. Z Naturforsch B. 1968 Mar;23(3):369–376. [PubMed] [Google Scholar]


