Abstract
Resolution of inflammation is an emerging new strategy to reduce damage following ischemic stroke. Lipoxin A4 (LXA4) is an anti-inflammatory, pro-resolution lipid mediator with high affinity binding to its receptor ALX. Since LXA4 is rapidly inactivated, potent analogs have been created, including the ALX agonist BML-111. We hypothesized that post-ischemic intravenous administration of BML-111 would provide protection to the neurovascular unit and reduce neuroinflammation in a rat stroke model. Animals were subjected to 90 min of middle cerebral artery occlusion (MCAO) and BML-111 was injected 100 min and 24 h after stroke onset and animals sacrificed at 48 h. Post-ischemic treatment with BML-111 significantly reduced infarct size, decreased vasogenic edema, protected against blood-brain barrier (BBB) disruption, and reduced hemorrhagic transformation. Matrix metalloproteinase (MMP)-9 and MMP-3 were significantly reduced following BML-111 treatment. Administration of BML-111 dramatically decreased microglial activation, as seen with CD68, and neutrophil infiltration and recruitment, as assessed by levels of myeloperoxidase (MPO) and intracellular adhesion molecule (ICAM)-1. The tight junction protein zona occludens-1 (ZO-1) was protected from degradation following treatment with BML-111. These results indicate that post-ischemic activation of ALX has pro-resolution effects that limit the inflammatory damage in the cerebral cortex and helps maintain BBB integrity after ischemic stroke.
Keywords: ischemia, blood-brain barrier, matrix metalloproteinases, vasogenic edema, hemorrhagic transformation, neuroinflammation, neutrophil recruitment, tight junctions, resolution
Introduction
Ischemic stroke is one of the leading causes of morbidity and mortality in the world. The severity of permanent brain damage following cerebral ischemia largely depends on the extent of secondary brain damage to the penumbra caused by spreading inflammation (Iadecola & Anrather 2011). Following ischemia, astrocytes and microglia produce inflammatory cytokines and chemokines that increase expression of intracellular adhesion molecules (ICAMs) and selectins on endothelial cells of the blood-brain barrier (BBB) and allow for the infiltration of peripheral immune cells (e.g. neutrophils, monocytes) (Ishikawa et al. 2004). These cells contribute to the production of inflammatory factors and amplify and expand the immune response and disrupt the BBB. Increased production of matrix metalloproteinases (MMPs) by pericytes, astrocytes, microglia, and invading neutrophils lead to a breakdown in the basal lamina and tight junction proteins (TJPs) that maintain the structural integrity of the BBB (Yang et al. 2007, Candelario-Jalil et al. 2011, Candelario-Jalil et al. 2009). Stroke patients with BBB disruption have less neurologic recovery and a higher likelihood of 90-day mortality (Desilles et al. 2013). Recent research suggests targeting neuroinflammation can provide protection and improve stroke outcome (Yoon et al. 2013, Culman et al. 2012, Joo et al. 2013).
Lipoxin A4 (LXA4) is an endogenous anti-inflammatory, pro-resolution molecule formed from arachidonic acid via lipoxygenase-mediated transcellular biosynthesis. LXA4 has high affinity binding with a G protein-coupled receptor, ALX (also termed FPRL1/FPR2) (Chiang et al. 2000). Activation of ALX limits neutrophil recruitment, increases the production of anti-inflammatory factors, and promotes clearance of inflammatory debris (Perretti et al. 2002, Godson et al. 2000, Levy et al. 2011). ALX is expressed in neutrophils, monocytes, and macrophages, as well as in resident brain astrocytes, microglia, and neural stem cells, suggesting these cells may be the targets of LXA4 anti-inflammatory activity in the brain (Maddox et al. 1997, Svensson et al. 2007, Wada et al. 2006, Sodin-Semrl et al. 2004). Since LXA4 is rapidly biosynthesized and enzymatically inactivated, stable and powerful analogs have been synthesized (Chiang et al. 2000). BML-111 (5(S),6(R),7-trihydroxyheptanoic acid methyl ester) is a commercially available ALX agonist that has been shown to inhibit neutrophil recruitment and to reduce inflammation in a number of peripheral inflammatory disorders, including arthritis, liver injury, and lung injury (Zhang et al. 2008, Conte et al. 2010, Zhang et al. 2007, Gong et al. 2012, Lee et al. 1991, Li et al. 2008).
It has been previously reported that LXA4, when injected directly into the brain immediately after the induction of ischemia, can provide protection to the BBB, reduce infarct size, and decrease inflammatory factors in rats (Sobrado et al. 2009, Ye et al. 2010, Wu et al. 2010, Wu et al. 2012). However, to our knowledge, no research has investigated the effects of administering the ALX agonist BML-111 after stroke onset on infarct size and BBB permeability following transient focal cerebral ischemia-reperfusion injury. The purpose of this work is to test the hypothesis that post-ischemic intravenous treatment with BML-111 will provide protection to the BBB and reduce neuroinflammation from ischemic stroke. In order to test this hypothesis, we measured infarct size, BBB permeability, MMPs, neuroinflammatory markers, and components of the BBB in vehicle- or BML-111-treated rats. Our results indicate that enhancing the resolution of the neuroinflammatory process after ischemic stroke by targeting the LXA4 pathway is a novel and promising approach to reduce neuroinflammation and neurovascular damage in stroke.
Materials and Methods
Animals
All experimental procedures were performed according to the guidelines and regulations of the University of Florida’s animal care services, the ARRIVE guidelines, and the guidelines of the National Institutes of Health (Bethesda, MD, USA) for the care and use of laboratory animals for experimental procedures. The institutional animal care and use committee approved our experimental protocol and appropriate measures were observed to minimize pain and stress to the animals. Adult male Wistar rats (9-11 weeks; 280-320 g; Harlan Laboratories, Indianapolis, IN, USA) were used in this study. All animals were acclimated to our animal facility for at least 7 days before surgery. Animals were housed in groups of two in polycarbonate cages in a room with a controlled environment and a 12 h light/dark cycle. Animals had free access to rodent pellet chow and water.
Rat transient focal cerebral ischemia model and drug administration
Focal cerebral ischemia was done by transient middle cerebral artery occlusion (MCAO) using the intraluminal filament method as previously described by our group (Candelario-Jalil et al. 2007). Briefly, rats were anesthetized with isoflurane in medical-grade oxygen. A midline vertical incision was made in the neck to expose the right common carotid artery (CCA), external carotid artery (ECA), and the internal carotid artery (ICA). The CCA was permanently ligated with a 4-0 silk suture and a vascular clip was temporarily placed over the pterygopalatine artery to prevent incorrect insertion of the monofilament. A loose tie was placed on the ICA and ECA bifurcation with a 4-0 silk suture and vascular clips were placed on the ICA and ECA. A small incision was made in the CCA approximately 2 mm proximal to the carotid bifurcation. A 4-0 silicone-coated filament (Cat. No. 403523PK10; Doccol Corporation, Sharon, MA, USA) was inserted through the incision in the CCA and gently pushed 18-20 mm inside the ICA until a mild resistance was felt. The occluding filament was left in place for 90 min while animals were allowed to recover from anesthesia. Eight to ten minutes before the end of the occlusion, animals were anesthetized with isoflurane and the filament was gently retracted to allow for reperfusion of the MCA territory. Animals were allowed to recover and for the next 48 h eat and drink freely. Rectal temperature was maintained at 37° ± 0.5°C with a heated mat during surgery. Animals were allowed to recover from anesthesia in a temperature-controlled chamber set at 37°C, and then transferred to their home cages for the duration of MCAO. Using this procedure, we obtained large and reproducible infarcted regions involving the cerebral cortex and the striatum.
A Micro-Renathane catheter was inserted into the right femoral vein at the time of MCAO surgery for drug administration. BML-111 (Cat. No. RA-108; Enzo Life Sciences, Ann Arbor, MI, USA) was freshly dissolved in saline. Rats were randomly assigned to vehicle or treatment conditions. Ten minutes after reperfusion (100 min after the onset of MCAO), rats in the BML-111 treatment condition were given a first dose of BML-111 (1 mg/kg) intravenously through the right femoral vein and a second dose was administered in the same fashion 24 h after reperfusion. This dose of BML-111 has been shown to potently and consistently reduce inflammation in peripheral disorders (Gong et al. 2012, Zhang et al. 2007, Zhang et al. 2008). Rats in the vehicle condition were on the same schedule but only administered the vehicle control (saline).
After 48 h of reperfusion, animals were deeply anesthetized with pentobarbital (150 mg/kg; i.p.) and a blood sample was withdrawn from the vena cava in a heparinized syringe. Blood (1.5 mL) was immediately mixed with heparin (50 μL, 1000 U/mL) and centrifuged for 10 min at 2,000 xg to separate and collect the plasma. Rats were perfused intracardially with ice-cold saline until no blood remained. Brains were harvested and sliced into six 2-mm slices. The fourth slice was separated into the ipsilateral and contralateral cerebral cortex and striatum to be used for molecular biology analyses. These samples were immediately frozen on dry ice and stored at −80°C until homogenization. All other slices were preserved for infarct size calculation.
Brain tissue homogenization
Brain tissue homogenates were prepared from rats with right focal cerebral ischemia. Unfixed frozen cerebral cortex and striatum samples from both the ipsilateral (stroke side) and contralateral hemispheres were weighed and homogenized in 1% sodium dodecyl sulfate (SDS) buffer containing 150 mM NaCl, 50 mM Tris-HCl pH 7.6, 1% IGEPAL® CA-630, and 1% sodium deoxycholate at 100 μL / 10 mg of brain tissue. Immediately before buffer was added to brain samples, HALT Protease Inhibitor Cocktail, HALT Phosphatase Inhibitor Cocktail (Cat. Nos. 78430 and 78428, respectively; Thermo Fisher Scientific, Rockford, IL, USA), and 0.5 M EDTA were added at 10 μL/mL of homogenization buffer. Chilled tissue was homogenized with a Tissue-Tearor homogenizer (Cat. No. 985370; BioSpec, Inc, Bartlesville, OK, USA) then sonicated twice using a Vibra-Cell™ sonicator (Model VCX130PB; Sonics & Materials, Inc, Newtown, CT, USA). Resulting tissue homogenates were centrifuged at 14,000 xg for 20 min at 4° C in an Eppendorf Microcentrifuge Model 5430R, and the supernatants aliquoted and stored at −80° C until use.
Infarct volume and brain swelling calculation
Infarct volume was calculated as previously reported (Candelario-Jalil et al. 2007). Briefly, the rats were deeply anesthetized and brains were removed and sectioned into six 2-mm-thick slices (anterior to posterior, first to sixth) using a rat brain slicer matrix (Zivic Instruments, Pittsburgh, PA, USA). All except the fourth slice were incubated for 30 min in a 2% solution of 2,3,5-triphenyltetrazolium chloride (TTC; Sigma-Aldrich, St. Louis, MO, USA) at room temperature and fixed in a 4% paraformaldehyde solution in phosphate-buffered saline, pH 7.4. Five stained sections per animal were placed directly onto the scanning screen of a color flatbed scanner (HP Scanjet 8300) within 24 h. Since the fourth slice was homogenized for molecular analyses, the back of the third slice was imaged for the fourth slice infarct data. After acquiring images, infarct volume was measured by an investigator blinded to the treatment conditions by manually outlining in Adobe Photoshop CS3 the margins of surviving normal gray matter in both hemispheres and subtracting the healthy tissue area in the ipsilateral hemisphere from the total area of the contralateral hemisphere (Swanson et al. 1990). This indirect method accurately measures infarct size and minimizes the error due to edema, which enlarges and distorts the margins of the infarcted region. Cortical and striatal infarct area was calculated for each slice, and the total volume of infarct for each animal was calculated by multiplying the infarcted area by the thickness of the slice and summing the volume of the six slices.
Percent brain swelling was calculated as described previously (Strbian et al. 2006). Briefly, volumetric growth of the ipsilateral hemisphere was compared to the contralateral hemisphere as percentage of brain swelling = [(ipsilateral hemisphere volume / contralateral hemisphere volume) − 1] × 100.
ELISAs
The permeability of the BBB was investigated by the extravasation of IgG from the blood into the brain parenchyma using an IgG ELISA kit from Immunology Consultants Laboratory, Inc (Cat. No. E-25G; Portland, OR, USA) according to the manufacturer’s instructions. Brain tissue homogenates were loaded at 50 μg total protein per well.
Extravasation of hemoglobin (Hb) into the brain was also measured as an indication of hemorrhagic transformation, micro-hemorrhaging, and BBB permeability using a Hb ELISA kit (Immunology Consultants Laboratory, Inc, Cat. No. E-25HM) following the instructions from the manufacturer. Brain tissue homogenates were loaded at 15 μg total protein per well.
Cell Culture
HT-1080 human fibrosarcoma cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in DMEM:F12 medium (Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Cat. No. 10082-147; Life Technologies), 100 U/mL penicillin and 100 μg/mL streptomycin in a humidified incubator at 37° C and 5% CO2. Once cells were at 80-85% confluence, they were washed with Dulbecco’s PBS and fresh media without FBS was added. After 24 h, cell culture media was collected and spun down at 5,000 xg for 10 min at 4° C. Aliquots of the media were prepared and stored at −80° C until use.
Gelatin substrate zymography for MMP-2 and MMP-9
Determination of MMP-2 and MMP-9 activity was done on brain homogenates and HT-1080 conditioned media using gelatin substrate zymography as previously described (Zhang & Gottschall 1997). Brain homogenates (50 μg total protein) were mixed with 10 μL zymogram loading buffer (Cat. No. 161-0764; Bio-Rad, Hercules, CA, USA) were loaded in the gels and HT-1080 conditioned media (15 μL) at 1:10 dilution in zymogram sample buffer was used as a positive control. Proteins were separated by electrophoresis in a SDS-PAGE gel (8%) containing 0.1% (w/v) gelatin (Cat. No. G-2500; Sigma-Aldrich) at a constant 150 V. Gels were then washed twice in 2.5% Triton X-100 for 20 minutes to remove SDS then incubated for 24 h at 37°C in zymogram incubation buffer (50 mM Tris-HCl pH 7.6 containing 200 mM NaCl, 10 mM CaCl2, 0.02% Brij® L23, 0.02% NaN3). Gels were then stained with Coomassie Brilliant Blue R solution (Cat. No. B6529; Sigma-Aldrich) for 1 h then destained in 10% acetic acid for one day. Gels were scanned using an HP Scanjet 8300 scanner. Densitometric analysis of lytic zones at 94 and 88 kDa for MMP-9 and 72 kDa for MMP-2 were performed using ImageJ (NIH). Rat MMP-9 is comprised of two bands running in the gels at 94 kDa (glycosylated form) and 88 kDa (intermediate form) (Zhang & Gottschall 1997).
Fluorometric immunocapture assay of MMP-3 enzymatic activity
Levels of active MMP-3 were determined using a fluorescence resonance energy transfer (FRET) peptide immunocapture assay as previously described (Hawkins et al. 2013, Candelario-Jalil et al. 2011). Briefly, 96-well plates were coated in protein A/G and then incubated at room temperature for 2 h with rabbit polyclonal anti-MMP-3 (Cat. No. sc-6839-R; Santa Cruz Biotechnology, Dallas, TX, USA).
Homogenized brain tissue was added at 50 μg total protein per well and allowed to incubate overnight at 4°C. Wells were washed and 5-FAM/QXL™520 FRET peptide was added (Cat. No. 60580-01; AnaSpec, San Jose, CA, USA). Plates were incubated for 24 h at 37°C, then relative fluorescence units (RFUs) were read and monitored at excitation / emission wavelengths of 485 / 528 nm in a Synergy HT Multi-mode microplate fluorescence reader (BioTek, Winooski, VT, USA) running Gen5™ data analysis software. A substrate control well was used to subtract baseline fluorescence from sample wells.
Immunoblotting
Equal protein amounts (50 μg) of brain tissue homogenates were separated by SDS-PAGE in appropriate reducing conditions for myeloperoxidase (MPO), CD68, glial fibrillary acidic protein (GFAP), intracellular adhesion molecule-1 (ICAM-1), zona occludens-1 (ZO-1), occludin, claudin-5, and type IV collagen (col-IV). After separation, proteins were transferred to Immobilon-FL polyvinylidene fluoride (PVDF) membranes using a Trans-Blot Turbo apparatus (Bio-Rad) at 25 V for 30 min. Membranes were blocked at room temperature for 1 h in 5% non-fat milk in TBS. Membranes were then incubated at 4°C overnight with one of the following primary antibodies diluted in 5% non-fat milk in TBST: anti-MPO (Cat. No. sc-16128-R; Santa Cruz Biotechnology; 1:700), anti-CD68 (MCA341R; AbD Serotec, Raleigh, NC, USA; 1:750), anti-GFAP (MCA5C10, EnCor Biotechnology Inc, Gainesville, FL, USA; 1:5000), anti-ICAM-1 (AF583; R&D Systems, Minneapolis, MN, USA; 1:2000), anti-ZO-1 (61-7300; Invitrogen, Grand Island, NY, USA; 1:1000), anti-occludin (71-1500; Invitrogen; 1:1000), anti-claudin-5 (35-2500; Invitrogen; 1:1000), or anti-collagen-IV (ab6586; Abcam; 1:2000). Membranes were then incubated with an IRDye 800CW secondary antibody (Li-Cor, Lincoln, NE, USA; 1:30,000) and protein bands were visualized with an Odyssey infrared scanner (Li-Cor). Actin normalization was done by incubating membranes with anti-actin (A1978; Sigma-Aldrich; 1:10,000) for 1 h followed by 1 h with IRDye 680LT (Li-Cor; 1:40,000).
Statistical methods
GraphPad Prism 5 (GraphPad Software Incorporated, San Diego, CA, USA) was used for statistical analysis. To assess statistical significance, a Student’s t-test, one-way ANOVA or two-way ANOVA followed by Bonferroni posttests were used. Bar graphs are shown as the mean ± SEM. Probability P < 0.05 was considered statistically significant.
Results
Infarct size and edema is decreased in ALX agonist-treated rats
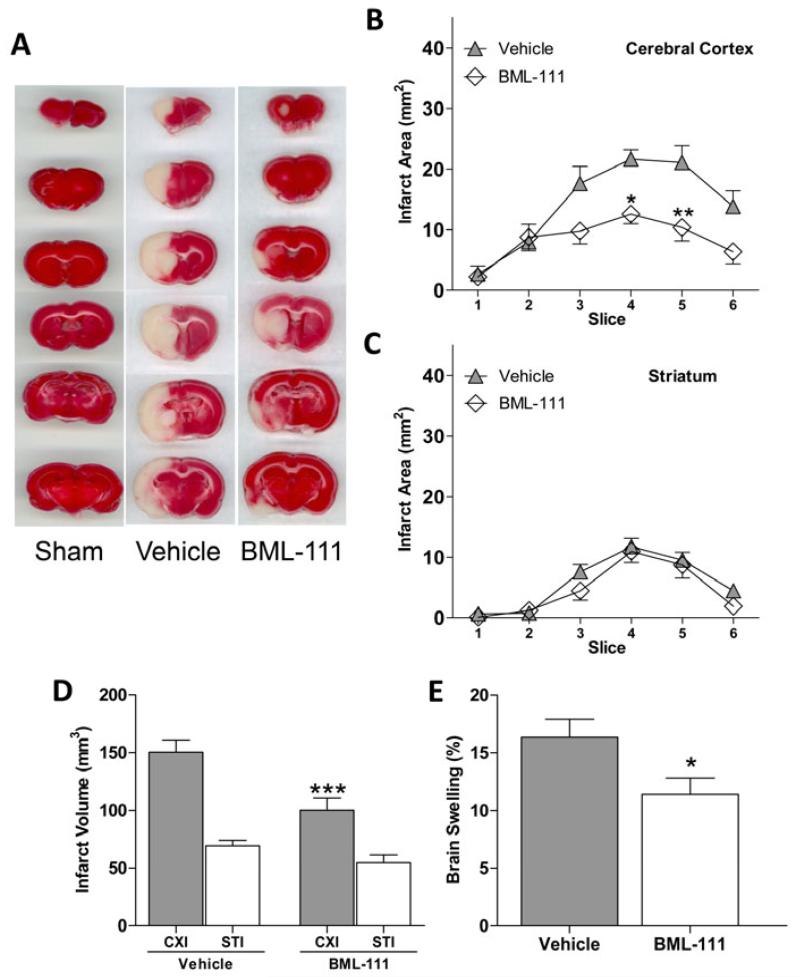
Infarct size was measured using TTC staining at 48 h of reperfusion (represented in Figure 1A). Post-treatment with BML-111 is sufficient to reduce the area of infarcted tissue in the cerebral cortex (Figure 1B, P < 0.05 and P < 0.01, respectively), and no change in infarct area in the striatum was observed (Figure 1C). Figure 1D shows a large decrease in infarct volume in the cerebral cortex when BML-111 was administered compared to animals that received the vehicle (P < 0.001), and no significant change was observed in the striatum. Brain swelling was also significantly reduced with BML-111 treatment compared to vehicle-treated rats (Figure 1E, P < 0.05).
Figure 1. Effects on infarct size and edema from ALX agonist treatment.
(A) Representative TTC staining of brain slices 1-6 from sham, vehicle, and BML-111 groups. (B) Comparison between vehicle and BML-111-treated rats of the area of the infarct in the cerebral cortex and (C) the striatum in six brain slices. Treatment with BML-111 had a significant effect on infarct area (two-way ANOVA; P < 0.0001, n = 12-15) in the cerebral cortex but not the striatum. Area of infarct in cerebral cortex slices 4 and 5 from rats treated with BML-111 were significantly smaller than the slices from vehicle animals. Bonferroni posttests, * P < 0.05, ** P < 0.01 versus vehicle, n = 12-15. (D) Comparison of the volume of infarct in vehicle- and BML-111-treated rats. Administration of BML-111 significantly reduced infarct volume in the cerebral cortex but not the striatum compared to vehicle. Student’s t test, ***P < 0.001 versus ipsilateral cerebral cortex of vehicle animals, n = 12-15. (E) Brain swelling in rats treated with the vehicle or BML-111. Brain swelling was significantly lessened in rats that received BML-111 compared to those that received the vehicle. Student’s t test, * P < 0.05 versus vehicle, n = 12-15. CXI/CXC = cerebral cortex ipsilateral/contralateral. STI/STC = striatum ipsilateral/contralateral.
BML-111 protects the BBB and reduces hemorrhagic transformation
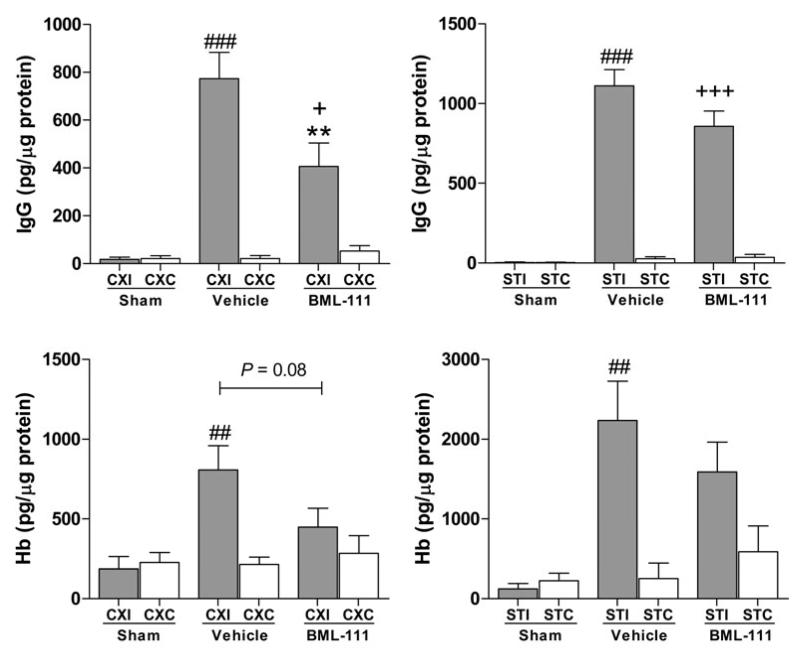
Blood-brain barrier permeability was measured by examining IgG extravasation into the brain parenchyma. Administration of BML-111 post-ischemically greatly reduced levels of IgG in the ipsilateral cerebral cortex compared to injection of the vehicle (Figure 2A, P < 0.01). No change was seen between treatment groups in the ipsilateral striatum (Figure 2B). Levels of IgG in the vehicle group ipsilateral cerebral cortex were significantly greater than the sham group while there was no significant difference between BML-111 treatment and sham ipsilateral cerebral cortex levels (Figure 2A). In the striatum, both BML-111 and vehicle treatment groups have significantly more IgG in the ipsilateral striatum compared to the sham group (Figure 2B). In both vehicle and BML-111 treatment groups, IgG levels were significantly elevated in the ipsilateral regions compared to the contralateral regions (Figure 2A and 2B).
Figure. 2. Effects of BML-111 on BBB permeability and hemorrhagic transformation.
Rats treated with BML-111 had reduced IgG in the ipsilateral cerebral cortex (A), but not the striatum (B), compared to rats in the vehicle group. Contralateral IgG levels were significantly lower compared to the ipsilateral hemisphere levels for both vehicle and BML-111 groups. Contralateral levels of IgG are not different from the sham group. One-way ANOVA with Bonferroni posttests, ** P < 0.01 versus ipsilateral vehicle-injected rats; ### P < 0.001 versus contralateral vehicle group; + P < 0.05, +++ P < 0.001 versus contralateral BML-111 group, n = 12 – 15. C) Hemoglobin (Hb) extravasation was lessened with BML-111 treatment in the ipsilateral cerebral cortex compared to vehicle-treated animals (P = 0.08) and was not significantly different from sham levels of Hb, n =12 – 15. D) No significant reduction was observed in the ipsilateral striatum between groups, n = 9 – 10. In both brain regions, vehicle-treated animals had significantly higher Hb extravasation in the ipsilateral hemisphere compared to the contralateral hemisphere, whereas no significant difference was seen between hemispheres in the BML-111 group. Sham levels of Hb were not different from contralateral levels of Hb. Student’s t test and one-way ANOVA with Bonferroni posttests, * P < 0.05 versus ipsilateral vehicle levels; ## P < 0.01 versus contralateral vehicle levels. CXI/CXC = cerebral cortex ipsilateral/contralateral. STI/STC = striatum ipsilateral/contralateral.
To study the effects of BML-111 treatment on hemorrhagic transformation, Hb extravasation into the brain was measured using a highly sensitive ELISA kit. Injections of BML-111 10 min and 24 h after 90 min of MCAO decreased amounts of Hb in the ipsilateral cerebral cortex compared to the vehicle-treated rats, as seen in Figure 2C, but did not reach statistical significance (P = 0.08). Again, this reduction was not seen in the ipsilateral striatum (Figure 2D). For vehicle-injected animals, levels of Hb in the ipsilateral cerebral cortex and striatum were significantly increased compared to the contralateral regions; however, Hb levels from BML-111-treated animals were not significantly raised in the ipsilateral regions compared to the contralateral regions (Figure 2C and 2D). Hb levels following BML-111 and vehicle treatments were not significantly increased compared to sham in the ipsilateral cerebral cortex (Figure 2C). In the ipsilateral striatum, vehicle-treated animals had significantly more Hb compared to the sham, while BML-111-treated rats do not have significantly raised levels of Hb compared to the sham (Figure 2D). Since no changes were observed in the sham condition compared to the contralateral hemisphere for BBB opening markers IgG and Hb, for the following experiments the contralateral region of the brain was used as the control.
BML-111 reduces MMP activity
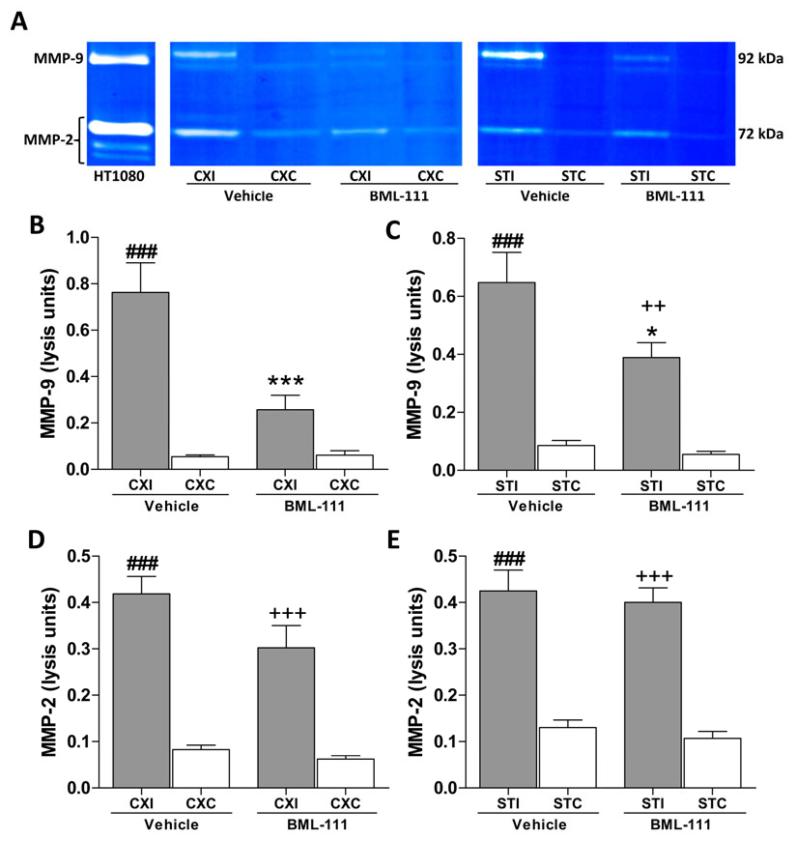
We investigated the effects of activation of ALX with BML-111 treatment on the levels of MMP-2/-9 in the cortex and striatum of rats following a 90 min transient focal cerebral ischemia and 48 h of reperfusion. Gelatin-substrate zymography was used to estimate levels of MMP-9 and MMP-2 as determined by the densitometric analysis of the 88 kDa and 94 kDa bands for MMP-9 and a 72 kDa band for MMP-2.
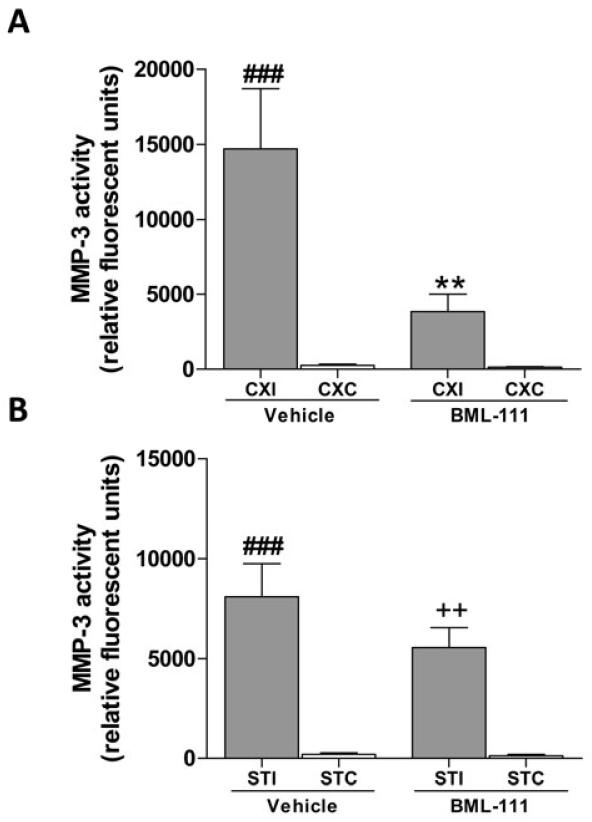
Figure 3A represents a typical zymogram comparing MMP-2/-9 in BML-111 and vehicle groups. Activation of ALX post-ischemia using the agonist BML-111 significantly reduced the levels of MMP-9 in the ipsilateral cerebral cortex and striatum compared to vehicle-treated animals (Figure 3B and 3C, P < 0.001 and P < 0.05, respectively). In the ipsilateral cerebral cortex of BML-111-treated rats, levels of MMP-9 did not significantly differ from the respective contralateral cerebral cortex whereas levels of MMP-9 in the vehicle rats’ cerebral cortex were significantly higher than the contralateral region (Figure 3B). However, in the striatum, MMP-9 was elevated in the stroke side for both treatment groups compared to the contralateral striatum (Figure 3C). No significant changes in MMP-2 levels were observed in the ipsilateral cerebral cortex or the striatum between treatments, despite a large increase in ipsilateral amounts of MMP-2 compared to the respective contralateral regions (Figure 3D and 3E). Using a novel fluorometric immunocapture assay, MMP-3 (stromelysin-1) activity was also measured. Levels of active MMP-3 were greatly reduced in the ipsilateral cerebral cortex of rats treated with BML-111 compared with rats that received the vehicle (Figure 4A, P < 0.01). MMP-3 activity was not significantly higher in the ipsilateral cortex of BML-111-treated rats compared to the contralateral cortex, whereas activity was increased in the ipsilateral cortex of vehicle-treated rats compared to the contralateral cortex (Figure 4A). No significant change was detected in MMP-3 levels in the ipsilateral striatum between treatments, and for both treatments the ipsilateral MMP-3 activity was significantly greater compared to contralateral levels (Figure 4B).
Figure 3. MMP-2 and -9 activity in BML-111- and vehicle-treated rats with a 90 min MCAO and 48 h reperfusion.
A) Representative gelatin zymogram showing the effects of treatment with the ALX agonist BML-111 on MMP-2 and -9 activity. HT1080 cultured media was loaded as a positive control for normalization to allow comparisons across multiple gels. Rat MMP-2 bands run at 72 kDa and MMP-9 comprises of two bands running in the gels at 94 kDa and 88 kDa. B and C) Administration of BML-111 reduced levels of MMP-9 in the ipsilateral cerebral cortex and the striatum compared to rats that received the vehicle. MMP-9 levels in the vehicle-treated animals’ ipsilateral cerebral cortex and striatum where significantly higher than the corresponding contralateral region, while only the ipsilateral striatum of BML-111-treated rats were higher than the respective contralateral region. One-way ANOVA with Bonferroni posttests, * P < 0.05, *** P < 0.001 versus ipsilateral vehicle region; ### P < 0.001 versus contralateral vehicle levels; ++ P < 0.01 versus contralateral BML-111-treated levels, n = 11 – 15. D and E) No significant effects were observed on levels of MMP-2 following treatment with the ALX agonist BML-111. Levels of MMP-2 were significantly elevated in the ipsilateral regions compared to their respective contralateral regions in both the cerebral cortex and striatum. One-way ANOVA with Bonferroni posttests, ### P < 0.001 versus contralateral levels in the vehicle group; +++ P < 0.001 versus contralateral levels in the BML-111 group, n = 11 – 15. CXI/CXC = cerebral cortex ipsilateral/contralateral. STI/STC = striatum ipsilateral/contralateral.
Figure 4. Activity of MMP-3 after treatment with BML-111 or the vehicle following transient focal cerebral ischemia.
Fluorometric immunocapture assay of MMP-3 activity in the cerebral cortex and striatum of rats treated with BML-111 or the vehicle. A) Administration of BML-111 greatly reduced the activity of MMP-3 in the affected cerebral cortex compared to MMP-3 activity in the cerebral cortex of vehicle-treated rats. Levels of MMP-3 activity were not significantly elevated in the ipsilateral cortex of the BML-111 group compared to the contralateral hemisphere. MMP-3 activity was significantly higher in the vehicle treatment condition in the ipsilateral cerebral cortex compared to the contralateral region. B) No effect was observed between treatment groups in the ipsilateral striatum. Both treatment conditions had elevated MMP-3 activity in the ipsilateral striatum compared to the contralateral striatum. One-way ANOVA with Bonferroni posttests, ** P < 0.01 versus ipsilateral vehicle cortex; ### P < 0.001 versus contralateral vehicle region; ++ P < 0.01 versus contralateral BML-111-treatment region, n = 11 – 14. CXI/CXC = cerebral cortex ipsilateral/contralateral. STI/STC = striatum ipsilateral/contralateral.
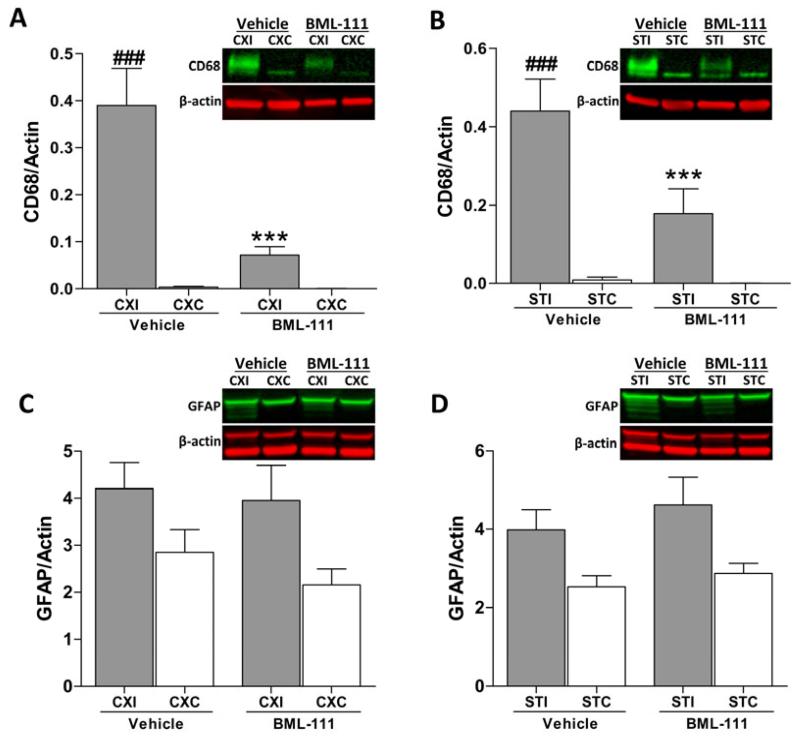
ALX agonist decreases inflammatory response in microglia
To determine whether glial activation from inflammation was attenuated by BML-111 administration, immunoblots were done to examine levels of CD68, a marker for microglial activation, and glial fibrillary acidic protein (GFAP) which is elevated during astrogliosis. In rats that received the vehicle, CD68 was dramatically increased in the ipsilateral cerebral cortex and striatum in contrast to the contralateral areas (Figure 5A and 5B, P < 0.001). With administration of BML-111, CD68 expression was greatly reduced in both the cerebral cortex and striatum compared to the vehicle treatment group, and ipsilateral levels were not different from contralateral levels (Figure 5A and 5B, P < 0.001). No difference was observed in GFAP levels between BML-111 and vehicle groups, and no significant change was seen in the ipsilateral hemisphere compared to the contralateral hemisphere (Figure 5C and 5D).
Figure 5. Post-ischemic administration of BML-111 reduces inflammatory response from microglia.
A-B) Microglial activation, as seen with the marker CD68, was significantly reduced in the cerebral cortex and striatum of rats treated with the ALX agonist BML-111 compared to rats that received the vehicle. CD68 was also not significantly increased in the ipsilateral hemisphere of the BML-111 group compared to the contralateral side, whereas microglial activation was greatly increased in the ipsilateral hemisphere of the vehicle rats compared to the contralateral hemisphere. Representative immunoblots are shown where CD68 was quantified as a band running at 90-110 kDa and normalized to β-actin. One-way ANOVA with Bonferroni posttests, *** P < 0.001 versus ipsilateral vehicle group; ### P < 0.001 versus contralateral vehicle region, n = 11 – 15. C-D) Glial fibrillary acidic protein (GFAP) was used as a marker for astrogliosis. Treatment with BML-111 did not alter astrogliosis compared to vehicle administration. No difference was observed between ipsilateral and contralateral hemispheres. A representative immunoblot is shown in which GFAP was quantified as a 55 kDa band with all lower molecular weight bands that are products from alternate transcripts from the single gene. One-way ANOVA with Bonferroni posttests, n = 12 – 15. CXI/CXC = cerebral cortex ipsilateral/contralateral. STI/STC = striatum ipsilateral/contralateral.
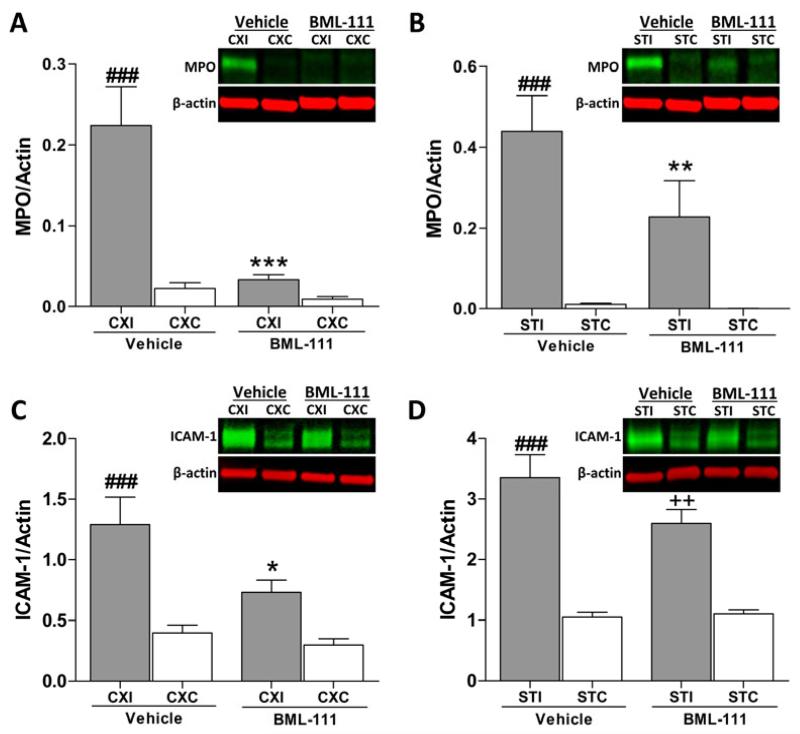
Post-ischemic BML-111 treatment inhibits neutrophil recruitment into the ischemic brain
To elucidate whether treatment with BML-111 would reduce recruitment of neutrophils, levels of MPO and ICAM-1 were determined using immunoblotting. Following a 90 min transient middle cerebral artery occlusion and 48 h of reperfusion, MPO was elevated in the vehicle group in both the ipsilateral cerebral cortex and striatum compared to the contralateral regions (Figure 6A and 6B, P < 0.001). Treatment with the ALX agonist BML-111 attenuated MPO expression in both the cerebral cortex and striatum compared to the vehicle group (P < 0.001 and P < 0.01, respectively) such that there was not a significant difference compared to the contralateral hemisphere of the BML-111 group (Figure 6A and 6B). Figure 6C shows a reduction in ICAM-1 in the cerebral cortex of BML-111-treated animals compared to the elevated levels in the vehicle cerebral cortex (P < 0.05). In the striatum, no reduction was observed between vehicle and BML-111 groups (Figure 6D). Both vehicle and BML-111 treatment groups have significantly higher levels of ICAM-1 in the ipsilateral striatum compared to the contralateral side (Figure 6D, P < 0.001 and P < 0.01, respectively).
Figure 6. Effects of treatment with ALX agonist BML-111 on markers of inflammation after a 90 min ischemic stroke.
A-B) Treatment with BML-111 significantly reduced levels of myeloperoxidase (MPO) in the ipsilateral cerebral cortex and striatum compared to rats treated with the vehicle. Levels of MPO in the ipsilateral hemisphere were elevated in the vehicle group but not in the BML-111 group compared to the contralateral hemisphere. Representative immunoblots are shown, with the heavy chain of MPO quantified as a 55-60 kDa band. One-way ANOVA with Bonferroni posttests, *** P < 0.001, ** P < 0.01 versus ipsilateral vehicle region; ### P < 0.001 versus vehicle contralateral region, n = 11 – 15. C-D) Levels of intracellular adhesion molecule (ICAM)-1 were significantly decreased in the ipsilateral cerebral cortex, but not the ipsilateral striatum, of BML-111-treated rats compared to vehicle animals. ICAM-1 expression was increased in all ipsilateral regions compared to contralateral regions except for the cerebral cortex of animals that received BML-111. Representative immunoblots are shown, with ICAM-1 as a 70-85 kDa band. One-way ANOVA with Bonferroni posttests, * P < 0.05 versus ipsilateral vehicle cortex; ### P < 0.001 versus vehicle contralateral region; ++ P < 0.01 versus BML-111 contralateral striatum, n = 11 – 15. CXI/CXC = cerebral cortex ipsilateral/contralateral. STI/STC = striatum ipsilateral/contralateral.
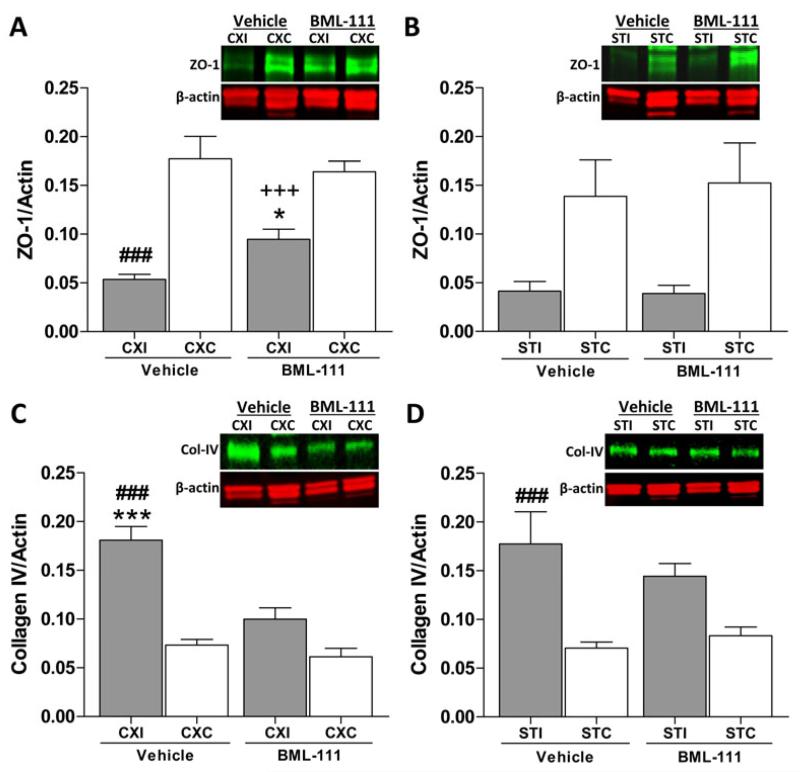
BML-111 protects ZO-1 from degradation and inhibits collagen-IV increase
Using immunoblotting, we examined the effects of activating ALX on the preservation of tight junction protein zona occludens-1 (ZO-1) and a basement membrane component, collagen-IV (col-IV) after a transient focal cerebral ischemia. Treatment with BML-111 did provide protection from degradation to ZO-1 in the ipsilateral cerebral cortex compared to the vehicle group (Figure 7A, P < 0.05). Levels of ZO-1 in the ipsilateral cerebral cortex for both groups were significantly lower than the contralateral region (Figure 7A, P < 0.001). No differences were observed in the striatum between groups or between hemispheres, probably due to high variability found in the ZO-1 content in contralateral regions (Figure 7B).
Figure 7. Tight junction proteins and type IV collagen after treatment with BML-111 following 90 min MCAO.
A) Zona occludens-1 (ZO-1) is protected from degradation in the ipsilateral cerebral cortex when BML-111 is administered compared to vehicle treatment, but not to the levels in the unaffected cerebral cortex. B) No protection to ZO-1 is seen in the striatum following treatment with BML-111. No significant differences were observed between the ipsilateral and contralateral hemispheres. Representative immunoblots are shown with ZO-1 quantified as a band running at 220 kDa. One-way ANOVA with Bonferroni posttests, * P < 0.05 versus ipsilateral vehicle group; ### P < 0.001 versus contralateral vehicle region; +++ P < 0.001 versus contralateral BML-111 region, n = 12 – 15. C) A significant increase in collagen type IV (Col-IV) was seen in the vehicle-treated group compared to the BML-111-treated group in the ipsilateral cerebral cortex and the contralateral cerebral cortex of vehicle-treated animals. Col-IV did not differ in the ipsilateral cerebral cortex compared to the contralateral region for that group. D) Ipsilateral striatum levels of Col-IV did not differ between groups. Col-IV was elevated in the ipsilateral striatum of vehicle-treated animals compared to the respective contralateral area. Immunoblots show intact Col-IV running at 220 kDa. One-way ANOVA with Bonferroni posttests, *** P < 0.001 versus ipsilateral BML-111 treatment; ### P < 0.001 versus contralateral vehicle region, n = 11 – 15. CXI/CXC = cerebral cortex ipsilateral/contralateral. STI/STC = striatum ipsilateral/contralateral.
Amount of intact col-IV was markedly increased in the vehicle group ipsilateral cerebral cortex compared to the contralateral cerebral cortex and to the BML-111 treatment group ipsilateral cerebral cortex (Figure 7C, P < 0.001). Col-IV in the ipsilateral cerebral cortex of rats that were administered BML-111 was not different from the respective contralateral region (Figure 7C). In the striatum, vehicle-treated animals had an elevated amount of col-IV on the ipsilateral side compared to the contralateral area (Figure 7D, P < 0.001). Post-ischemic treatment with BML-111 did not significantly alter the levels of col-IV in the ipsilateral striatum compared to the respective contralateral region or to the vehicle ipsilateral striatum (Figure 7D).
Tight junction proteins occludin and claudin-5 were also examined and no effects from treatment were observed (Supplementary Figure 1).
Discussion
In the present study, we examined the effects of post-ischemic administration of BML-111, an ALX agonist, in a rat model of focal cerebral ischemia. We chose a time point of 48 h to determine whether BML-111 could reduce the inflammatory effects of ischemic stroke in the acute phase of the injury, as we have shown before that BBB permeability is maximal at this time point in our rat model (Candelario-Jalil et al. 2007). Our results show that treatment with BML-111 significantly reduces infarct size and provides protection to the cerebral cortex, likely by decreasing BBB permeability, reducing levels of MMP-9 and MMP-3, limiting microglial activation and neutrophil infiltration, and protecting the tight junction protein ZO-1. These results support the hypothesis that post-ischemic injections of BML-111 provide neuroprotection and reduce inflammation following ischemic stroke.
During ischemia, tissue in the immediate area of the occlusion, or the core infarct, will succumb within minutes to necrotic cell death. Inflammatory cells migrating to the penumbra, the potentially recoverable tissue surrounding the infarct core, will cause progressive damage and apoptotic neuronal cell death. In our rat model of ischemic stroke, occlusion of the middle cerebral artery results in rapid cell death in the lateral striatum, where the majority of the infarct core is located, while most of the outlying cerebral cortex will succumb to delayed cell death mainly due to spreading inflammation. Our results show that BML-111 reduced infarct size in the cerebral cortex and not the striatum, suggesting that BML-111 attenuated the spreading inflammation into the cerebral cortex.
Neuroinflammation alters the permeability of the BBB through changes in endothelial status, cytokine and chemokine expression, and peripheral immune cell recruitment (Zlokovic 2008). Disruption of the BBB and high levels of MMPs are associated with edema, hemorrhagic transformation, and worse outcome in stroke victims (Desilles et al. 2013, Warach & Latour 2004, Latour et al. 2004). Previous studies have shown a biphasic opening of the BBB in focal cerebral ischemia in rats where an early opening occurs around 2-3 h after reperfusion, and a late opening 48 h following reperfusion (Candelario-Jalil et al. 2007, Rosenberg et al. 1998). Gelatinase A (MMP-2) is implicated in the early opening whereas gelatinase B (MMP-9) is maximally elevated during the late opening (Rosenberg et al. 1998, Yang et al. 2007). MMP-9 is released from microglia and astrocytes as a zymogen and must be cleaved by stromelysin-1 (MMP-3) to become catalytically active (Ogata et al. 1992) while invading neutrophils release active MMP-9 (Rosell et al. 2008, Gidday et al. 2005). MMP-9 breaks down the extracellular matrix (ECM) and tight junction proteins (TJPs) of the BBB (Candelario-Jalil et al. 2011, Yang et al. 2007). MMP-3 has also been associated with opening of the BBB in mice 24 h after intracerebral injections of lipopolysaccharide (LPS) (Gurney et al. 2006). In ischemia, MMP-3 is a critical mediator of BBB damage and intracranial bleeding after tPA treatment of ischemic stroke (Suzuki et al. 2007).
In our study, rats were sacrificed 48 h after reperfusion, during the late opening phase of the BBB. At this time point, we have previously shown that there is a large increase in BBB permeability associated with a dramatic increase in neutrophil infiltration and vasogenic edema (Candelario-Jalil et al. 2007). Injections of BML-111 reduced levels of MMP-9 in both the cerebral cortex and striatum and MMP-3 in the cerebral cortex of ischemic rat brains, suggesting this may be a mechanism through which BML-111 decreased edema, BBB permeability, and hemorrhagic transformation in the cerebral cortex. MMP-2 levels were elevated in the ipsilateral hemisphere compared to the contralateral hemisphere perhaps implying MMP-2 is contributing to BBB damage but is not a critical factor.
Occlusion of a major blood vessel stops oxygen and glucose from reaching the nearby brain tissue, creating an excitotoxic environment which activates microglia and results in astrogliosis, triggering an inflammatory cascade (Iadecola & Anrather 2011). CD68 is expressed on activated microglia and GFAP is increased during astrogliosis. We found that activation of ALX with BML-111 reduced levels of CD68 in both the cerebral cortex and striatum, demonstrating that BML-111 decreases microglial activation and thus reduces inflammation. Administration of BML-111 did not significantly reduce GFAP; however, this finding may be due to high variability of GFAP content in the samples.
Pro-inflammatory cytokines and chemokines produced by reactive astrocytes and activated microglia will recruit neutrophils into the parenchyma and enhance endothelial adhesion molecule expression on the surface of vascular endothelial cells thus promoting neutrophilic extravasation (Iadecola & Anrather 2011, Zlokovic 2008). Our findings show BML-111 inhibited ICAM-1 expression in the cerebral cortex, suggesting that treatment is limiting neutrophil adhesion in this region. MPO is a marker for neutrophil infiltration 48 h following reperfusion (Breckwoldt et al. 2008). Our results show that treatment with BML-111 decreased MPO levels in the cerebral cortex and striatum, indicating activation of ALX inhibits neutrophil recruitment into the brain parenchyma following ischemic stroke.
BML-111 administration is likely reducing BBB permeability by decreasing MMP-9 and MMP-3 levels and inhibiting neutrophil extravasation which may provide protection to the TJPs of the BBB. Endothelial cells lining the vasculature are tightly zipped together by TJPs such as ZO-1, occludin, and claudin-5 (Hawkins & Davis 2005). Previous experiments indicate occludin is not necessary for tight junction (TJ) formation and will only localize to TJs, while claudin-5 is suspected to form the primary seal of TJs (Hawkins & Davis 2005, Kubota et al. 1999). Thus, occludin acts more as an addition support structure of the TJ, though disruption of occludin is associated with BBB permeability after hypoxia (Hawkins & Davis 2005, Brown & Davis 2005). ZO-1 is an accessory cytoplasmic TJP that links the transmembrane TJPs occludin and claudin-5 to the actin cytoskeleton, and dissociation of ZO-1 from the TJ complex is associated with increased permeability after hypoxic insult (Fanning et al. 1998, Fischer et al. 2002). We found that administering BML-111 post-ischemically protected ZO-1 from degradation, but not occludin or claudin-5, suggesting activating ALX prevents dissociation of ZO-1 from the TJ complex but does not inhibit degradation of occludin or claudin-5. However, we did not observe a change in occludin or claudin-5 between the ipsilateral and contralateral hemispheres, indicating that they were not significantly degraded by ischemia in either group.
Brain vasculature and endothelial cells are supported by an underlying ECM comprised mostly of collagen IV, fibronectin, and laminin (Hawkins & Davis 2005, Zlokovic 2008). We examined the effects of BML-111 treatment on col-IV degradation. Our results show that col-IV in the ipsilateral hemisphere is no different in the cerebral cortex and striatum compared to the contralateral hemisphere with treatment of BML-111. However, without treatment, levels of col-IV are dramatically increased in the cerebral cortex compared to the BML-111 group. Some studies have shown ECM proteins like col-IV to have increased expression following ischemia (Ji & Tsirka 2012, Jucker et al. 1993) while others report reduced levels of col-IV (Hamann et al. 2002, Burggraf et al. 2003). Differences in study design, such as length of ischemia, reperfusion, and time of sacrifice can contribute to the difference in col-IV after ischemia. Treatment with BML-111 inhibits the increase of col-IV in the ipsilateral cerebral cortex. Early angiogenesis may be responsible for the increase in col-IV in the ipsilateral cerebral cortex in vehicle rats. Early formation of new blood vessels with incomplete TJs and ECMs may contribute to BBB leakage and edema. In our study, BML-111 treatment could be limiting early angiogenesis and therefore reducing new col-IV production. Inhibiting early angiogenesis could also contribute to the effects of BML-111 administration on reducing BBB permeability and brain edema.
In conclusion, these results demonstrate that post-ischemic intravenous injections of BML-111 provide neurovascular protection and reduce infarct size 48 h after reperfusion in a rat model of ischemic stroke. Specifically, administration of the ALX agonist BML-111 decreased BBB permeability by resolving microglial activation, which led to reduced neutrophil infiltration, decreased levels of MMP-9 and MMP-3, and protection of the TJs. BML-111 may also be limiting early angiogenesis, indicated by the lack of elevation of col-IV in the cerebral cortex. While BML-111 did attenuate levels of MMP-9, CD68, and MPO in the striatum, it was not sufficient to significantly reduce infarct size or BBB permeability because so much of the striatum succumbs to necrosis very soon after occlusion. Our study highlights the possibilities of new therapeutics based on LXA4 analogues and ALX agonists to provide protection to the neurovascular unit in many diseases. Further research is necessary to elucidate whether targeting ALX to resolve neuroinflammation is protective up to weeks following ischemia and whether long-term functional recovery is possible.
Supplementary Material
Acknowledgements
This research was partly supported by a grant from the NIH (NINDS; R01 grant NS065849) to ECJ. Additional support for this project was provided by the Brain and Spinal Cord Injury Research Trust Fund, McKnight Brain Institute, University of Florida.
ABBREVIATIONS
- ALX
lipoxin A4 receptor
- BBB
blood-brain barrier
- CCA
common carotid artery
- Col-IV
collagen IV
- ECA
external carotid artery
- FBS
fetal bovine serum
- FRET
fluorescence resonance energy transfer
- GFAP
glial fibrillary acidic protein
- Hb
hemoglobin
- ICA
internal carotid artery
- ICAM-1
intracellular adhesion molecule-1
- IgG
immunoglobulin G
- LXA4
lipoxin A4
- MCAO
middle cerebral artery occlusion
- MMP
matrix metalloproteinase
- MPO
myeloperoxidase
- SDS
sodium dodecyl sulfate
- TJPs
tight junction proteins
- ZO-1
zona occludens-1
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
Supporting Information Additional supporting information may be found in the online version of this article on the publisher’s website.
References
- Breckwoldt MO, Chen JW, Stangenberg L, Aikawa E, Rodriguez E, Qiu S, Moskowitz MA, Weissleder R. Tracking the inflammatory response in stroke in vivo by sensing the enzyme myeloperoxidase. Proc Natl Acad Sci U S A. 2008;105:18584–18589. doi: 10.1073/pnas.0803945105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RC, Davis TP. Hypoxia/aglycemia alters expression of occludin and actin in brain endothelial cells. Biochem Biophys Res Commun. 2005;327:1114–1123. doi: 10.1016/j.bbrc.2004.12.123. [DOI] [PubMed] [Google Scholar]
- Burggraf D, Martens HK, Jager G, Hamann GF. Recombinant human tissue plasminogen activator protects the basal lamina in experimental focal cerebral ischemia. Thromb Haemost. 2003;89:1072–1080. [PubMed] [Google Scholar]
- Candelario-Jalil E, Gonzalez-Falcon A, Garcia-Cabrera M, Leon OS, Fiebich BL. Post-ischaemic treatment with the cyclooxygenase-2 inhibitor nimesulide reduces blood-brain barrier disruption and leukocyte infiltration following transient focal cerebral ischaemia in rats. J Neurochem. 2007;100:1108–1120. doi: 10.1111/j.1471-4159.2006.04280.x. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Thompson J, Taheri S, Grossetete M, Adair JC, Edmonds E, Prestopnik J, Wills J, Rosenberg GA. Matrix metalloproteinases are associated with increased blood-brain barrier opening in vascular cognitive impairment. Stroke. 2011;42:1345–1350. doi: 10.1161/STROKEAHA.110.600825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Fierro IM, Gronert K, Serhan CN. Activation of lipoxin A(4) receptors by aspirin-triggered lipoxins and select peptides evokes ligand-specific responses in inflammation. J Exp Med. 2000;191:1197–1208. doi: 10.1084/jem.191.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte FP, Menezes-de-Lima O, Jr., Verri WA, Jr., Cunha FQ, Penido C, Henriques MG. Lipoxin A(4) attenuates zymosan-induced arthritis by modulating endothelin-1 and its effects. Br J Pharmacol. 2010;161:911–924. doi: 10.1111/j.1476-5381.2010.00950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culman J, Nguyen-Ngoc M, Glatz T, Gohlke P, Herdegen T, Zhao Y. Treatment of rats with pioglitazone in the reperfusion phase of focal cerebral ischemia: a preclinical stroke trial. Exp Neurol. 2012;238:243–253. doi: 10.1016/j.expneurol.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Desilles JP, Rouchaud A, Labreuche J, et al. Blood-brain barrier disruption is associated with increased mortality after endovascular therapy. Neurology. 2013;80:844–851. doi: 10.1212/WNL.0b013e31828406de. [DOI] [PubMed] [Google Scholar]
- Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- Fischer S, Wobben M, Marti HH, Renz D, Schaper W. Hypoxia-induced hyperpermeability in brain microvessel endothelial cells involves VEGF-mediated changes in the expression of zonula occludens-1. Microvasc Res. 2002;63:70–80. doi: 10.1006/mvre.2001.2367. [DOI] [PubMed] [Google Scholar]
- Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD, Chan PH, Park TS. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol. 2005;289:H558–568. doi: 10.1152/ajpheart.01275.2004. [DOI] [PubMed] [Google Scholar]
- Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164:1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- Gong J, Guo S, Li HB, Yuan SY, Shang Y, Yao SL. BML-111, a lipoxin receptor agonist, protects haemorrhagic shock-induced acute lung injury in rats. Resuscitation. 2012;83:907–912. doi: 10.1016/j.resuscitation.2011.12.035. [DOI] [PubMed] [Google Scholar]
- Gurney KJ, Estrada EY, Rosenberg GA. Blood-brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol Dis. 2006;23:87–96. doi: 10.1016/j.nbd.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Hamann GF, Liebetrau M, Martens H, Burggraf D, Kloss CU, Bultemeier G, Wunderlich N, Jager G, Pfefferkorn T. Microvascular basal lamina injury after experimental focal cerebral ischemia and reperfusion in the rat. J Cereb Blood Flow Metab. 2002;22:526–533. doi: 10.1097/00004647-200205000-00004. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Hawkins KE, DeMars KM, Yang C, Rosenberg GA, Candelario-Jalil E. Fluorometric immunocapture assay for the specific measurement of matrix metalloproteinase-9 activity in biological samples: application to brain and plasma from rats with ischemic stroke. Mol Brain. 2013;6:14. doi: 10.1186/1756-6606-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Cooper D, Arumugam TV, Zhang JH, Nanda A, Granger DN. Platelet-leukocyte-endothelial cell interactions after middle cerebral artery occlusion and reperfusion. J Cereb Blood Flow Metab. 2004;24:907–915. doi: 10.1097/01.WCB.0000132690.96836.7F. [DOI] [PubMed] [Google Scholar]
- Ji K, Tsirka SE. Inflammation modulates expression of laminin in the central nervous system following ischemic injury. J Neuroinflammation. 2012;9:159. doi: 10.1186/1742-2094-9-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo SP, Xie W, Xiong X, Xu B, Zhao H. Ischemic postconditioning protects against focal cerebral ischemia by inhibiting brain inflammation while attenuating peripheral lymphopenia in mice. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker M, Bialobok P, Kleinman HK, Walker LC, Hagg T, Ingram DK. Laminin-like and laminin-binding protein-like immunoreactive astrocytes in rat hippocampus after transient ischemia. Antibody to laminin-binding protein is a sensitive marker of neural injury and degeneration. Ann N Y Acad Sci. 1993;679:245–252. doi: 10.1111/j.1749-6632.1993.tb18304.x. [DOI] [PubMed] [Google Scholar]
- Kubota K, Furuse M, Sasaki H, Sonoda N, Fujita K, Nagafuchi A, Tsukita S. Ca(2+)-independent cell-adhesion activity of claudins, a family of integral membrane proteins localized at tight junctions. Curr Biol. 1999;9:1035–1038. doi: 10.1016/s0960-9822(99)80452-7. [DOI] [PubMed] [Google Scholar]
- Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S. Early blood-brain barrier disruption in human focal brain ischemia. Ann Neurol. 2004;56:468–477. doi: 10.1002/ana.20199. [DOI] [PubMed] [Google Scholar]
- Lee TH, Lympany P, Crea AE, Spur BW. Inhibition of leukotriene B4-induced neutrophil migration by lipoxin A4: structure-function relationships. Biochem Biophys Res Commun. 1991;180:1416–1421. doi: 10.1016/s0006-291x(05)81354-3. [DOI] [PubMed] [Google Scholar]
- Levy BD, Zhang QY, Bonnans C, et al. The endogenous pro-resolving mediators lipoxin A4 and resolvin E1 preserve organ function in allograft rejection. Prostaglandins Leukot Essent Fatty Acids. 2011;84:43–50. doi: 10.1016/j.plefa.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YS, Wu P, Zhou XY, et al. Formyl-peptide receptor like 1: a potent mediator of the Ca2+ release-activated Ca2+ current ICRAC. Arch Biochem Biophys. 2008;478:110–118. doi: 10.1016/j.abb.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Maddox JF, Hachicha M, Takano T, Petasis NA, Fokin VV, Serhan CN. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein-linked lipoxin A4 receptor. J Biol Chem. 1997;272:6972–6978. doi: 10.1074/jbc.272.11.6972. [DOI] [PubMed] [Google Scholar]
- Ogata Y, Enghild JJ, Nagase H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem. 1992;267:3581–3584. [PubMed] [Google Scholar]
- Perretti M, Chiang N, La M, Fierro IM, Marullo S, Getting SJ, Solito E, Serhan CN. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat Med. 2002;8:1296–1302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell A, Cuadrado E, Ortega-Aznar A, Hernandez-Guillamon M, Lo EH, Montaner J. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008;39:1121–1126. doi: 10.1161/STROKEAHA.107.500868. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29:2189–2195. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- Sobrado M, Pereira MP, Ballesteros I, et al. Synthesis of lipoxin A4 by 5-lipoxygenase mediates PPARgamma-dependent, neuroprotective effects of rosiglitazone in experimental stroke. J Neurosci. 2009;29:3875–3884. doi: 10.1523/JNEUROSCI.5529-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodin-Semrl S, Spagnolo A, Mikus R, Barbaro B, Varga J, Fiore S. Opposing regulation of interleukin-8 and NF-kappaB responses by lipoxin A4 and serum amyloid A via the common lipoxin A receptor. Int J Immunopathol Pharmacol. 2004;17:145–156. doi: 10.1177/039463200401700206. [DOI] [PubMed] [Google Scholar]
- Strbian D, Karjalainen-Lindsberg ML, Tatlisumak T, Lindsberg PJ. Cerebral mast cells regulate early ischemic brain swelling and neutrophil accumulation. J Cereb Blood Flow Metab. 2006;26:605–612. doi: 10.1038/sj.jcbfm.9600228. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Nagai N, Umemura K, Collen D, Lijnen HR. Stromelysin-1 (MMP-3) is critical for intracranial bleeding after t-PA treatment of stroke in mice. J Thromb Haemost. 2007;5:1732–1739. doi: 10.1111/j.1538-7836.2007.02628.x. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Zattoni M, Serhan CN. Lipoxins and aspirin-triggered lipoxin inhibit inflammatory pain processing. J Exp Med. 2007;204:245–252. doi: 10.1084/jem.20061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Wada K, Arita M, Nakajima A, Katayama K, Kudo C, Kamisaki Y, Serhan CN. Leukotriene B4 and lipoxin A4 are regulatory signals for neural stem cell proliferation and differentiation. FASEB J. 2006;20:1785–1792. doi: 10.1096/fj.06-5809com. [DOI] [PubMed] [Google Scholar]
- Warach S, Latour LL. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke. 2004;35:2659–2661. doi: 10.1161/01.STR.0000144051.32131.09. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang YP, Guo P, Ye XH, Wang J, Yuan SY, Yao SL, Shang Y. A lipoxin A4 analog ameliorates blood-brain barrier dysfunction and reduces MMP-9 expression in a rat model of focal cerebral ischemia-reperfusion injury. J Mol Neurosci. 2012;46:483–491. doi: 10.1007/s12031-011-9620-5. [DOI] [PubMed] [Google Scholar]
- Wu Y, Ye XH, Guo PP, Xu SP, Wang J, Yuan SY, Yao SL, Shang Y. Neuroprotective effect of lipoxin A4 methyl ester in a rat model of permanent focal cerebral ischemia. J Mol Neurosci. 2010;42:226–234. doi: 10.1007/s12031-010-9355-8. [DOI] [PubMed] [Google Scholar]
- Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- Ye XH, Wu Y, Guo PP, Wang J, Yuan SY, Shang Y, Yao SL. Lipoxin A4 analogue protects brain and reduces inflammation in a rat model of focal cerebral ischemia reperfusion. Brain Res. 2010;1323:174–183. doi: 10.1016/j.brainres.2010.01.079. [DOI] [PubMed] [Google Scholar]
- Yoon JS, Lee JH, Tweedie D, Mughal MR, Chigurupati S, Greig NH, Mattson MP. 3,6′-dithiothalidomide improves experimental stroke outcome by suppressing neuroinflammation. J Neurosci Res. 2013;91:671–680. doi: 10.1002/jnr.23190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JW, Gottschall PE. Zymographic measurement of gelatinase activity in brain tissue after detergent extraction and affinity-support purification. J Neurosci Methods. 1997;76:15–20. doi: 10.1016/s0165-0270(97)00065-4. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wan J, Li H, et al. Protective effects of BML-111, a lipoxin A(4) receptor agonist, on carbon tetrachloride-induced liver injury in mice. Hepatol Res. 2007;37:948–956. doi: 10.1111/j.1872-034X.2007.00154.x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang X, Wu P, et al. BML-111, a lipoxin receptor agonist, modulates the immune response and reduces the severity of collagen-induced arthritis. Inflamm Res. 2008;57:157–162. doi: 10.1007/s00011-007-7141-z. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.