Abstract
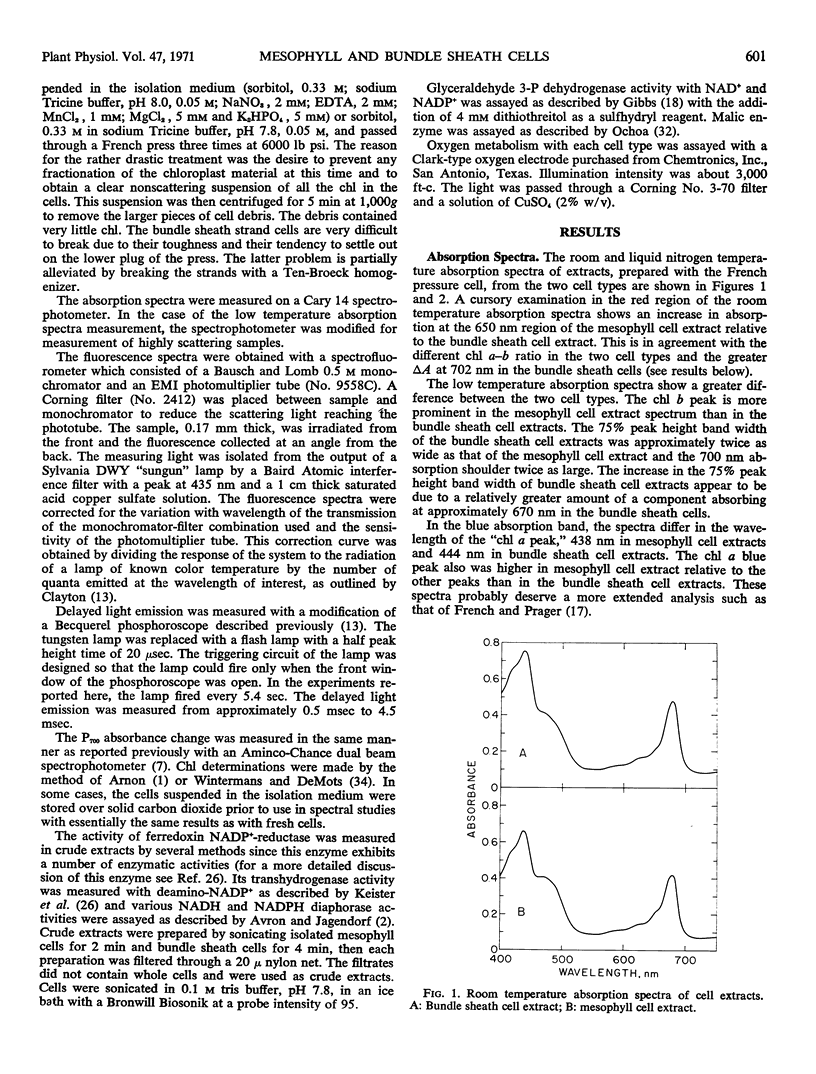
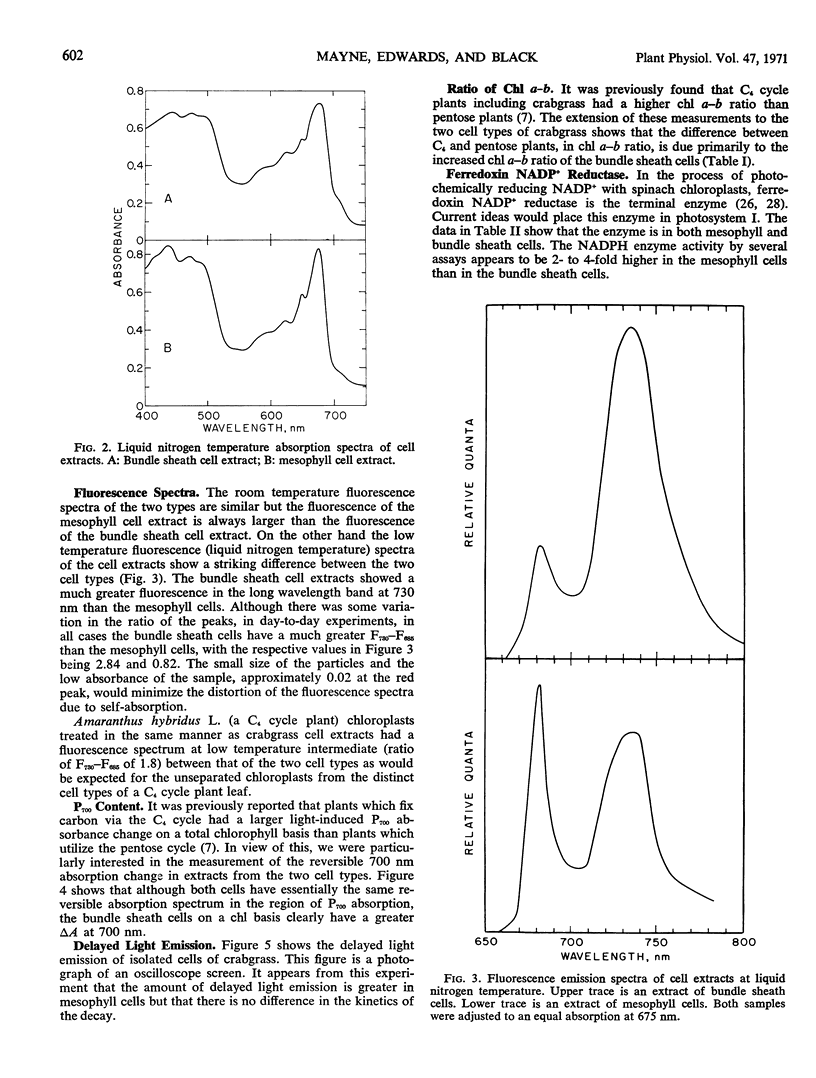
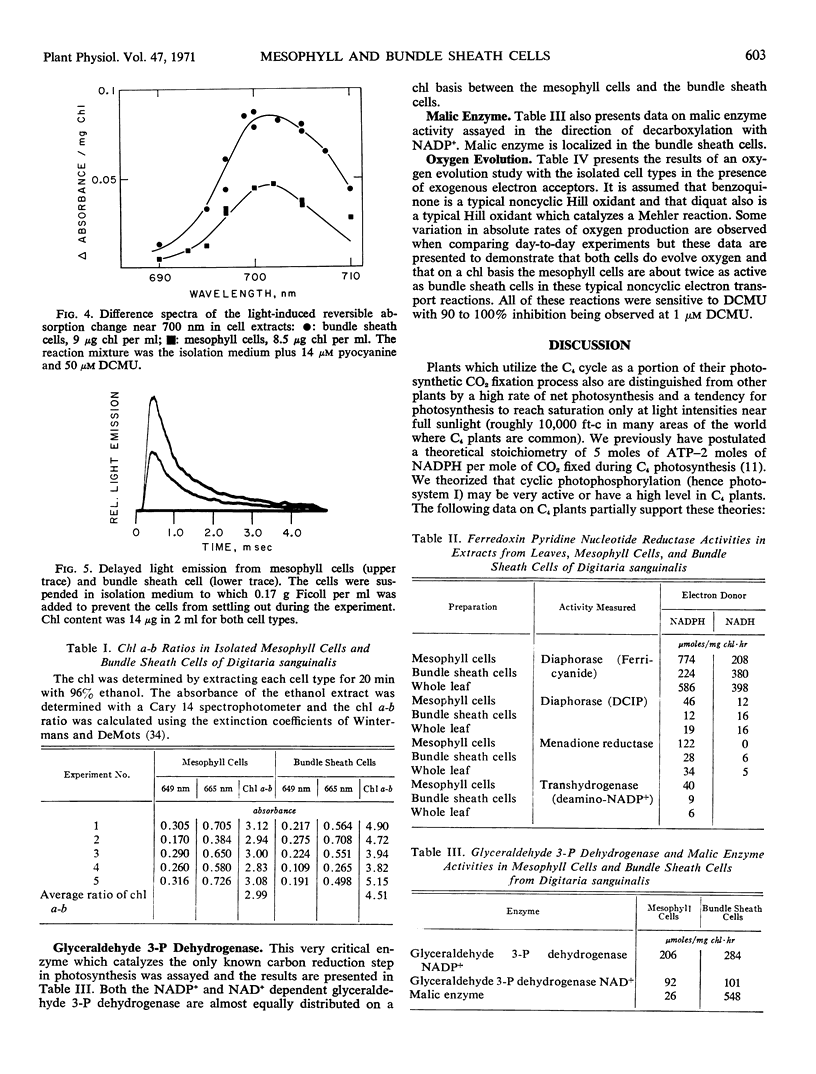
Isolated mesophyll cells and bundle sheath cells of Digitaria sanguinalis were used to study the light-absorbing pigments and electron transport reactions of a plant which possesses the C4-dicarboxylic acid cycle of photosynthesis. Absorption spectra and chlorophyll determinations are presented showing that mesophyll cells have a chlorophyll a-b ratio of about 3.0 and bundle sheath cells have a chlorophyll a-b ratio of about 4.5. The absorption spectrum of bundle sheath cells has a greater absorption in the 700 nm region at liquid nitrogen temperature, and there is a relatively greater amount of a pigment absorbing at 670 nm in the bundle sheath cells compared to the mesophyll cells. Fluorescence emission spectra, at liquid nitrogen temperature, of mesophyll cells have a fluorescence 730 nm-685 nm ratio of about 0.82 and bundle sheath cells have a ratio of about 2.84. The reversible light-induced absorption change in the region of P700 absorption is similar in both cell types but bundle sheath cells exhibit about twice as much total P700 change as mesophyll cells on a total chlorophyll basis. The delayed light emission of bundle sheath cells is about one-half that of mesophyll cells. Both mesophyll cells and bundle sheath cells evolve oxygen in the presence of Hill oxidants with the mesophyll cells exhibiting about twice the activity of bundle sheath cells, and both activities are inhibited by 1 μM 3-(3,4-dichlorophenyl)-1, 1-dimethylurea. Ferredoxin nicotinamide adenine dinucleotide phosphate reductase is present in both cells although it is about 3- or 4-fold higher in mesophyll cells than in bundle sheath cells. Glyceraldehyde 3-P dehydrogenases, both nicotinamide adenine dinucleotide and nicotinamide adenine dinucleotide phosphate, are equally distributed in the two cell types on a chlorophyll basis. Malic enzyme is localized in the bundle sheath cells.
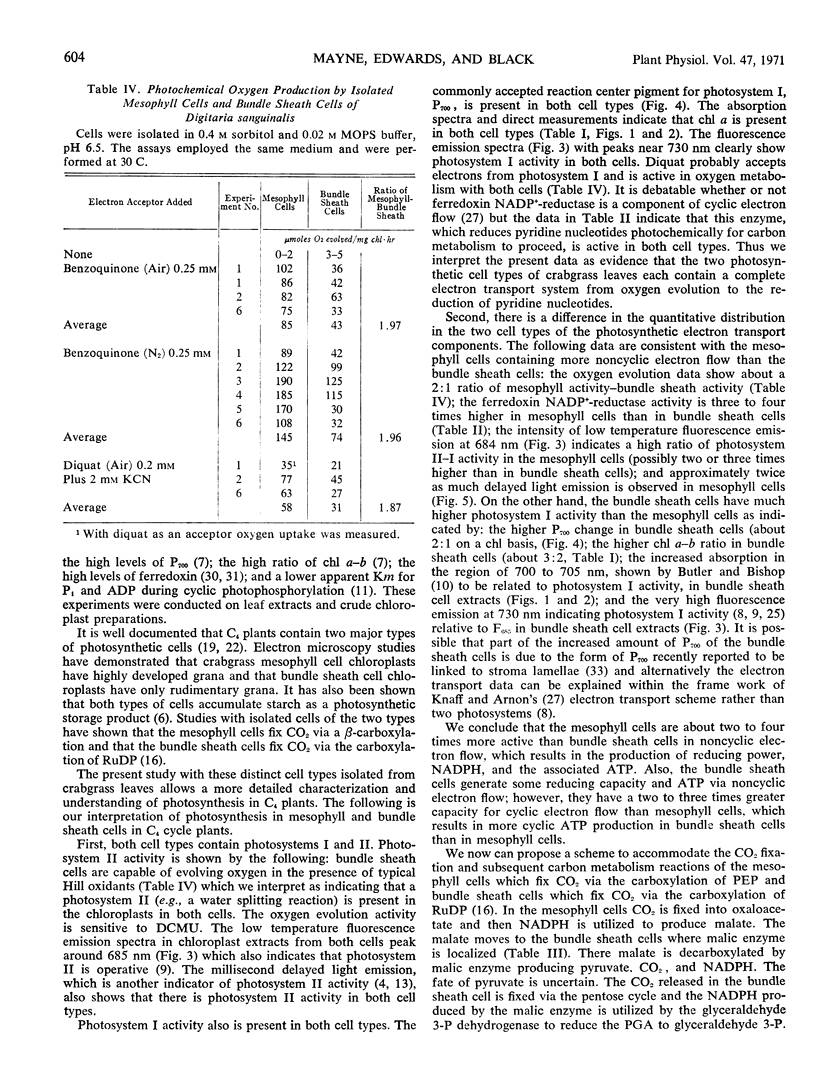
We interpret the data as evidence for the presence of a complete chloroplast electron transport system from oxygen evolution to pyridine nucleotide reduction in both mesophyll and bundle sheath cells. However, there is a quantitative difference in the distribution of photosystem I and photosystem II components in the two photosynthetic cells with about a 3-fold higher photosystem I-II ratio in the bundle sheath cells than in the mesophyll cells. A scheme is proposed to accommodate photosynthetic CO2 fixation and electron transport activities in the mesophyll cells via a β-carboxylation and in the bundle sheath cells via carboxylation of ribulose-1, 5-diphosphate.
Full text
PDF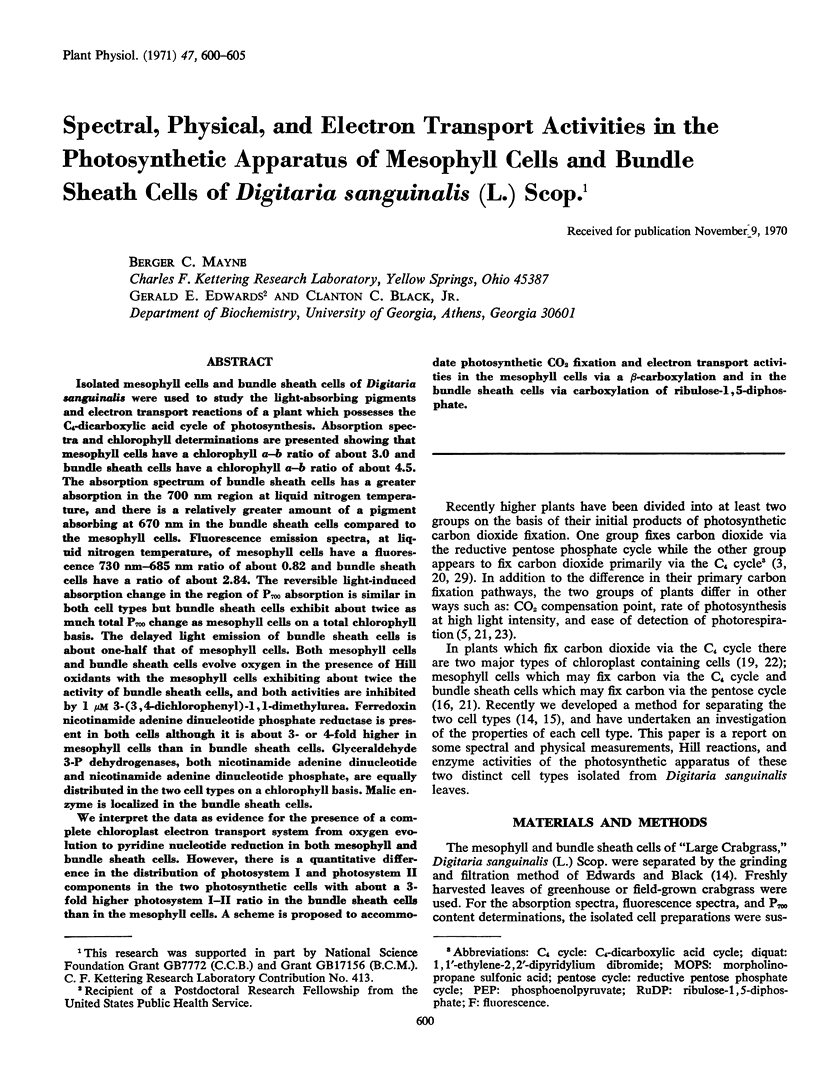

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M., JAGENDORF A. T. A TPNH diaphorase from chloroplasts. Arch Biochem Biophys. 1956 Dec;65(2):475–490. doi: 10.1016/0003-9861(56)90207-7. [DOI] [PubMed] [Google Scholar]
- BUTLER W. L. A far-red absorbing form of chlorophyll. in vivo. Arch Biochem Biophys. 1961 May;93:413–422. doi: 10.1016/0003-9861(61)90287-9. [DOI] [PubMed] [Google Scholar]
- Bertsch W., Azzi J. R., Davidson J. B. Delayed light studies on photosynthetic energy conversion. I. Identification of the oxygen-evolving photoreaction as the delayed light emitter in mutants of Scenedesmus obliquus. Biochim Biophys Acta. 1967 Jul 5;143(1):129–143. doi: 10.1016/0005-2728(67)90116-8. [DOI] [PubMed] [Google Scholar]
- Black C. C., Mayne B. C. P(700) activity and chlorophyll content of plants with different photosynthetic carbon dioxide fixation cycles. Plant Physiol. 1970 Jun;45(6):738–741. doi: 10.1104/pp.45.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black C. C., Mollenhauer H. H. Structure and distribution of chloroplasts and other organelles in leaves with various rates of photosynthesis. Plant Physiol. 1971 Jan;47(1):15–23. doi: 10.1104/pp.47.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. M., Brown R. H., Black C. C. Photosynthetic CO(2) Fixation Products and Activities of Enzymes Related to Photosynthesis in Bermudagrass and Other Plants. Plant Physiol. 1971 Feb;47(2):199–203. doi: 10.1104/pp.47.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. E., Black C. C. Isolation of Mesophyll Cells and Bundle Sheath Cells from Digitaria sanguinalis (L.) Scop. Leaves and a Scanning Microscopy Study of the Internal Leaf Cell Morphology. Plant Physiol. 1971 Jan;47(1):149–156. doi: 10.1104/pp.47.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. E., Lee S. S., Chen T. M., Black C. C. Carboxylation reactions and photosynthesis of carbon compounds in isolated mesophyll and bundle sheath cells of Digitaria sanguinalis (L.) Scop. Biochem Biophys Res Commun. 1970 May 11;39(3):389–395. doi: 10.1016/0006-291x(70)90589-9. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. Photosynthesis by sugar-cane leaves. A new carboxylation reaction and the pathway of sugar formation. Biochem J. 1966 Oct;101(1):103–111. doi: 10.1042/bj1010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEISTER D. L., SAN PIETRO A., STOLZENBACH F. E. Pyridine nucleotide transhydrogenase from spinach. I. Purification and properties. J Biol Chem. 1960 Oct;235:2989–2996. [PubMed] [Google Scholar]
- Ke B., Vernon L. P. Fluorescence of the subchloroplast particles obtained by the action of Triton X-100. Biochemistry. 1967 Jul;6(7):2221–2226. doi: 10.1021/bi00859a045. [DOI] [PubMed] [Google Scholar]
- Knaff D. B., Arnon D. I. A concept of three light reactions in photosynthesis by green plants. Proc Natl Acad Sci U S A. 1969 Oct;64(2):715–722. doi: 10.1073/pnas.64.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortschak H. P., Hartt C. E., Burr G. O. Carbon Dioxide Fixation in Sugarcane Leaves. Plant Physiol. 1965 Mar;40(2):209–213. doi: 10.1104/pp.40.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. S., Travis J., Black C. C., Jr Characterization of ferredoxin from nutsedge, Cyperus rotundus L., and other species with a high photosynthetic capacity. Arch Biochem Biophys. 1970 Dec;141(2):676–689. doi: 10.1016/0003-9861(70)90188-8. [DOI] [PubMed] [Google Scholar]
- Sane P. V., Park R. B. Purification of photosystem I reaction centers from spinach stroma lamellae. Biochem Biophys Res Commun. 1970 Oct 9;41(1):206–210. doi: 10.1016/0006-291x(70)90489-4. [DOI] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]
- Woo K. C., Anderson J. M., Boardman N. K., Downton W. J., Osmond C. B., Thorne S. W. Deficient Photosystem II in Agranal Bundle Sheath Chloroplasts of C(4) Plants. Proc Natl Acad Sci U S A. 1970 Sep;67(1):18–25. doi: 10.1073/pnas.67.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]


