Abstract
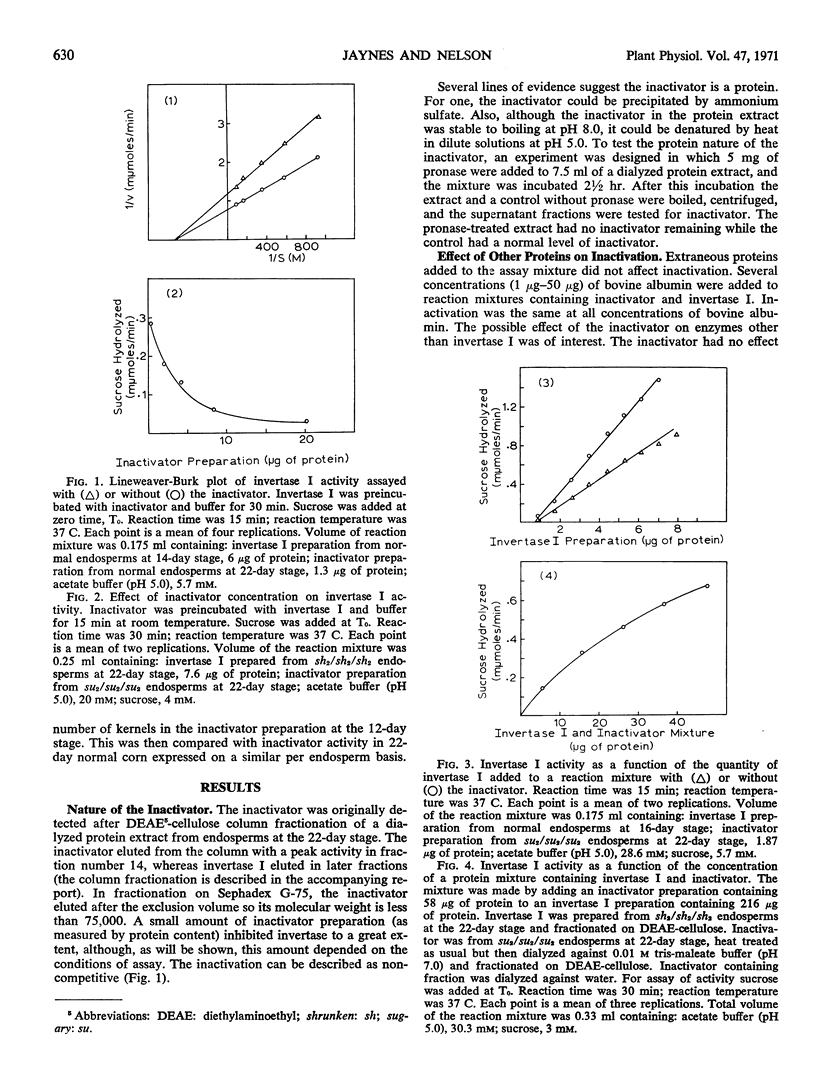
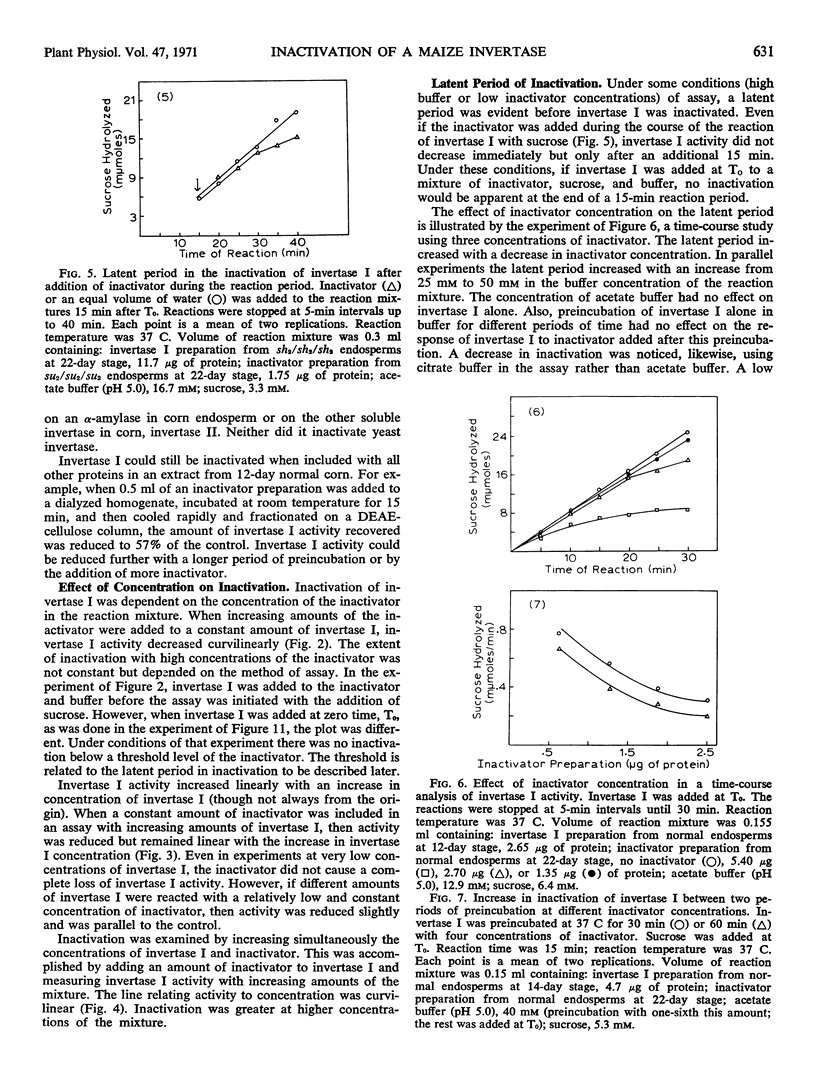
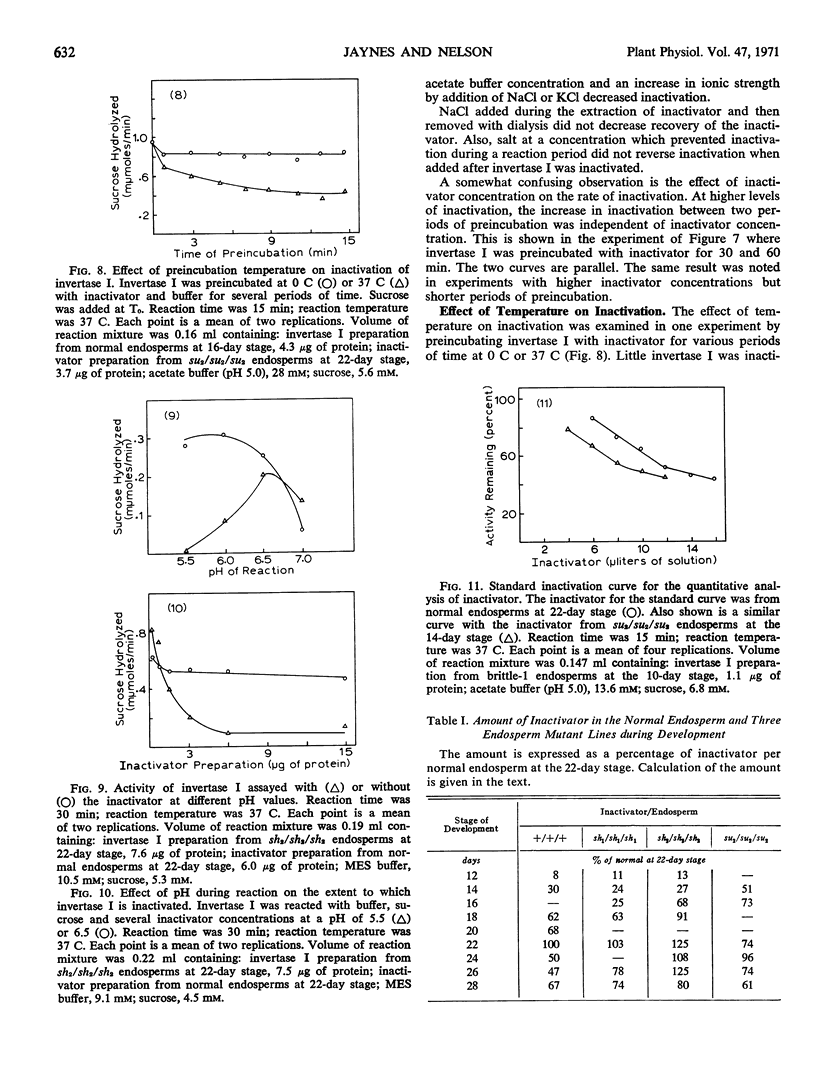
A protein present in the developing endosperm of maize (Zea mays L.) causes a loss of invertase activity under certain conditions of incubation. This protein, designated an inactivator, inactivates invertase I of maize even in the presence of other proteins. No inactivation of invertase II of maize or yeast invertase has been observed. The inactivator and invertase I are found only in the endosperm. The quantity of inactivator increases in the normal endosperm during development while invertase I activity decreases. However, the altered levels of invertase I activity in several endosperm mutant lines do not result from different quantities of inactivator. The inactivator can decrease invertase I activity during a preincubation period before addition of sucrose; inactivation is noncompetitive. Invertase I activity decreases curvilinearly with an increase in inactivator concentration. At high buffer concentrations or low inactivator concentrations in the reaction mixture, a latent period is observed when invertase I is not inactivated. Inactivation increases with an increase in temperature and a decrease in pH.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Jaynes T. A., Nelson O. E. Invertase Activity in Normal and Mutant Maize Endosperms during Development. Plant Physiol. 1971 May;47(5):623–628. doi: 10.1104/pp.47.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressey R. Invertase inhibitor from potatoes: purification, characterization, and reactivity with plant invertases. Plant Physiol. 1967 Dec;42(12):1780–1786. doi: 10.1104/pp.42.12.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressey R. Separation and properties of potato invertase and invertase inhibitor. Arch Biochem Biophys. 1966 Mar;113(3):667–674. doi: 10.1016/0003-9861(66)90246-3. [DOI] [PubMed] [Google Scholar]
- Pressey R., Shaw R. Effect of temperature on invertase, invertase inhibitor, and sugars in potato tubers. Plant Physiol. 1966 Dec;41(10):1657–1661. doi: 10.1104/pp.41.10.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIMKE R. T. THE IMPORTANCE OF BOTH SYNTHESIS AND DEGRADATION IN THE CONTROL OF ARGINASE LEVELS IN RAT LIVER. J Biol Chem. 1964 Nov;239:3808–3817. [PubMed] [Google Scholar]
- SCHLESINGER M. J. IN VITRO COMPLEMENTATION AND THE SUBUNIT STRUCTURE OF E. COLI ALKALINE PHOSPHATASE. Brookhaven Symp Biol. 1964 Dec;17:66–79. [PubMed] [Google Scholar]
- SCHLESINGER M. J., LEVINTHAL C. Hybrid protein formation of E. coli alkaline phosphatase leading to in vitro complementation. J Mol Biol. 1963 Jul;7:1–12. doi: 10.1016/s0022-2836(63)80014-5. [DOI] [PubMed] [Google Scholar]
- SWICK R. W. Measurement of protein turnover in rat liver. J Biol Chem. 1958 Apr;231(2):751–764. [PubMed] [Google Scholar]
- Zucker M. Sequential Induction of Phenylalanine Ammonia-lyase and a Lyase-inactivating System in Potato Tuber Disks. Plant Physiol. 1968 Mar;43(3):365–374. doi: 10.1104/pp.43.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]


