Abstract
Objective
To examine biomarkers of methylmercury (MeHg) intake in women and infants from seafood-consuming populations globally and characterize the comparative risk of fetal developmental neurotoxicity.
Methods
A search was conducted of the published literature reporting total mercury (Hg) in hair and blood in women and infants. These biomarkers are validated proxy measures of MeHg, a neurotoxin found primarily in seafood. Average and high-end biomarkers were extracted, stratified by seafood consumption context, and pooled by category. Medians for average and high-end pooled distributions were compared with the reference level established by a joint expert committee of the Food and Agriculture Organization (FAO) and the World Health Organization (WHO).
Findings
Selection criteria were met by 164 studies of women and infants from 43 countries. Pooled average biomarkers suggest an intake of MeHg several times over the FAO/WHO reference in fish-consuming riparians living near small-scale gold mining and well over the reference in consumers of marine mammals in Arctic regions. In coastal regions of south-eastern Asia, the western Pacific and the Mediterranean, average biomarkers approach the reference. Although the two former groups have a higher risk of neurotoxicity than the latter, coastal regions are home to the largest number at risk. High-end biomarkers across all categories indicate MeHg intake is in excess of the reference value.
Conclusion
There is a need for policies to reduce Hg exposure among women and infants and for surveillance in high-risk populations, the majority of which live in low-and middle-income countries.
Résumé
Objectif
Examiner les biomarqueurs de l'ingestion de méthylmercure (MeHg) chez les femmes et les enfants des populations consommant des poisson et fruits de mer au niveau mondial et caractériser le risque comparatif de la neurotoxicité sur le développement du fœtus.
Méthodes
Une recherche a été effectuée dans la documentation publiée rapportant les quantités totales de mercure (Hg) dans les cheveux et le sang des femmes et des enfants. Ces biomarqueurs ont été validés comme étant des mesures indirectes du MeHg, une neurotoxine que l'on trouve principalement dans les poissons et fruits de mer. Les biomarqueurs moyens et terminaux ont été extraits, stratifiés par contexte de consommation de poisons et fruits de mer et groupés par catégorie. Les médianes pour les distributions groupées des biomarqueurs moyens et terminaux ont été comparées avec le niveau de référence établi par un comité mixte d'experts de l'Organisation des Nations Unies pour l'alimentation et l'agriculture (FAO) et l'Organisation mondiale de la Santé (OMS).
Résultats
Les critères de sélection ont été satisfaits par 164 études concernant des femmes et des enfants dans 43 pays. Les biomarqueurs moyens groupés suggèrent une ingestion de MeHg plusieurs fois supérieure à la référence FAO/OMS chez les riverains consommateurs de poissons et vivant à proximité d’une zone d'orpaillage à petite échelle et bien au-delà de la référence chez les consommateurs de mammifères marins dans les régions arctiques. Dans les régions côtières de l'Asie du Sud-Est, du Pacifique occidental et de la Méditerranée, les biomarqueurs moyens se rapprochent de la référence. Bien que les deux premiers groupes aient un risque de neurotoxicité plus important que les derniers groupes, les régions côtières abritent le plus grand nombre de personnes à risque. Les biomarqueurs terminaux dans toutes les catégories indiquent que l'ingestion de MeHg est supérieure à la valeur de référence.
Conclusion
Il y a un besoin de politiques pour réduire l'exposition au Hg chez les femmes et les enfants, ainsi que pour surveiller les populations à haut risque, dont la majorité vit dans les pays à revenu faible et intermédiaire.
Resumen
Objetivo
Examinar los biomarcadores de la ingesta de metilmercurio (MeHg) en mujeres y niños procedentes de poblaciones que consumen pescados y mariscos a nivel global y describir el riesgo comparativo de neurotoxicidad del desarrollo fetal.
Métodos
Se realizó una búsqueda de la literatura publicada que informa sobre el mercurio total (Hg) en el cabello y la sangre de mujeres y niños. Estos biomarcadores son medidas indirectas validadas de MeHg, una neurotoxina que se encuentra sobre todo en el pescado y marisco. Se extrajeron biomarcadores de gama media y alta, los cuales se estratificaron por contexto de consumo de pescado y marisco y se agruparon por categorías. Se compararon las medianas de las distribuciones por grupos de gama media y alta con el nivel de referencia establecido por un comité mixto de expertos de la Organización para la Agricultura y la Alimentación (FAO) y la Organización Mundial de la Salud (OMS).
Resultados
164 estudios de mujeres y niños de 43 países cumplieron los criterios de selección. El grupo de biomarcadores de gama media indica una ingesta de MeHg varias veces superior a la referencia de la FAO/OMS en los ribereños que consumen pescado que viven cerca de una pequeña mina de oro, y muy superior a la referencia en los consumidores de mamíferos marinos en las regiones árticas. En las regiones costeras del sudeste de Asia, el Pacífico occidental y el Mediterráneo, los biomarcadores de gama media se acercan a la referencia. Aunque el riesgo de neurotoxicidad es mayor en los dos grupos anteriores que en el último, las regiones costeras albergan el mayor número de personas en riesgo. En todas las categorías, los biomarcadores de alta gama indican que la ingesta de MeHg es superior al valor de referencia.
Conclusión
Se necesitan políticas que reduzcan la exposición al Hg entre mujeres y niños, así como una vigilancia en las poblaciones de alto riesgo, la mayoría de las cuales viven en países de bajos y medianos ingresos.
ملخص
الغرض
فحص الواصمات البيولوجية لمدخول ميثيل الزئبق (MeHg) لدى المرأة والطفل من السكان الذين يتناولون المأكولات البحرية على مستوى العالمي، وتمييز المخاطر المقارنة للسمية العصبية التنموية للجنين.
الطريقة
يشير بحث تم إجراؤه في المؤلفات المنشورة إلى إجمالي الزئبق (Hg) في شعر ودم النساء والرضع. ويتم التحقق من هذه الواصمات البيولوجية من خلال التدابير غير المباشرة لميثيل الزئبق، وتوجد السمية العصبية بشكل أساسي في المأكولات البحرية. وتم استخلاص الواصمات البيولوجية المتوسطة والعليا، وتم تقسيمها إلى طبقات حسب سياق استهلاك المأكولات البحرية، وتم تجميعها حسب الفئات. وتم مقارنة متوسطات التوزيعات المتوسطة والعليا التي تم تجميعها مع المستوى المرجعي المحدد من قبل لجنة خبراء مشتركة تابعة لمنظمة الأغذية والزراعة ومنظمة الصحة العالمية.
النتائج
استوفت 164 دراسة للنساء والرضع من 43 دولة معايير الاختيار. وتشير الواصمات البيولوجية التي تم تجميعها إلى مدخول ميثيل زئبق يتجاوز مرات عديدة مرجع منظمتي الأغذية والزراعة والصحة العالمية لدى سكان الشواطئ الذين يتناولون الأسماك ويعيشون بالقرب من مناجم الذهب صغيرة الحجم، وبشكل زائد عن المرجع الخاص بمستهلكي الثدييات البحرية في مناطق القطب الشمالي. وفي المناطق الساحلية لجنوب شرق آسيا، وغرب المحيط الهادي والبحر المتوسط، يقترب متوسط الواصمات البيولوجية من المرجع. ورغم أن المجموعتين السابقتين معرضتان لمخاطر أعلى للإصابة بالسمية العصبية عن المجموعة الأخيرة، إلا أن المناطق الساحلية تعد موطناً لأكبر عدد معرض للخطر. وتشير الواصمات البيولوجية العليا عبر جميع الفئات إلى أن مدخول ميثيل الزئبق (MeHg) يتجاوز القيمة المرجعية.
الاستنتاج
هناك حاجة لسياسات تحد من التعرض للزئبق بين النساء والرضع، والترصد بالنسبة للسكان المعرضين لمخاطر عالية، والذين يعيش أكثرهم في البلدان المنخفضة الدخل والمتوسطة الدخل.
摘要
目的
调查在全球范围内妇女和婴儿从海产品消费中摄取的甲基汞(MeHg)的生物标志物,表征胎儿发育性神经中毒的相对风险。
方法
对报告妇女和婴儿毛发和血管中的总汞(Hg)含量的已发表文献进行检索。这些生物标志物是对MeHg经过验证的间接量度,MeHg是一种主要在水产品中发现的神经毒素。提取平均和高端生物标志物,并按海鲜消费环境进行分层,按类别汇集。将平均和高端汇集分布的中位值与联合国粮农组织(FAO)和世卫组织(WHO)联合专家委员会制定的参考水平进行比较。
结果
来自43个国家的164个有关妇女和婴儿的研究符合入选标准。汇集的平均生物标志物显示,居住在靠近小型金矿河边的鱼类消费人群中摄入MeHg超过FAO/WHO参考值数倍,在北极圈地区海洋哺乳动物的消费人群摄入量也大大超过参考水平。在东南亚、西太平洋和地中海沿海地区,平均生物标志物接近参考水平。尽管前两组的神经中毒风险比后者更高,沿海地区却是风险数量最多的地方。各个类别中,高端生物标记物表明MeHg摄入量超过了参考值。
结论
需要通过政策来减少妇女和婴儿的汞接触,同时对高风险人群进行监测,这些人群绝大多数在中低收入国家。
Резюме
Цель
Изучить биомаркеры поступления метилртути (MeHg) у женщин и детей из группы населения, потребляющего морепродукты, в мировом масштабе и охарактеризовать сравнительный риск отдаленного нейротоксического действия на плод.
Методы
Был проведен поиск опубликованной литературы, в которой сообщалось об общем содержании ртути (Hg) в волосах и крови женщин и детей. Эти биомаркеры являются подтвержденными репрезентативными индикаторами содержания MeHg – нейротоксина, обнаруживаемого главным образом в морепродуктах. После отбора биомаркеры среднего и высокого уровней были разделены по контексту потребления морепродуктов и сгруппированы по категориям. Медианные значения распределений биомаркеров для среднего и высокого уровней сравнивались с контрольным уровнем, установленным объединенным экспертным комитетом Продовольственной и сельскохозяйственной организации ООН (ФАО) и Всемирной организацией здравоохранения (ВОЗ).
Результаты
Критериям выбора соответствовали 164 исследования женщин и детей из 43 стран. Сгруппированные биомаркеры среднего уровня позволяют заключить, что поступление MeHg в несколько раз превышает контрольный уровень ФАО/ВОЗ у представителей населения прибрежных районов, потребляющих морепродукты и проживающих вблизи небольших месторождений золота, и значительно выше контрольного уровня – у потребителей морских млекопитающих в Арктике. В прибрежных районах Юго-Восточной Азии, Западной части Тихого океана и Средиземноморье биомаркеры среднего уровня близки к контрольному уровню. Несмотря на то, что две первые группы подвержены более высокому риску нейротоксичности, чем вторая, в указанных прибрежных районах проживает наибольшее число подверженных риску. Биомаркеры высокого уровня во всех категориях указывают на то, что поступление MeHg превышает контрольный уровень.
Вывод
Необходима разработка стратегий уменьшения воздействия Hg на женщин и детей и эпидемиологического надзора над населением, составляющим группу повышенного риска, большая часть которого проживает в странах с низким и средним уровнями доходов.
Introduction
The World Health Organization (WHO) considers mercury (Hg) among the top 10 chemicals of “major public health concern”.1 Evidence of ubiquitous Hg contamination globally led to the recent Minamata Mercury Convention, a binding international treaty to control anthropogenic Hg emissions.2 A principal form of Hg to which general populations are exposed is methylmercury (MeHg). Transformation of Hg emissions to organic MeHg takes place in the aquatic environment, where MeHg bioaccumulates in food webs. In human beings MeHg exposure occurs predominantly through the consumption of seafood (including freshwater and marine varieties, shellfish and marine mammals).3–6 MeHg is a neurotoxin particularly harmful to the developing fetal brain.3–6 A large body of research has demonstrated an association of exposure in utero with developmental neurotoxicity (e.g. deficits in fine motor skills, language and memory) among populations that consume seafood regularly.3,7–9 Such studies have been used to develop health-based reference doses below which no appreciable risk of harm is thought to occur, including the provisional tolerable weekly intake (PTWI), established by the Joint Expert Committee on Food Additivies (JECFA) of the Food and Agriculture Organization (FAO) and WHO.6,10 Recent research suggests harm at doses associated with relatively infrequent seafood consumption.11
Seafood species vary in MeHg content depending on contamination source, trophic level and other factors.12–14 Seafood, on the other hand, is an important source of nutrients, including neuroprotective omega-3 polyunsaturated fatty acids.15 Research on the benefits and harms of seafood highlights the importance of choosing species low in MeHg and high in these polyunsaturated fatty acids and of ensuring that consumers have sufficient information to make such choices.15,16 Well-designed seafood advisories can be helpful to this end,17,18 but they exist in a small number of countries, most of which are high-income.19 An estimated 400 million women of reproductive age in the world rely on seafood for at least 20% of their intake of animal protein; a large share of them live in low- and middle-income countries where access to information on MeHg content in seafood is not widely available.20–22 Although the research conducted in the last two decades has highlighted the risk in subsistence fishing communities that practise artisanal and small-scale gold mining23 and among Arctic peoples whose diet consists of apex marine predators such as the pilot whale,24 few researchers have compared MeHg exposures globally in women who consume seafood.
Human exposure to chemical contaminants can be characterized by examining biomarkers.25 Total Hg in hair (THHg) and total Hg in blood (TBHg) are both validated biomarkers of MeHg intake correlated with seafood consumption in general human populations.4,26 Our goal was to review and synthesize the evidence from published studies reporting THHg and TBHg biomarkers to systematically compare global MeHg exposure among women and their infants from seafood-consuming populations. By identifying populations at higher risk, we aim to provide policy-makers with scientific evidence for the prioritization of risk reduction messages and targeted population surveillance.
Methods
Based on a pre-defined study protocol,27 we performed a systematic electronic search of the peer-reviewed scientific literature (Box 1). Studies were selected in two stages: title and abstract screening, followed by full text review after application of exclusion criteria. We excluded studies not involving women or infants from general populations and not reporting a central THHg or TBHg biomarker estimate. When multiple articles reported on a single sample, we chose the most recent one with complete data. To ensure robust summary statistics, we excluded studies with less than 40 participants.
Box 1. Literature search strategy for global systematic review of methylmercury exposure from seafood in women and infants.
1. “fetus” OR “infant” OR “newborn” OR “maternal” OR “mother” OR “pregnant” OR “women”
2. “fish” OR “marine” OR “shellfish” OR “seafood”
3. “mercury” OR “methylmercury” OR “methyl AND mercury” OR “biomonitoring”
Combined terms: 1 AND 2 AND 3.
Note: The following databases were searched for studies published from January 1991 to September 2013: PubMed, Embase, SCOPUS, Web of Science, TOXNET and LILACS. References were hand-checked and there were no restrictions on language or study design.
We extracted data on study design, population characteristics, measures of average (geometric mean or median) and high-end (90th or 95th percentile or maximum) biomarkers, exposure conditions and main covariates examined. Extracted biomarkers were organized into three subpopulation groups: non-pregnant women; pregnant women and mothers who had recently given birth; and infants (up to 12 months of age). Because biomarkers for more than one subpopulation with different levels of exposure were often reported in the same study, the subpopulation was our main level of analysis.
We stratified subpopulations into six mutually exclusive categories based on predictors of the body burden of MeHg. The most important of these predictors are seafood consumption frequency and seafood MeHg content. In most seafood species MeHg represents the largest fraction of total Hg (inorganic Hg representing a much smaller share). Thus, seafood MeHg concentration is commonly measured as total Hg in tissue.3,4 Seafood consumption estimates were reported in some studies; data on total Hg concentrations were rarely provided. Research suggests the following general hierarchy: marine mammals, other apex marine predators and some industrially-contaminated fish [containing several parts per million (ppm)]; large marine fish [containing up to 1 or more ppm]; most commercially purchased marine and freshwater fish [often containing less than 0.5 ppm] and most shellfish [often containing less than 0.2 ppm].23,24,28–31 Seafood intake is generally higher in coastal regions than inland30,32 and seafood from globalized commercial sources predominates in many urban areas.14 We therefore generated six categories based on the following proxy predictors, reported in most studies: seafood source; seafood type; likely Hg contamination pathway; and residential context. Four categories included populations consuming seafood that was mainly self-caught and two included populations consuming seafood that was commercially purchased primarily (Table 1).
Table 1. Methylmercury exposure categoriesa for women and infants from seafood-consuming populations.
| Category/subcategory | Predominant Hg pathway to seafood | Predominant seafood type | Seafood intake range (kg per month)b | Residential context |
|---|---|---|---|---|
| Locally self-caught seafood is important share of diet | ||||
| Arctic – Traditional diet – Mixed diet |
Unique polar meteorology and Hg deposition/mobilization, Arctic food-chain (marine mammals as apex predators) | Traditional: marine fish and marine mammals Mixed: marine fish and non-seafood protein sources, few if any marine mammals |
0.6–7.1 | Far northern Arctic, where people rely on apex Hg-contaminated marine mammals and fish |
| Gold mining – Rural riverine – Urban |
Artisanal and small-scale gold mining, soil lixiviation, forest fires releasing Hg emissions | Rural: high share of locally-caught freshwater fish Urban: mixed diet including non-seafood protein, low share of locally-caught freshwater fish |
0.6–14.9 | Rural and urban tropical areas near artisanal and small-scale gold mining, where the diet includes fish from rivers contaminated by gold mining activity |
| Fishing | Local and general global transport of Hg emissions | Marine and freshwater fish and shellfish | 0.1–3.8 | Recreational or subsistence fishing areas near rivers, reservoirs or lakes without a particular Hg contamination source |
| Industry | Local Hg-emitting industry (chloralkali, power generation, mining other than gold mining) | Marine and freshwater fish and shellfish | 0.2–5.8 | Recreational or subsistence fishing areas near water bodies with active or disused industrial facilities |
| Seafood consumed is mostly from commercial sources (i.e. non-self-caught)c | ||||
| Coastal – Atlantic – Mediterraneand – Pacific |
Local and general global transport of Hg emissions in all three regions; natural Hg emission sources in the Mediterranean | Marine and freshwater fish and shellfish | 0.3–5.6 | Atlantic, Mediterranean or Pacific coastal areas where seafood intake is frequent |
| Inland | Local and general global transport of Hg emissions | Marine and freshwater fish and shellfish | Very little–2.0 | Inland areas where seafood intake is low |
Hg, mercury.
a Exposure categories based on proxy predictors reported in selected studies.
b Estimated per capita seafood intake ranges were derived from data reported in selected studies. They were converted to kg per month for comparability.
c Several subpopulations consume an important share of self-caught marine seafood in addition to commercially-purchased varieties.
d Because Indian Ocean and Persian Gulf subpopulations were not numerous and reported seafood intake and total Hg biomarkers similar to those of the more numerous Mediterranean subpopulations, the former were included with the latter.
As recommended in guidelines for the systematic review of observational studies,27 we evaluated study quality by examining the risk of bias in three areas: selection of participants (selection methods and reporting of exposure characteristics); exposure measurement (laboratory methods and quality control); and statistical methods and covariate analysis (evaluation of distribution shape, reporting of seafood intake and exposure to non-seafood sources of Hg).
We derived two summary distributions – central and upper bound – for each exposure category by pooling average and high-end biomarkers. For comparability, all TBHg biomarkers were converted to THHg-equivalent at a hair-to-blood ratio of 250:1.3,5 We summarized resulting statistical distributions using medians and percentiles. To interpret results, we compared distribution medians with the THHg-equivalent value of the PTWI dose (approximately 2.2 μg/g) established by the JECFA.10 We also determined the share of subpopulations with average and high-end biomarkers over this reference. In sensitivity analysis we evaluated the impact on pooled biomarkers taking into account differences in participant selection, exposure measurement and statistical methods identified in the quality review. Given substantial heterogeneity in population exposure conditions, study designs and reporting, we did not undertake a meta-analysis. All data analysis was performed in Stata10 (StataCorp, College Station, United States of America).
Results
Selected studies
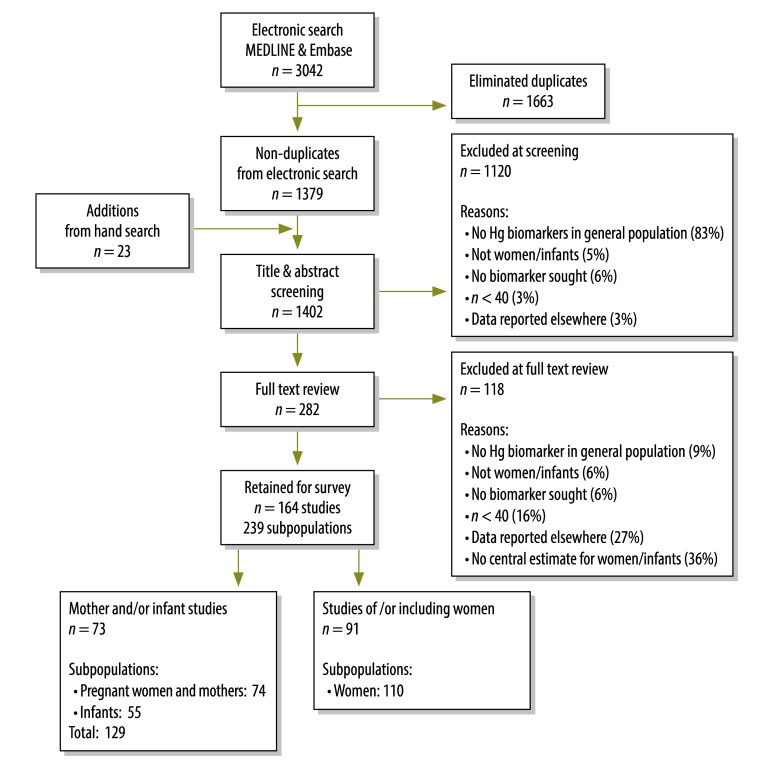
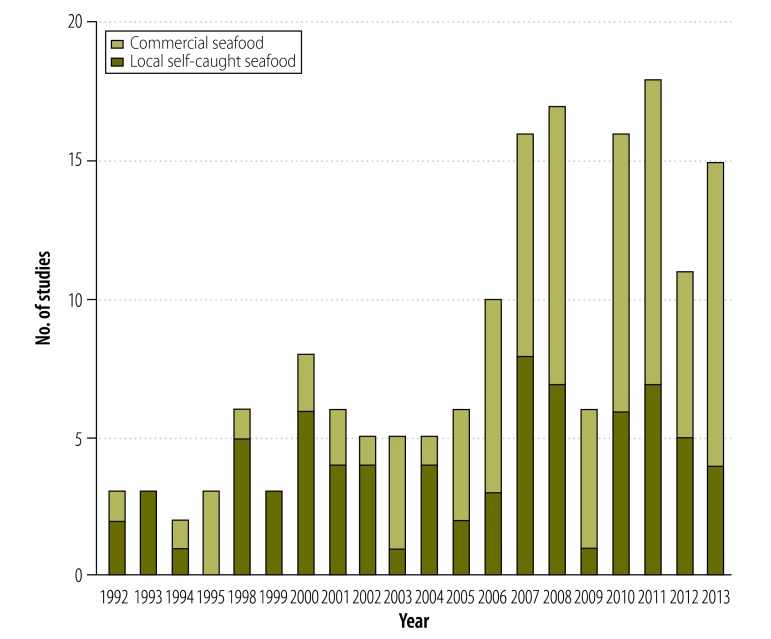
Of 3042 articles identified in the published literature, we screened 1402 non-duplicates (1379 were identified by electronic search and 23 by hand search); we excluded 1120 and we reviewed the full texts of the remaining 282, from which we excluded 118 (Fig. 1). The remaining 164 articles, which reported total Hg biomarkers for 239 distinct subpopulations, were included in this review. Selected articles report biomarker concentrations for 63 943 women and infants from 43 countries (Table 2). Most (73%) studies were cross-sectional and over half (56%) reported THHg measures; the majority (79%) were published after 2001. Studies published in 1991–2001 were conducted primarily in populations consuming self-caught seafood; since 2001, the number of studies in consumers of seafood that is predominantly commercially purchased has increased notably in both absolute and relative terms (Fig. 2). The characteristics of the selected studies are provided in Table 3 and Table 4 (both available at: http://www.who.int/bulletin/volumes/92/04/13-116152).
Fig. 1.
Selection of articles for the review of studies on methylmercury exposure in women and infants from seafood-consuming populations
Table 2. Summary of studies assessing total mercury in hair (THHg) or total mercury in blood (TBHg) among women and infants from seafood-consuming populations, by exposure category.
| Study characteristics | No. of studies | Exposure categories |
||||||
|---|---|---|---|---|---|---|---|---|
| Self-caught seafood |
Commercially-purchased seafood |
|||||||
| Arctic | Gold mining | Fishing | Industrya | Coastal | Inland | |||
| Population studied | ||||||||
| Mothers and/or infantsb | 73 | 9 | 10 | 3 | 5 | 37 | 9 | |
| Women in general | 91 | 3 | 19 | 9 | 15 | 32 | 13 | |
| All | 164 | 12 | 29 | 12 | 20 | 69 | 22 | |
| Study design | ||||||||
| Cross-sectional | 119 | 10 | 28 | 9 | 13 | 44 | 15 | |
| Other | 45 | 2 | 1 | 3 | 7 | 25 | 7 | |
| Biomarker reported | ||||||||
| Reporting THHg biomarkersc | 92 | 1 | 27 | 5 | 16 | 37 | 6 | |
| Reporting TBHg biomarkersb | 72 | 11 | 2 | 7 | 4 | 32 | 16 | |
| Reporting of seafood data | ||||||||
| Some | 84 | 6 | 14 | 10 | 11 | 37 | 6 | |
| None | 80 | 6 | 15 | 2 | 9 | 32 | 16 | |
| Publication date | ||||||||
| Published in 1991–2001 | 34 | 6 | 10 | 3 | 4 | 9 | 2 | |
| Published in 2002–2013 | 130 | 6 | 19 | 9 | 16 | 60 | 20 | |
| Subpopulation studiedd | ||||||||
| Infants | 55 | 7 | 9 | 3 | 3 | 27 | 6 | |
| Pregnant women or mothers | 74 | 10 | 13 | 2 | 4 | 35 | 10 | |
| Non-pregnant women | 110 | 4 | 21 | 9 | 18 | 40 | 18 | |
| All | 239 | 21 | 43 | 14 | 25 | 102 | 34 | |
| Study participants | ||||||||
| Average participants per study | 390 | 495 | 350 | 263 | 152 | 448 | 48 | |
| Average participants per subpopulation | 268 | 283 | 236 | 236 | 121 | 303 | 316 | |
| Total no. of participants | 63 943 | 5935 | 10 152 | 3161 | 3035 | 30 915 | 10 745 | |
| Countries represented | 43 | 5 | 6 | 5 | 17 | 23 | 16 | |
a Other than gold mining.
b Mother and infant studies include pregnant women, mothers who have recently given birth and infants (i.e. children up to 12 months of age).
c Some studies reported both TBHg and THHg biomarkers. When both were reported, THHg biomarkers were extracted.
d Of these studies, 48 reported on two or more distinctly-defined exposed subpopulations of more than 40 non-pregnant women, pregnant women, women who had recently given birth, or infants (i.e. children up to 12 months of age).
Fig. 2.
Number of selected studies reporting total mercury in hair (THHg) or total mercury in blood (TBHg) in women and infants from seafood-consuming populations, by predominant seafood type (local self-caught or commercially purchased) and year of publication
Table 3. Characteristics of studies assessing total mercury in hair (THHg) or total mercury in blood (TBHg) in women and infants consuming self-caught seafood, by exposure category and subcategory.
| Studies, by category and subcategory | Study design | Location | Seafood intakea (kg per month) | Subpopulation | n | THHg, averageb (μg/g) | THHg, High-endb (μg/g) |
|---|---|---|---|---|---|---|---|
| Gold miningc | |||||||
| Gold mining: rural riverine | |||||||
| Monrroy et al. 200833 | Cross-sectional | Bolivia (Plurinational State of), Beni valley | 2.2 | W | 163 | 3.9 | 20.0 |
| Barbieri et al. 200934 | Cross-sectional | Bolivia (Plurinational State of), Beni valley | 5.1 | W | 77 | 2.5 | – |
| Boischio et al. 199335 | Cross-sectional | Brazil, upper Madeira (river) | – | W | 70 | 10.0 | 125.0 |
| Barbosa et al. 199836 | Cross-sectional | Brazil, upper Madeira (river) | – | MO | 98 | 12.8 | 94.7 |
| Lebel et al. 199837 | Cross-sectional | Brazil, Tapajos | 6.9 | W | 46 | 11.2 | 26.6 |
| Grandjean et al. 199938 | Cross-sectional | Brazil, Tapajos | 10.2 | W | 114 | 11.6 | – |
| Amorim et al. 200039 | Cross-sectional | Brazil, Tapajos | – | W | 46 | 10.8 | – |
| Boischio et al. 200040 | Cross-sectional | Brazil, Madeira | – | MO | 90 | 12.6d | 28.3 |
| Dolbec et al. 200041 | Cross-sectional | Brazil, Tapajos | 9.0 | W | 40 | 8.7 | – |
| Harada et al. 200142 | Cross-sectional | Brazil, Barreiras | – | W | 44 | 16.4d | 53.8 |
| Crompton et al. 200243 | Cross-sectional | Brazil, Jacareacanga | – | W | 113 | 6.7d | – |
| Santos et al. 200244 | Cross-sectional | Brazil, Sai Cinza | 5.1 | W | 192 | 14.7 | 90.4 |
| Santos et al. 200345 | Cross-sectional | Brazil, Pakaanova | – | W | 549 | 8.55 | 39.4 |
| Santos et al. 200746 | Cross-sectional | Brazil, Itaituba | – | IN | 1510 | 4.2d | – |
| MO | 1510 | 2.9d | – | ||||
| Passos et al. 200847 | Cross-sectional | Brazil, Tapajos | – | W | 121 | 16.3d | 150.0 |
| Grotto et al. 201048 | Cross-sectional | Brazil, Tapajos | – | W | 54 | 8.8 | – |
| Fillion et al. 201149 | Cross-sectional | Brazil, Tapajos | – | W | 126 | 9.4 | – |
| Dórea et al. 201250 | Cross-sectional | Brazil, Bom Futuro | 1.6 | IN | 166 | 1.6 | – |
| Barcelos et al. 201351 | Cross-sectional | Brazil, Tapajos | 14.9 | W | 193 | 16.3 | – |
| Marques et al. 201352 | Cross-sectional | Brazil, Madeira (river) | – | IN | 396 | 3.0 | 18.5 |
| Brazil, Madeira (river) | 4.3 | MO | 396 | 12.1 | 130.7 | ||
| Brazil, Madeira (tin region) | – | IN | 294 | 0.8 | 2.0 | ||
| Brazil, Madeira (tin region) | 0.9 | MO | 294 | 4.5 | 11.9 | ||
| Brazil, Madeira (rural) | – | IN | 67 | 2.0 | 8.8 | ||
| Brazil, Madeira (rural) | 2.6 | MO | 67 | 7.8 | 41.1 | ||
| Vieira et al. 201353 | Cross-sectional | Brazil, Porto Velho (river) | 4.4 | MO | 75 | 8.2 | 20.1 |
| Olivero-Verbel et al. 201154 | Cross-sectional | Colombia, Antioquia | – | W | 757 | 1.4 | 10.0 |
| Cordier et al. 199855 | Cross-sectional | French Guiana | – | PW | 109 | 1.6 | 22.0 |
| Cordier et al. 200256 | Cross-sectional | French Guiana, upper Maroni | 10.2 | W | 90 | 12.7 | – |
| French Guiana, Camopi | – | W | 63 | 6.7 | – | ||
| French Guiana, Awala | – | W | 55 | 2.8 | – | ||
| Fujimura et al. 201257 | Cross-sectional | French Guiana, upper Maroni | 8.63 | W | 234 | 9.9d | 26.6 |
| Bose-O’Reilly et al. 201058 | Ecological | Indonesia, Kalimantan | – | W | 64 | 2.5 | 29.6 |
| Gold mining: urban | |||||||
| Hacon et al. 200059 | Cross-sectional | Brazil, Alta Floresta | 0.6 | MO | 75 | 1.1d | 8.2 |
| Marques et al. 200760 | Cross-sectional | Brazil, Porto Velho | 0.7 | IN | 100 | 0.2 | – |
| 0.7 | MO | 100 | 0.1 | – | |||
| Dorea et al. 201250 | Cross-sectional | Brazil, Porto Velho | 1.4 | IN | 82 | 1.8 | – |
| Marques et al. 201352 | Cross-sectional | Brazil, Madeira (urban) | – | IN | 676 | 1.5 | 4.8 |
| 1.7 | MO | 676 | 5.4 | 24.1 | |||
| Vieira et al. 201353 | Cross-sectional | Brazil, Porto Velho (urban) | 0.7 | MO | 82 | 1.3 | 6.1 |
| Mohan et al. 200561 | Cross-sectional | Surinam, Paramaribo | – | IN | 39 | 1.6d | 19.6 |
| – | MO | 39 | 0.8d | 15.4 | |||
| Arctice | |||||||
| Arctic: Traditional diet | |||||||
| Dewailly et al. 200162 | Cross-sectional | Canada, Nunavik | – | W | 284 | 4.2 | 28.0 |
| Muckle et al. 200163 | Cohort | Canada, Nunavik | – | IN | 95 | 4.6 | 24.3 |
| – | MO | 130 | 2.6 | 11.1 | |||
| Lucas et al. 200464 | Cross-sectional | Canada, Nunavik | 4.9 | IN | 439 | 3.5 | – |
| Butler-Walker et al. 200665 | Cross-sectioal | Canada, Northwest Territories (Inuit) | – | IN | 132 | 1.7 | 19.0 |
| 3.5 | MO | 132 | 0.9 | 8.5 | |||
| Fontaine et al. 200866 | Cross- sectional | Canada, Nunavik | 1.5 | W | 308 | 2.1 | 41.1 |
| Grandjean et al. 199267 | Cohort | Denmark, Faroe Islands | – | IN | 1020 | 6.1 | – |
| 2.2 | MO | 1020 | 4.5 | – | |||
| Bjerregaard et al. 200068 | Cross-sectional | Denmark, Greenland (Disko Bay) | – | IN | 178 | 6.3 | 45.3 |
| 7.1 | MO | 180 | 3.2 | 18.9 | |||
| Nielsen et al. 201269 | Cross-sectional | Denmark, Greenland | – | W | 1040 | 3.7 | 42.5 |
| Arctic: Mixed diet | |||||||
| Butler-Walker et al. 200665 | Cross-sectional | Canada, Northwest Territories (Caucasian) | – | IN | 124 | 0.3 | 3.2 |
| 0.6 | MO | 124 | 0.2 | 1.1 | |||
| Odland et al. 199970 | Cross-sectional | Norway, northern (Norwegian) | – | MO | 81 | 0.6 | 0.6 |
| Norway, northern (Russian) | – | MO | 151 | 0.4 | 1.4 | ||
| Hansen et al. 201171 | Cross-sectional | Norway, northern | – | MO | 211 | 0.3 | 0.9 |
| Klopov et al. 199872 | Cross-sectional | Russian Federation, Norilsk-Sakelhard | – | IN | 42 | 3.1d | – |
| 1.5 | MO | 42 | 3.9d | – | |||
| Arnold et al. 200573 | Cross-sectional | United States, Alaska | – | MO | 150 | 0.5 | 6.4 |
| – | W | 52 | 0.6 | 7.8 | |||
| Industryf | |||||||
| Nilson et al. 200174 | Cross-sectional | Brazil, Itapessuma | – | W | 84 | 1.9d | 12.5 |
| Kuno et al. 201075 | Cross-sectional | Brazil, São Paulo state | 0.2 | W | 265 | 0.3 | 1.1 |
| Bruhn et al. 199476 | Cross-sectional | Chile, 8th district | – | PW | 59 | 1.7 | 7.1 |
| Li et al. 200677 | Ecological | China, Chanchung | 0.6 | W | 69 | 0.5d | 10.5 |
| Zhang et al. 200678 | Cross-sectional | China, Wujiazhan | – | W | 40 | 0.6 | – |
| Tang et al. 200879 | Cohort | China, Tongliang | – | IN | 110 | 1.8d | 9.9 |
| Fang et al. 201280 | Cross-sectional | China, Zhejiang | 1.9 | W | 50 | 0.8d | 3.0 |
| Pawlas et al. 201381 | Cross-sectional | China, Guiyang | – | W | 49 | 2.2 | 35.0 |
| Olivero-Verbel et al. 200882 | Cross-sectional | Colombia, Cartagena (bay) | 4.3 | W | 258 | 1.0 | – |
| Madeddu et al. 200883 | Case control | Italy, Sicily Augusta | – | W | 100 | 1.2 | 5.0 |
| Deroma et al. 201384 | Cohort | Italy, Venice (region) | – | IN | 70 | 0.7 | – |
| – | MO | 79 | 1.2 | – | |||
| Hsiao et al. 201185 | Cross-sectional | Kazakhstan, Temirtau | 1.1 | W | 174 | 0.4 | 4.6 |
| Lim et al. 201086 | Cohort | Republic of Korea, Sinha-Banud | 0.4 | W | 852 | 0.7 | – |
| Trasande et al. 201087 | Cross-sectional | Mexico, Lake Chapala | – | W | 91 | 0.5 | – |
| Elhamri et al. 200788 | Cross-sectional | Morocco, Martil | 1.2 | W | 40 | 1.4 | 7.9 |
| Lacayo et al. 199189 | Cross-sectional | Nicaragua, Lake Xolotlan | – | W | 40 | 3.4 | – |
| Bravo et al. 201090 | Cross-sectional | Romania, Babeni | 1.5 | W | 38 | 1.0 | – |
| Palkovicova et al. 200891 | Cohort | Slovakia, eastern | – | IN | 99 | 0.2 | 0.64 |
| – | MO | 99 | 0.2 | 0.73 | |||
| Pawlas et al. 201381 | Cross-sectional | Slovakia, Baska Bystrica | – | W | 52 | 0.6 | 3.3 |
| Oskarsson et al. 199492 | Cross-sectional | Sweden, Boliden | – | MO | 124 | 0.3d | – |
| Chang et al. 200893 | Cross-sectional | China, Taiwan, Tainan | 5.8 | W | 99 | 3.7 | – |
| Lincoln et al. 201194 | Cross-sectional | United States, Louisiana (gulf) | 1.5 | W | 44 | 0.7 | 3.6 |
| Rojas et al. 200795 | Case control | Venezuela (Bolivarian Republic of), Valencia | – | W | 50 | 0.9d | 4.31 |
| Fishingg | |||||||
| Black et al. 201196 | Cross-sectional | Botswana, Okavango delta | 2.6 | W | 60 | 0.1 | 0.9 |
| Girard et al. 199597 | Cross-sectional | Canada, St James | – | MO | 991 | 2.5 | – |
| Mahaffey et al. 199898 | Cross-sectional | Canada, St Lawrence | 0.6 | W | 99 | 0.04 | – |
| Belles-Isles et al. 200299 | Cohort | Canada, St Lawrence | 3.8 | IN | 40 | 0.5 | 2.8 |
| Cole et al. 2004100 | Cross-sectional | Canada, Ontario | 2.2 | W | 38 | 1.5 | 5.4 |
| Morrissette et al. 2004101 | Cohort | Canada, St Lawrence (river) | 0.6 | IN | 101 | 0.1 | 0.4 |
| 0.6 | MO | 101 | 0.1 | 0.3 | |||
| Abdelouahab et al. 2008102 | Cross-sectional | Canada, St Lawrence (river) | 1.2 | W | 87 | 0.4 | 3.9 |
| Jenssen et al. 2012103 | Cross-sectional | Norway | 2.2 | W | 100 | 0.9 | 4.0 |
| Johnsson et al. 2004104 | Cross-sectional | Sweden, Hagfors | – | W | 51 | 0.7 | – |
| Stewart et al. 2000105 | Cohort | United States, New York (state) | – | W | 296 | 0.5 | 0.7 |
| Knobeloch et al. 2007106 | Cross-sectional | United States, Wisconsin | 1.3 | W | 1050 | 0.4 | 5.3 |
| Schantz et al. 2010107 | Cross-sectional | United States, Wisconsin | 0.1 | W | 79 | 0.4 | 3.3 |
IN, infants; MO, mothers; PW, pregnant women; W, women.
a Seafood intake as reported in studies, converted to kg per month (assuming average meal size of 170 g if not stated) and shown for mothers if reported for both mothers and infants; not all studies reported seafood intake.
b Biomarker concentrations shown as THHg, either as reported or as converted from TBHg using the hair-to-blood ratio of 250:1. All THHg concentrations are rounded to one decimal place. Average THHg is the geometric mean or median (unless noted with “d”); high-end THHg is the maximum or the 95th or 90th percentile.
c Women and infants near tropical small-scale gold mining sites who consume freshwater fish from Hg-contaminated rivers.
d The average is the arithmetic mean and was not included in main pooling results.
e Women and infants living in the Arctic or far-Northern regions consuming apex marine foods, including marine mammals.
f Women and infants periodically consuming marine and freshwater fish caught locally from water bodies contaminated by mercury-emitting industry.
g Women and infants periodically consuming marine and freshwater fish caught locally from water bodies not affected by industrial emissions.
Table 4. Characteristics of studies assessing total mercury in hair (THHg) or total mercury in blood (TBHg) in women and infants consuming seafood that is predominantly commercially purchased, by exposure category and subcategory.
| Studies, by category and subcategory | Study design | Location | Seafood intakea (kg/mo) | Subpopulation | n | THHg, averageb (μg/g) | THHg, high-endb (μg/g) |
|---|---|---|---|---|---|---|---|
| Coastalc | |||||||
| Coastal: Atlantic | |||||||
| Carneiro et al. 2011108 | Cross-sectional | Brazil, Porto Alegre | 0.5 | W | 107 | 0.1d | – |
| Legrand et al. 2005109 | Cross-sectional | Canada, Bay of Fundy | 1.5 | W | 77 | 0.5d | 0.7 |
| Albert et al. 2010110 | Risk assessment | France, north-western | – | PW | 125 | 0.7 | 2.8 |
| Drouillet-Pinard et al. 2010111 | Cohort | France, Poitiers | – | IN | 645 | 0.4 | – |
| Cohort | 1.4 | MO | 645 | 0.5 | – | ||
| Vahter et al. 2000112 | Cohort | Sweden, Solna | – | IN | 148 | 0.4 | 1.2 |
| Cohort | – | MO | 148 | 0.2 | 0.7 | ||
| Björnberg et al. 2003113 | Cross-sectional | Sweden, Uppsala | – | IN | 123 | 0.3 | 1.4 |
| Cross-sectional | 0.8 | MO | 123 | 0.4 | 1.5 | ||
| Rosborg et al. 2003114 | Cross-sectional | Sweden (acid region) | – | W | 47 | 0.4 | 3.5 |
| Cross-sectional | Sweden (alkaline region) | – | W | 43 | 0.3 | 1.0 | |
| Brantsaeter et al. 2010115 | Cohort | Norway, Baerum | 1.2 | MO | 119 | 0.4 | 1.1 |
| Gerhardsson et al. 2010116 | Cross-sectional | Norway, Simrishamn | 0.7 | PW | 50 | 0.2 | – |
| Renzoni et al. 1998117 | Cross-sectional | Portugal, Maderia | – | W | 181 | 8.6 | 42.6 |
| Ramon et al. 2011118 | Cohort | Spain, Asturias | 2.7 | IN | 340 | 2.7 | 17.3 |
| Cohort | Spain, Gipuzkoa | 2.4 | IN | 529 | 1.9 | 12.5 | |
| Oskarsson et al. 199492 | Cross-sectional | Sweden, Homsund | – | MO | 79 | 0.3d | – |
| Björnberg et al. 2005119 | Cross-sectional | Sweden | 2.1 | W | 127 | 0.7 | 6.6 |
| Pawlas et al. 201381 | Cross-sectional | Sweden, southern | – | W | 54 | 1.4 | 9.8 |
| Bates et al. 2007120 | Cross-sectional | United Kingdom | 0.7 | W | 44 | 0.2 | – |
| Dewailly et al. 2012121 | Cross-sectional | United Kingdom (Bermuda) | – | MO | 49 | 1.1 | 5.0 |
| Stern et al. 2001122 | Cross-sectional | United States, New Jersey | 1.2 | MO | 143 | 0.3d | 8.0 |
| Ortiz-Roque et al. 2004123 |
Cross-sectional | United States, Puerto Rico | 2.0 | W | 45 | 0.4 | – |
| Cross-sectional | United States, Vieques | 3.6 | W | 41 | 0.3 | – | |
| Oken et al. 2005124 | Cohort | United States, eastern Massachusetts | 0.9 | MO | 135 | 0.1 | 0.6 |
| McKelvey et al. 2007125 | Cross-sectional | United States, New York City | 1.5 | W | 1049 | 0.7 | 2.8 |
| Karouna-Renier et al. 2008126 | Cross-sectional | United States, Florida panhandle | – | PW | 83 | 0.2 | 10.7 |
| – | W | 515 | 0.3 | 22.1 | |||
| Lederman et al. 2008127 | Cross-sectional | United States, New York City (non-Asian) | – | IN | 178 | 0.7 | – |
| United States, New York City (Chinese) | – | MO | 83 | 1.1 | – | ||
| United States, New York City (non-Asian) | – | MO | 176 | 0.4 | – | ||
| Caldwell et al. 2009128 | Cross-sectional | United States (national) | – | W | 1888 | 0.2 | 1.1 |
| Wells et al. 2011129 | Cross-sectional | United States, Maryland | – | IN | 300 | 0.3 | – |
| King et al. 2013130 | Cross-sectional | United States, Pawtucket | – | IN | 538 | 0.1 | 9.8 |
| Traynor et al. 2013131 | Cross-sectional | United States, Duval County, Florida | 2.1 | W | 698 | 0.3 | 3.0 |
| Coastal: Mediterranean, Indian Ocean, Persian Gulf | |||||||
| Babi et al. 2000132 | Cross-sectional | Albania, Tirana | 0.3 | W | 47 | 0.6 | 2.0 |
| Miklavčič et al. 2013133 | Cohort | Croatia, Rijeka | 0.8 | IN | 210 | 0.7 | 8.0 |
| 0.8 | MO | 255 | 0.5 | 5.3 | |||
| Gibičar et al. 2006134 | Cohort | Greece, islands | 1.5 | PW | 246 | 1.4 | 17.5 |
| Vardavas et al. 2011135 | Cohort | Greece, Heraklion Crete | – | PW | 47 | 0.4 | 1.7 |
| Miklavčič et al. 2013133 | Cohort | Greece, Lesvos and Chios | 1.0 | MO | 391 | 1.5 | 8.3 |
| Fakour et al. 2010136 | Cohort | Islamic Republic of Iran, Mahshahr | 1.3 | W | 195 | 3.0d | 26.5 |
| Salehi et al. 2010137 | Cross-sectional | Islamic Republic of Iran, Mahshahr | 2.9 | PW | 149 | 2.0 | 10.0 |
| Barghi et al. 2012138 | Cross-sectional | Islamic Republic of Iran, Noushahr | 3.9 | PW | 59 | 0.3 | 0.6 |
| Okati et al. 2012139 | Cross-sectional | Islamic Republic of Iran, Mazandaran | – | IN | 93 | 1.9d | 6.9 |
| 1.1 | MO | 93 | 3.6d | 9.0 | |||
| Díez et al. 2008140 | Cross-sectional | Italy, Naples | – | W | 114 | 0.5 | 1.5 |
| Maddedu et al. 200883 | Case control | Italy, Sicily, Catalina | – | W | 100 | 0.9 | 4.2 |
| Miklavĉiĉ et al. 2013133 | Cohort | Italy, Trieste | 1.2 | IN | 614 | 1.0 | 8.3 |
| Cohort | 1.2 | MO | 871 | 0.6 | 10.0 | ||
| Bou-Olayan et al. 1994141 | Cross-sectional | Kuwait | 2.2 | W | 68 | 4.1d | 25.0 |
| Khassouani et al. 2001142 | Cross-sectional | Morocco, Rabat | – | W | 70 | 1.6d | – |
| Myers et al. 1995143 | Cohort | Seychelles, Mahe | – | PW | 740 | 5.9 | 26.7 |
| Channa et al. 2013144 | Cross-sectional | South Africa, KwaZulu-Natal | – | IN | 350 | 0.2 | 4.6 |
| Cross-sectional | – | MO | 350 | 0.2 | 3.1 | ||
| Rudge et al. 2009145 | Cross-sectional | South Africa | – | IN | 62 | 1.2 | 9.7 |
| – | MO | 62 | 0.7 | 8.8 | |||
| Soria et al. 1992146 | Cross-sectional | Spain, Seville | – | W | 50 | 2.9d | 20.0 |
| Ramon et al. 2011118 | Cohort | Spain, Valencia | 2.1 | IN | 554 | 2.4 | 16.5 |
| Spain, Sabadell | 2.3 | IN | 460 | 1.6 | 15.0 | ||
| Unuvar et al. 2007147 | Cohort | Turkey, Istanbul | 1.1 | IN | 143 | 0.1 | – |
| 1.1 | MO | 143 | 0.1 | – | |||
| Coastal: Pacific coast | |||||||
| Choy et al. 2002148 | Case control | China, Hong Kong Special Administrative Region | – | W | 155 | 1.7 | – |
| Fok et al. 2007149 | Cohort | China, Hong Kong Special Administrative Region | 1.3 | IN | 1057 | 2.2 | – |
| 1.3 | MO | 1057 | 1.2 | – | |||
| Gao et al. 2007150 | Cohort | China | 2.9 | IN | 408 | 1.4 | – |
| 2.9 | MO | 408 | 1.3 | – | |||
| Liu et al. 2008151 | Cross-sectional | China, 5 cities | 2.1 | W | 321 | 0.7 | 8.5 |
| Dewailly et al. 2008152 | Cross-sectional | French Polynesia, Tahiti | 5.6 | IN | 234 | 2.6 | 12.1 |
| Nakagawa et al. 1995153 | Cross-sectional | Japan, Tokyo | – | W | 177 | 1.9 | – |
| Iwasaki et al. 2003154 | Cross-sectional | Japan, Akita | – | W | 154 | 1.7 | 5.8 |
| Yasutake et al. 2003155 | Cross-sectional | Japan | – | W | 1666 | 1.4 | 25.8 |
| Arakawa et al. 2006156 | Cohort | Japan, Sendai | 2.6 | MO | 180 | 2.0 | 9.4 |
| Ohno et al. 2007157 | Cohort | Japan, Akita | – | W | 59 | 1.5 | 3.6 |
| Sakamoto et al. 2007158 | Cross-sectional | Japan, 3 cities | – | IN | 115 | 2.5 | – |
| – | MO | 115 | 1.3 | – | |||
| Sakamoto et al. 2008159 | Biomarker valid | Japan, Fukuoka | – | IN | 40 | 0.4 | – |
| – | MO | 40 | 0.4 | – | |||
| Miyake et al. 2011160 | Cohort | Japan, Osaka | – | W | 582 | 1.5 | 3.2 |
| Kim et al. 2006161 | Case control | Republic of Korea, Seoul | – | IN | 63 | 1.0 | 5.0 |
| – | MO | 63 | 0.6 | 7.4 | |||
| Kim et al. 2008162 | Cross-sectional | Republic of Korea (coastal) | 4.4 | W | 111 | 0.8 | – |
| Jo et al. 2010163 | Cross-sectional | Republic of Korea, Busan | 4.4 | W | 146 | 1.9 | 11.4 |
| Kim et al. 2010164 | Cross-sectional | Republic of Korea, 3 cities | 4.4 | IN | 312 | 3.7 | – |
| Lee et al. 2010165 | Cohort | Republic of Korea, 3 cities | 4.4 | IN | 417 | 1.4 | 6.0 |
| 4.4 | PW | 417 | 0.8 | 4.6 | |||
| Kim et al. 2011166 | Cohort | Republic of Korea, 3 cities | – | IN | 797 | 1.3 | 2.3 |
| – | MO | 797 | 0.8 | 1.4 | |||
| Kim et al. 2012167 | Cross-sectional | Republic of Korea | – | W | 2964 | 1.0 | – |
| You et al. 2012168 | Cross-sectional | Republic of Korea, Busan and Ulsan | – | W | 200 | 4.7 | – |
| Eom et al. 2013169 | Cross-sectional | Republic of Korea (coastal) | – | W | 308 | 1.1 | – |
| Hong et al. 2013170 | Cross-sectional | Republic of Korea, Seoul | – | W | 79 | 1.4d | – |
| Kim et al. 2013171 | Cross-sectional | Republic of Korea (urban) | 1.5 | W | 117 | 0.9 | – |
| Republic of Korea (coastal) | 1.5 | W | 114 | 0.9 | – | ||
| Republic of Korea (rural) | 1.5 | W | 105 | 0.7 | – | ||
| Marsh et al. 1995172 | Cohort | Peru, Mancora | – | MO | 131 | 7.1 | 28.5 |
| Hsu et al. 2007173 | Cross-sectional | China, Taiwan, Taipei | – | IN | 65 | 2.3 | 7.0 |
| 1.9 | MO | 65 | 2.2 | 5.3 | |||
| Chien et al. 2010174 | Risk assessment | China, Taiwan (northern) | 1.5 | W | 263 | 1.7 | 16.3 |
| Sato et al. 2006175 | Cross-sectional | United States, Honolulu, Hawaii | 0.6 | IN | 188 | 0.7d | 5.0 |
| Tsuchiya et al. 2009176 | Cohort | United States, Washington state (Koreans) | 1.8 | W | 108 | 0.6 | – |
| United States, Washington state (Japanese) | 1.8 | W | 106 | 1.2 | – | ||
| Inlande | |||||||
| Gundacker et al. 2006177 | Cross-sectional | Austria, Vienna | – | W | 78 | 0.6d | – |
| Rudge et al. 2011178 | Cross-sectional | Brazil, São Paulo state | – | MO | 155 | 0.2 | 1.1 |
| Rhainds et al. 1999179 | Cross-sectional | Canada, southern Quebec | – | IN | 109 | 0.2 | 3.4 |
| Pawlas et al. 201381 | Cross-sectional | Croatia, Koprivnica | – | W | 60 | 0.4 | 7.6 |
| Puklová et al. 2010180 | Cross-sectional | Czech Republic | 0.5 | W | 163 | 0.2 | 2.3 |
| Cerna et al. 2012181 | Cross-sectional | Czech Republic | – | W | 494 | 0.2 | 0.7 |
| Pawlas et al. 201381 | Cross-sectional | Czech Republic | – | W | 51 | 0.9 | 8.0 |
| Khassouani et al. 2001142 | Cross-sectional | France, Angers | – | W | 62 | 0.9 | – |
| Huel et al. 2008182 | Cohort | France, Paris | – | MO | 81 | 1.2 | 2.9 |
| Deroma et al. 201384 | Cohort | Italy, northern | – | IN | 58 | 0.9 | – |
| – | MO | 72 | 0.9 | – | |||
| Eom et al. 2013169 | Cross-sectional | Republic of Korea (inland) | – | W | 886 | 0.8 | – |
| Pawlas et al. 201381 | Cross-sectional | Morocco, Fez | – | W | 50 | 1.0 | 9.1 |
| Anwar et al. 2007183 | Cross-sectional | Pakistan, Lahore | 0.7 | W | 75 | 0.2 | 2.5 |
| Jędrychowski et al. 2007184 | Cross-sectional | Poland, Krakov | – | IN | 313 | 0.1 | – |
| Poland | 0.7 | MO | 313 | 0.2 | – | ||
| Pawlas et al. 201381 | Cross-sectional | Poland, Wroclaw | – | W | 51 | 0.7 | 2.9 |
| Al-Saleh et al. 2006185 | Case control | Saudi Arabia | – | W | 185 | 0.9d | 5.4 |
| Al-Saleh et al. 2008186 | Case control | Saudi Arabia, Riyadh | – | W | 434 | 0.9d | 7.6 |
| Al-Saleh et al. 2011187 | Cross-sectional | Saudi Arabia, Riyadh | – | IN | 1561 | 0.6 | 1.9 |
| Saudi Arabia, Riyahd | – | MO | 1574 | 0.5 | 2.2 | ||
| Al-Saleh et al. 2013188 | Cross-sectional | Saudi Arabia | – | MO | 150 | 0.3 | – |
| Miklavčič et al. 2011189 | Cohort | Slovenia, Ljubljana | – | IN | 446 | 0.4 | – |
| Cohort | Slovenia, Ljubljana | 0.8 | MO | 574 | 0.3 | – | |
| Miklavčič et al. 2013133 | Cohort | Slovenia, Ljubljana | 1.3 | MO | 446 | 0.4 | 3.5 |
| Pawlas et al. 201381 | Cross-sectional | Slovenia, Ljubljana | – | W | 50 | 0.7 | 13.0 |
| Díez et al. 2009190 | Cohort | Spain, Madrid | 1.4 | IN | 57 | 1.5 | 5.1 |
| Díez et al. 2011191 | Case control | Spain, Toledo | 2.0 | W | 64 | 2.5 | – |
| Bjermo et al. 2013192 | Cross-sectional | Sweden | – | W | 145 | 0.2 | 0.7 |
| Gerhardsson et al. 2010116 | Cross-sectional | Sweden, Hasselholm | 0.4 | PW | 50 | 0.2 | – |
| Knobeloch et al. 2005193 | Cross-sectional | United States, 12 states | 0.7 | W | 414 | 0.3 | 1.6 |
| Xue et al. 2007194 | Cohort | United States, Michigan | 0.6 | MO | 1024 | 0.1 | – |
| Pollack et al. 2011195 | Cross-sectional | United States, western New York state | – | W | 252 | 0.3 | – |
| Pollack et al. 2012196 | Cross-sectional | United States, Buffalo | – | W | 248 | 0.4 | – |
IN, infants; MO, mothers; PW, pregnant women; W, women.
a Seafood intake as reported in studies, converted to kg per month (assuming average meal size of 170 g if not stated) and shown for mothers if reported for both mothers and infants; not all studies reported seafood intake.
b Biomarker concentrations shown as THHg, either as reported or as converted from TBHg using the hair-to-blood ratio of 250:1. All THHg concentrations are rounded to one decimal place. Average THHg is the geometric mean or median (unless noted with “d”); high-end THHg is the maximum or the 95th or 90th percentile.
c Women and infants living in coastal regions and consuming marine and freshwater seafood mainly purchased from local and global markets.
d The average is the arithmetic mean and was not included in the main pooled results.
e Women and infants living inland and consuming marine and freshwater seafood mainly purchased from local and global markets.
Pooled biomarker concentrations
For 43 subpopulations of women and infants living near small-scale gold mining sites in Bolivia (Plurinational State of),33,34 Brazil,35–53,59,60 Colombia,54 French Guiana,55–57 Indonesia58 and Surinam61 the pooled central distribution median THHg biomarker concentration was 5.4 µg/g (upper bound median: 23.1) (Table 5). Values were higher (8.2 µg/g; upper bound: 27.5) in the subgroup of rural riverine dwellers reliant on local freshwater fish and lower (1.4 µg/g; upper bound: 11.8) among urban dwellers consuming less fish. For 21 subpopulations from Arctic regions, including in Canada,62–66 Denmark (Greenland and the Faroe Islands),67–69 Norway,70,71 the Russian Federation72 and the United States (state of Alaska),73 the pooled central distribution median result was 2.1 µg/g (upper bound: 9.8); values were higher (3.6 µg/g; upper bound: 24.3) for marine mammal and other self-caught seafood consumers and lower (0.4 µg/g; upper bound: 1.4) among those with a diet including less seafood and less reliant on these traditional foods.
Table 5. Pooled total THHg biomarker distributions in women and infants from seafood-consuming populations, by exposure category and subcategory.
| Category and subcategory | No. of subpopulations | No. of participants | Central distributiona |
Upper bound distributiona |
|||
|---|---|---|---|---|---|---|---|
| THHg (μg/g)b 25th, 50th 75th, 95th percentile | Percentage > PTWIc | THHg (μg/g)b 25th, 50th, 75th, 95th percentile | Percentage > PTWIc | ||||
| Gold mining | 43 | 10 152 | 1.80, 5.36, 10.00, 14.70 | 77 | 11.94, 23.07, 39.40, 125.00 | 98 | |
| Rural | 34 | 8 283 | 2.50, 8.24, 11.20, 14.70 | 85 | 18.53, 27.45, 53.80, 130.70 | 97 | |
| Urban | 9 | 1 869 | 0.19, 1.41, 1.80, 5.36 | 44 | 6.09, 11.80, 19.60, 24.14 | 100 | |
| Arctic | 21 | 5 935 | 0.47, 2.09, 4.18, 6.33 | 52 | 2.30, 9.76, 26.13, 45.25 | 81 | |
| Traditional | 12 | 4 958 | 2.34, 3.61, 4.56, 6.33 | 75 | 18.90, 24.25, 41.08, 45.25 | 100 | |
| Mixed diet | 9 | 977 | 0.31, 0.40, 0.55, 0.64 | 11 | 0.93, 1.38, 6.35, 7.82 | 56 | |
| Industry | 25 | 3 035 | 0.25, 0.75, 1.27, 3.54 | 32 | 3.04, 4.62, 9.93, 35.00 | 89 | |
| Fishing | 14 | 3 161 | 0.13, 0.38, 0.70, 2.50 | 6 | 0.70, 2.75, 4.00, 5.38 | 71 | |
| Coastal | 102 | 30 915 | 0.36, 0.82, 1.51, 3.70 | 23 | 2.83, 6.76, 10.65, 26.46 | 86 | |
| Atlantic | 35 | 9 675 | 0.27, 0.35, 0.69, 2.70 | 16 | 1.16, 2.93, 9.75, 22.14 | 76 | |
| Mediterranean | 27 | 6 536 | 0.29, 0.65, 1.45, 5.90 | 32 | 4.18, 8.53, 16.50, 26.46 | 96 | |
| Pacific | 40 | 14 704 | 0.85, 1.34, 1.94, 4.66 | 23 | 2.83, 6.03, 10.65, 28.50 | 98 | |
| Inland | 34 | 10 745 | 0.31, 0.38, 0.77, 1.47 | 18 | 1.93, 2.90, 7.59, 13.00 | 79 | |
| Total | 239 | 63 943 | – | 34 | – | 86 | |
PTWI, provisional tolerable weekly intake; THHG, total mercury in hair.
a Central distribution reflects pooling of geometric mean and median biomarkers from reported studies; upper bound distribution reflects pooling of 90th, 95th percentiles and maximums from reported studies.
b Biomarkers measuring total mercury in blood converted to THHg equivalent at a hair-to-blood ratio of 250:1.
c Share of total subpopulations with a reported average or high-end biomarker greater than the PTWI equivalent of 2.2 μg/g of THHg.
For 25 subpopulations whose self-caught fish from local waterways is affected by Hg-emitting industries in Brazil,74,75 Chile,76 China,77–81 Colombia,82 Italy,83,84 Kazakhstan,85 Mexico,87 Morocco,88 Nicaragua,89 Norway,115 the Republic of Korea,86 Romania,90 Slovakia,81,91 Sweden,92 Taiwan, China,93 the United States94 and Venezuela (Bolivarian Republic of),95 the pooled central THHg median biomarker was 0.8 µg/g (upper bound: 4.6). In 14 subpopulations consuming fish periodically from non-industry-contaminated waters in Botswana,96 Canada,97–102 Norway,103 Sweden104 and the United States,105–107 the value was 0.4 µg/g (upper bound: 2.8).
For 102 coastal or island-dwelling subpopulations consuming seafood that is predominantly commercially purchased, the combined central median THHg concentration was 0.8 µg/g (upper bound: 6.8). On the Atlantic coast, the pooled result for 35 subpopulations in Brazil,108 Canada,99,109 France,110,111 Norway,115 Portugal,117 Spain,118 Sweden,81,92,112–114,119 the United Kingdom of Great Britain and Northern Ireland120,121 and the United States122–131 was 0.4 µg/g (upper bound: 2.9). For 27 subpopulations from the Mediterranean, Persian Gulf and Indian Ocean (combined because of similar THHg ranges and referred to as “Mediterranean”) in Albania,132 Croatia,133 Greece,133,135 the Islamic Republic of Iran,136–139 Italy,83,133,140 Kuwait,141 Morocco,142 Seychelles,143 South Africa,144,145 Spain146 and Turkey,147 the pooled central THHg concentration was 0.7 µg/g (upper bound: 8.5). For 40 Pacific coast subpopulations in China,148–151 Japan,153–160 Peru,172 the Republic of Korea,161–171 Taiwan, China174 and the United States,175,176 the pooled result was 1.3 µg/g (upper bound: 6.0).
For 34 subpopulations living in inland regions of Austria,177 Brazil,178 Canada,179 Croatia,81 the Czech Republic,81,180,181 France,142,182 Italy,84 Morocco,81 Pakistan,183 Poland,184 the Republic of Korea,169 Saudi Arabia,186–188 Slovenia,81,189 Spain,190,191 Sweden192 and the United States,193–196 the pooled central TTHg median was 0.4 µg/g (upper bound: 2.9).
Comparison with provisional tolerable weekly intake
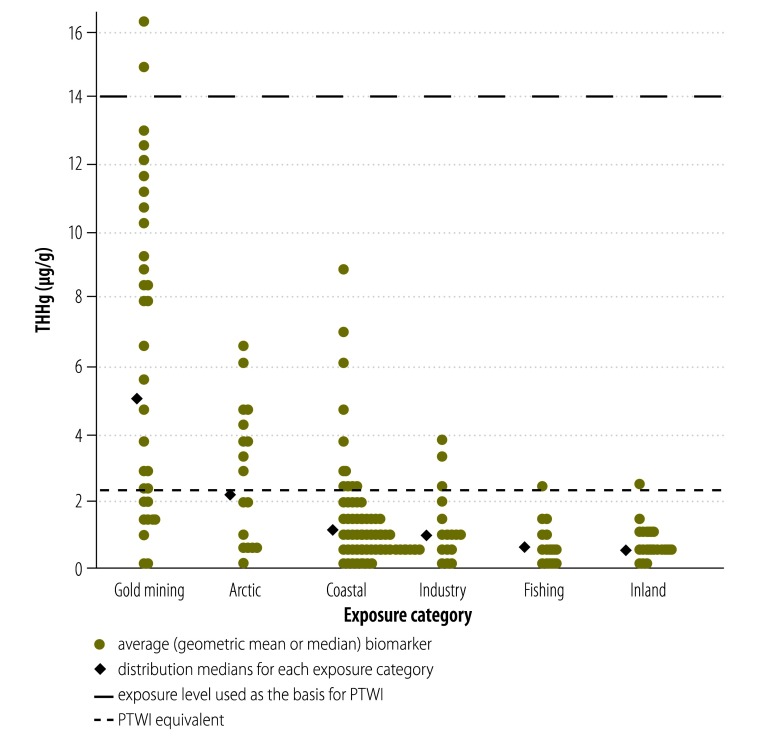
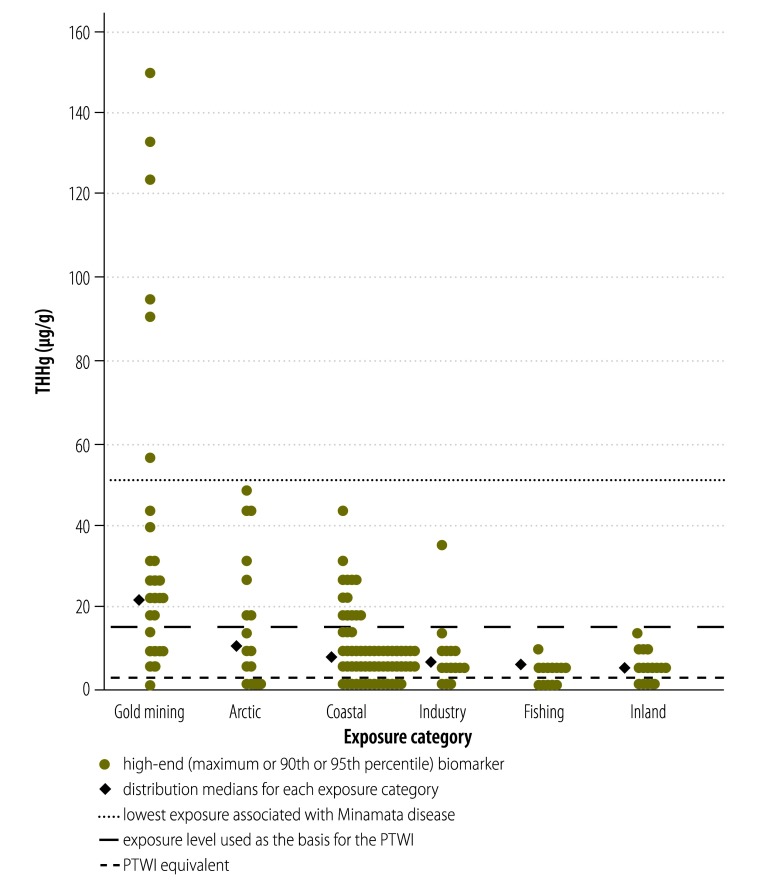
The median of the pooled central THHg biomarker distribution for women and infants in rural riverine communities near tropical gold mining sites reached nearly four times the FAO/WHO PTWI reference level of 2.2 ug/g (Fig. 3), while the upper-bound median reached more than 10 times this reference. Some individual high-end biomarkers exceeded 50 µg/g, the lower end of the range found in the neurological syndrome known as Minamata disease,4 associated with accidental industrial Hg poisoning in Japan in the 1950s and 1960s (Fig. 4). The median of the central THHg biomarker distribution in Arctic traditional food consumers exceeded the reference by 63%, while the upper bound median was over 10 times the value. For women and infants in the industry and fishing categories, central estimate medians were below the international reference, although the industry central median was twice that of the fishing category; most high-end biomarkers were above it. For those in the Pacific coastal subcategory, the 75th percentile approached the reference value; the upper bound median was nearly three times this value and nearly all high-end biomarkers exceeded it. Central biomarkers were below the PTWI in the Atlantic. However in many subpopulations in the Mediterranean they exceeded this reference, while the upper bound median was nearly four times the reference and most high-end biomarkers exceeded it. For the inland category, the central estimate median was well below the reference, but nearly 80% of the high-end biomarkers exceeded it.
Fig. 3.
Distributions of central estimate for total mercury in hair (THHg) reported in selected studies of women and infants from seafood-consuming populations, by exposure category
PTWI, provisional tolerable weekly intake.
Fig. 4.
Distributions of upper-bound total mercury in hair (THHg) reported in selected studies of women and infants from seafood-consuming populations, by exposure category
PTWI, provisional tolerable weekly intake.
Note: High-end biomarkers in the gold mining, Arctic and coastal categories reach into the range associated with observable neurological damage.
Study quality
A majority (78%) of selected studies were based on convenience samples taken from seafood-consuming populations. Some details of the seafood context were provided in most (71%) studies, but in the others this information was sparse. Laboratory protocols for THHg and TBHg detection were nearly universally reported (91%). Most (82%) protocols were based on cold vapour atomic absorption spectrometry (CV-AAS) or inductively-coupled plasma mass spectrometry (ICP-MS) and a majority (74%) reported laboratory quality control procedures. In 86% of studies, distributions were transformed to lognormal scale and summarized using geometric means or medians. More than half (55%) of the studies reported maximums as high-end estimates, while the remainder reported 90th or 95th percentiles. Only 51% of studies reported some seafood intake data and 25% evaluated non-seafood sources of Hg.
Discussion
We found that biomarkers of MeHg intake were of greatest health concern among three categories of seafood-consuming women and their infants: (i) rural riverside dwellers living near tropical small-scale gold mining with diets dependent on locally-caught freshwater fish; (ii) those in Arctic regions for whom apex food-chain marine mammals are a dietary staple; and (iii) coastal inhabitants, particularly in the Pacific and Mediterranean, who probably consume seafood that is primarily commercially sourced. In the first group, average Hg biomarkers suggest MeHg intake exceeds by several fold the level considered by WHO and FAO to pose no substantial risk of developmental neurotoxicity. In the second group, average biomarkers suggest MeHg intake well over the reference value. In the third group, biomarkers suggest an important share of the population approach or exceed the reference level. High-end biomarkers in all three groups indicate body burdens of MeHg in the range associated in epidemiological studies with observable neurological damage. While average biomarkers in other groups suggest that MeHg intake is below the recommended level, most upper bound biomarkers in these categories exceed the reference, which shows that even in groups with lower average exposure certain populations are at risk.
Before this study, few researchers had systematically compared the global exposures and risks linked to MeHg intake from seafood. Brune et al. reviewed Hg biomarker studies – published from 1976 to 1990 – of general populations exposed through various sources and found the highest values among seafood consumers in Greenland and Japan.197 Sioen et al. estimated contaminant and nutrient intake in general populations based on global seafood availability data and found the estimated MeHg intake to be highest in Japan and the Pacific islands, followed by the Nordic and Mediterranean regions.198 A recent European regional study examining biomarkers showed the highest MeHg exposure to be in Mediterranean countries.199 Our findings are broadly consistent with these studies and with the literature describing MeHg exposure and risk in specific subsistence fishing communities. This review adds to the evidence by synthesizing the findings from the two most recent decades of published international Hg biomarker data specifically for women and infants and by examining, in a single study, MeHg exposure in populations consuming self-caught and commercially purchased seafood.
Several limitations affect the interpretation of our results. Our goal was to compare MeHg exposure across various international groups of women and infants from seafood-consuming populations. However, incomplete reporting prevented us from evaluating the share of non-consumers of seafood in each study. Furthermore, most studies used convenience samples that may not have been representative of the populations from which they were taken. In sensitivity analysis we pooled biomarkers excluding the several large representative population surveys (which have a higher share of non-consumers of seafood than other studies). However, this did not alter our findings. Physiological differences in MeHg metabolism and elimination by life stage are well known200 and the FAO/WHO reference dose was established based on maternal biomarkers. Thus, in sensitivity analysis we also combined biomarkers excluding infants. This resulted in slightly lower medians for the Arctic and gold mining categories and higher ones for the coastal and inland categories.
TBHg is a better indicator of recent MeHg exposure than THHg, which is a better measure of longer-term MeHg exposure.3,4,6 Although this difference may be important among sporadic seafood consumers, the majority of our subpopulations were regular seafood consumers. Conversion of TBHg biomarkers to THHg equivalents is likely to have resulted in some measurement error. However, the range of hair-to-blood ratios reported in our studies was similar to the range on which the standard conversion ratio is based, which minimizes this bias.5 When we pooled only THHg biomarkers, medians were slightly higher across most categories (although some categories had few observations). Despite the use of laboratory methods that relied on commonly employed protocols, detection techniques are subject to variation3,11 and quality control practices were not uniformly reported. Sensitivity analysis examining only studies using CV-AAS or similar procedures resulted in slightly higher biomarkers for the Arctic category.
Population Hg biomarker distributions are often skewed to the right, so that central tendency is best captured by geometric means or medians.3 Thus, in reporting our main results we chose to exclude the small number of studies reporting only arithmetic means. Including arithmetic means yielded higher results for the inland category. To give greater weight to estimates from larger samples, we pooled biomarkers using sample-size weighting. Doing so yielded higher summary biomarkers in the Arctic and coastal categories. Variations in the share of MeHg in total Hg have been reported, both among frequent and infrequent seafood consumers,23,201 depending in part on exposure to Hg sources other than seafood (such as elemental Hg in dental amalgams or inorganic Hg compounds in skin-lightening creams).3,29 Most of the one quarter of selected studies examining non-seafood sources of Hg assessed the presence of dental amalgams, mainly in infrequent consumers of seafood; while this inorganic Hg source is best measured with urinary biomarkers, in cases where this exposure is important TBHg biomarkers may overestimate MeHg.26 We eliminated high outlier biomarkers due to suspected non-seafood sources wherever these were noted by authors (most were in subpopulations where skin-lightening creams were used). Nevertheless, other sources of Hg exposure influencing high-end measures cannot be excluded. These limitations in the underlying data suggest that our findings should be interpreted cautiously. However, most sensitivity analyses resulted in higher biomarker summary statistics than the main findings we report; we chose conservative assumptions for our main results.
Estimated IQ losses in infants born to seafood consuming mothers serve as an alternative means of characterizing the public health impact of MeHg exposure. As an illustration, we applied a dose–response relationship (0.18 infant IQ point lost for every ppm increase in maternal THHg)202 that has been used to estimate the economic costs associated with Hg contamination203,204 to our pooled upper bound biomarkers. The resulting interquartile range of estimated IQ loss spanned from 1 to 13 points for the gold mining, Arctic and coastal subpopulation categories. IQ losses at the higher end of this range may be sufficient to contribute to mild mental retardation, defined as an IQ between 50 and 69 points. Among subsistence fishing populations in the Amazon, an assessment of global burden of disease showed an incidence of mild mental retardation of up to 17.4 cases per 1000 infants205 and separate research identified MeHg-associated deficits in memory and learning in adults.206 IQ losses in the lower end of the range may contribute to borderline intellectual functioning, characterized by memory and executive function deficits.207 Although such minor losses in IQ may go unnoticed in an individual, they can cause an important shift in intellectual capacity at the population level, as documented in the case of lead.208 IQ loss represents only one facet of the neurological harm resulting from MeHg; our analysis did not include recent research suggesting neurological effects at lower dose11 or other documented effects, such as adverse cardiovascular outcomes.209
Systematic reviews provide an opportunity to identify gaps in a body of research. Small-scale gold mining is practiced in 70 countries,210 but we found Hg biomarker studies meeting our criteria in only six. We identified studies in 23 coastal countries, although per capita seafood consumption data suggest that many other such countries warrant study.20 Although reviews of subsistence fishing populations in the Amazon and Arctic are available, few have been conducted for coast-dwelling frequent seafood consumers (e.g. in south-eastern Asia or the Mediterranean) or for fishing populations near abandoned chloralkali plants and other aquatic sources of Hg contamination. We found population-based Hg biomonitoring surveys in only a handful of countries; most are high-income and have relatively low per capita seafood consumption.
It was beyond the scope of this review to assess time trends in Hg biomarkers. Without major policy changes, projections indicate that global anthropogenic Hg emissions are likely to increase.211 Moreover, modelling suggests that any reduction in Hg emissions is likely to take time to translate into reduced MeHg in seafood.212 Declines in Hg biomarkers in humans have been observed in association with changes in seafood consumption habits in various populations. This finding reinforces the importance of carefully designed public health messages intended to reduce MeHg exposure.199,212 In subsistence fishing populations, the cultural importance of seafood harvesting and the scarcity of alternative animal protein sources suggest the existence of complex tradeoffs in guiding seafood consumption and the need for well-targeted messages. In predominantly urban seafood-consuming coastal populations, commercial seafood advisories may be an appropriate choice for reaching at-risk populations.19 Because of seafood’s important nutritional benefits, all such messages should aim to encourage a shift away from large apex predator species and towards those with lower MeHg and higher polyunsaturated fatty acid content, rather than to reduce seafood intake.
Conclusion
In this review of biomarkers of MeHg intake in women and infants from 164 studies across 43 countries, we found a very high risk in tropical riverine populations near gold mining sites and in traditional Arctic populations. In both groups, biomarkers suggest average MeHg intake exceeds the FAO/WHO recommendation, although their share of the global total of seafood-consuming women and infants is likely to be fairly small. We also found an elevated risk among seafood consumers in the coastal regions of south-eastern Asia, the western Pacific and the Mediterranean; a large share of the world’s seafood-consuming women and their infants is likely to be found in this group because of its large population. In other populations for whom data were available, average indicators of risk were lower and generally within international intake recommendations. However, women and infants with high exposure to MeHg were evident across all exposure categories. Although sources of bias were present, these results should help to set broad priorities for preventive policy and research.
The findings of this review underscore the importance of WHO’s call for enhanced population monitoring and risk communication to women of reproductive age regarding healthful seafood choices.1 One of the provisions of the Minamata Convention aims to protect vulnerable populations from Hg exposure through public education and other measures.213 The Convention is a potentially important strategic tool to reach the populations at highest risk through development of seafood advisory risk messages for commercial seafood consumers, targeted community-based interventions for subsistence fishing groups and regular population surveillance.
Competing interests:
None declared.
References
- 1.World Health Organization [Internet]. Mercury and health (Fact sheet No. 361). Geneva: WHO; 2013. Available from: http://www.who.int/mediacentre/factsheets/fs361/en/ [accessed 11 October 2013]
- 2.“Minamata” Convention agreed by nations: global mercury agreement to lift health threats from lives of millions world-wide Geneva: United Nations Environment Programme; 2013. Available from: http://www.unep.org/hazardoussubstances/Portals/9/Mercury/Documents/INC5/press_release_mercury_Jan_19_2013.pdf [accessed 11 October 2013].
- 3.Committee on Toxicological Effects of Methylmercury, National Research Council of the United States, National Academies of Science. Toxicological effects of methylmercury Washington: National Academies Press; 2000. [Google Scholar]
- 4.Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36:609–62. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- 5.Environmental health criteria document 101: methylmercury Geneva: International Program for Chemical Safety, World Health Organization; 1990. [Google Scholar]
- 6.United Nations Environment Programme. DTIE Chemicals Branch. Guidance for identifying populations at risk from mercury exposure Geneva: World Health Organization, Department of Food Safety, Zoonoses and Foodborne Diseases; 2008. Available from: http://www.who.int/foodsafety/publications/chem/mercuryexposure.pdf [accessed 11October 2013].
- 7.Crump KS, Kjellström T, Shipp AM, Silvers A, Stewart A. Influence of prenatal mercury exposure upon scholastic and psychological test performance: benchmark analysis of a New Zealand cohort. Risk Anal. 1998;18:701–13. doi: 10.1023/B:RIAN.0000005917.52151.e6. [DOI] [PubMed] [Google Scholar]
- 8.Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997;19:417–28. doi: 10.1016/S0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- 9.Myers GJ, Marsh DO, Davidson PW, Cox C, Shamlaye CF, Tanner M, et al. Main neurodevelopmental study of Seychellois children following in utero exposure to methylmercury from a maternal fish diet: outcome at six months. Neurotoxicology. 1995;16:653–64. [PubMed] [Google Scholar]
- 10.Joint FAO/WHO Expert Committee on Food Additives. In: Sixty-first meeting, Rome, 10–19 June 2003: summary and conclusions Food and Agriculture Organization of the United Nations & World Health Organization; 2003. Available from: ftp://ftp.fao.org/es/esn/jecfa/jecfa61sc.pdf [accessed 11 October 2013].
- 11.Karagas MR, Choi AL, Oken E, Horvat M, Schoeny R, Kamai E, et al. Evidence on the human health effects of low-level methylmercury exposure. Environ Health Perspect. 2012;120:799–806. doi: 10.1289/ehp.1104494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahaffey KR. Fish and shellfish as dietary sources of methylmercury and the omega-3 fatty acids, eicosahexaenoic acid and docosahexaenoic acid: risks and benefits. Environ Res. 2004;95:414–28. doi: 10.1016/j.envres.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Mergler D, Anderson HA, Chan LHM, Mahaffey KR, Murray M, Sakamoto M, et al. Panel on Health Risks and Toxicological Effects of Methylmercury Methylmercury exposure and health effects in humans: a worldwide concern. Ambio. 2007;36:3–11. doi: 10.1579/0044-7447(2007)36[3:MEAHEI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Selin NE, Sunderland EM, Knightes CD, Mason RP. Sources of mercury exposure for US seafood consumers: implications for policy. Environ Health Perspect. 2010;118:137–43. doi: 10.1289/ehp.0900811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Food and Nutrition Board, Institute of Medicine of the National Academies. Nesheim MC, Yaktine AL, editors. Seafood choices: balancing benefits and risks Washington: National Academies Press; 2006. [Google Scholar]
- 16.Mahaffey KR, Sunderland EM, Chan HM, Choi AL, Grandjean P, Mariën K, et al. Balancing the benefits of n-3 polyunsaturated fatty acids and the risks of methylmercury exposure from fish consumption. Nutr Rev. 2011;69:493–508. doi: 10.1111/j.1753-4887.2011.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimshack JP, Ward MB. Mercury advisories and household health trade-offs. J Health Econ. 2010;29:674–85. doi: 10.1016/j.jhealeco.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Lando AM, Zhang Y. Awareness and knowledge of methylmercury in fish in the United States. Environ Res. 2011;111:442–50. doi: 10.1016/j.envres.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Sheehan MC. Risk of developmental neurotoxicity due to methylmercury in seafood: examining global exposures, susceptibility and policy Johns Hopkins Bloomberg School of Public Health, Health Policy and Management Department; 2011. [Dissertation]. [Google Scholar]
- 20.The state of world fisheries and aquaculture: world review of fisheries and aquaculture – part 1 Rome: Food and Agriculture Organization; 2010. Available from: http://www.fao.org/docrep/013/i1820e/i1820e01.pdf [accessed 12 October 2013].
- 21.United Nations Department of Economic and Social Affairs. Population Division, Population Estimates and Projections Section [Internet]. World population prospects: the 2012 revision – population by age groups – female. New York: United Nations; 2013. Available from: http://esa.un.org/wpp/Excel-Data/population.htm [accessed 12 October 2013].
- 22.United Nations Environment Programme. Vital water graphics: an overview of the state of the world’s fresh and and marine waters 2nd ed. New York: United Nations; 2008. Available from: http://www.unep.org/dewa/vitalwater/article176.html [accessed 12 October 2013].
- 23.Passos CJ, Mergler D. Human mercury exposure and adverse health effects in the Amazon: a review. Cad Saude Publica. 2008;24:S503–20. doi: 10.1590/s0102-311x2008001600004. [DOI] [PubMed] [Google Scholar]
- 24.Van Oostdam J, Donaldson SG, Feeley M, Arnold D, Ayotte P, Bondy G, et al. Human health implications of environmental contaminants in Arctic Canada: A review. Sci Total Environ. 2005;351-352:165–246. doi: 10.1016/j.scitotenv.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 25.Committee on Human Biomonitoring for Environmental Toxicants, National Research Council of the United States National Academies of Science. Human biomonitoring for environmental chemicals Washington: National Academies Press; 2006. [Google Scholar]
- 26.Berglund M, Lind B, Björnberg KA, Palm B, Einarsson O, Vahter M. Inter-individual variations of human mercury exposure biomarkers: a cross-sectional assessment. Environ Health. 2005;4:20. doi: 10.1186/1476-069X-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 28.Pirrone N, Mahaffey K. Dynamics of mercury pollution on regional and global scales: atmospheric processes and human exposures around the world New York: Springer; 2005. [Google Scholar]
- 29.Mahaffey KR, Clickner RP, Bodurow CC. Blood organic mercury and dietary mercury intake: National Health and Nutrition Examination Survey, 1999 and 2000. Environ Health Perspect. 2004;112:562–70. doi: 10.1289/ehp.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groth E 3rd. Ranking the contributions of commercial fish and shellfish varieties to mercury exposure in the United States: implications for risk communication. Environ Res. 2010;110:226–36. doi: 10.1016/j.envres.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Balshaw S, Edwards J, Daughtry B, Ross K. Mercury in seafood: mechanisms of accumulation and consequences for consumer health. Rev Environ Health. 2007;22:91–113. doi: 10.1515/reveh.2007.22.2.91. [DOI] [PubMed] [Google Scholar]
- 32.Mahaffey KR, Clickner RP, Jeffries RA. Adult women’s blood mercury concentrations vary regionally in the United States: association with patterns of fish consumption (NHANES 1999–2004). Environ Health Perspect. 2009;117:47–53. doi: 10.1289/ehp.11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monrroy SX, Lopez RW, Roulet M, Benefice E. Lifestyle and mercury contamination of Amerindian populations along the Beni river (lowland Bolivia). J Environ Health. 2008;71:44–50. [PubMed] [Google Scholar]
- 34.Barbieri FL, Cournil A, Gardon J. Mercury exposure in a high fish eating Bolivian Amazonian population with intense small-scale gold-mining activities. Int J Environ Health Res. 2009;19:267–77. doi: 10.1080/09603120802559342. [DOI] [PubMed] [Google Scholar]
- 35.Boischio AAP, Barbosa A.Exposição ao mercúrio orgânico em populações Ribeirinhas do Alto Madeira, Rondônia, 1991: resultados preliminares. Cad Saude Publica 19939155–60.Portugese10.1590/S0102-311X1993000200006 [DOI] [PubMed] [Google Scholar]
- 36.Barbosa AC, Silva SRL, Dórea JG. Concentration of mercury in hair of indigenous mothers and infants from the Amazon basin. Arch Environ Contam Toxicol. 1998;34:100–5. doi: 10.1007/s002449900291. [DOI] [PubMed] [Google Scholar]
- 37.Lebel J, Mergler D, Branches F, Lucotte M, Amorim M, Larribe F, et al. Neurotoxic effects of low-level methylmercury contamination in the Amazonian Basin. Environ Res. 1998;79:20–32. doi: 10.1006/enrs.1998.3846. [DOI] [PubMed] [Google Scholar]
- 38.Grandjean P, White RF, Nielsen A, Cleary D, de Oliveira Santos EC. Methylmercury neurotoxicity in Amazonian children downstream from gold mining. Environ Health Perspect. 1999;107:587–91. doi: 10.1289/ehp.99107587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amorim MIM, Mergler D, Bahia MO, Dubeau H, Miranda D, Lebel J, et al. Cytogenetic damage related to low levels of methyl mercury contamination in the Brazilian Amazon. An Acad Bras Cienc. 2000;72:497–507. doi: 10.1590/S0001-37652000000400004. [DOI] [PubMed] [Google Scholar]
- 40.Boischio AA, Henshel D. Fish consumption, fish lore, and mercury pollution–risk communication for the Madeira River people. Environ Res. 2000;84:108–26. doi: 10.1006/enrs.2000.4035. [DOI] [PubMed] [Google Scholar]
- 41.Dolbec J, Mergler D, Sousa Passos CJ, Sousa de Morais S, Lebel J. Methylmercury exposure affects motor performance of a riverine population of the Tapajós river, Brazilian Amazon. Int Arch Occup Environ Health. 2000;73:195–203. doi: 10.1007/s004200050027. [DOI] [PubMed] [Google Scholar]
- 42.Harada M, Nakanishi J, Yasoda E, Pinheiro MCN, Oikawa T, de Assis Guimarâes G, et al. Mercury pollution in the Tapajos River basin, Amazon: mercury level of head hair and health effects. Environ Int. 2001;27:285–90. doi: 10.1016/S0160-4120(01)00059-9. [DOI] [PubMed] [Google Scholar]
- 43.Crompton P, Ventura AM, de Souza JM, Santos E, Strickland GT, Silbergeld E. Assessment of mercury exposure and malaria in a Brazilian Amazon riverine community. Environ Res. 2002;90:69–75. doi: 10.1006/enrs.2002.4358. [DOI] [PubMed] [Google Scholar]
- 44.Santos ECO, de Jesus IM, Câmara VdeM, Brabo E, Loureiro ECB, Mascarenhas A, et al. Mercury exposure in Munduruku Indians from the community of Sai Cinza, State of Pará, Brazil. Environ Res. 2002;90:98–103. doi: 10.1006/enrs.2002.4389. [DOI] [PubMed] [Google Scholar]
- 45.Santos ECO, Câmara VdeM, Brabo EdaS, Loureiro ECB, de Jesus IM, Fayal K, et al. Avaliação dos níveis de exposição ao mercúrio entre índios Pakaanóva, Amazônia, Brasil. Cad Saude Publica 200319199–206.Portugese 10.1590/S0102-311X2003000100022 [DOI] [PubMed] [Google Scholar]
- 46.Santos EO, Jesus IM, Câmara VdeM, Brabo EdaS, Jesus MI, Fayal KF, et al. Correlation between blood mercury levels in mothers and newborns in Itaituba, Pará State, Brazil. Cad Saude Publica. 2007;23(Suppl 4):S622–9. doi: 10.1590/S0102-311X2007001600022. [DOI] [PubMed] [Google Scholar]
- 47.Passos CJ, Da Silva DS, Lemire M, Fillion M, Guimarães JR, Lucotte M, et al. Daily mercury intake in fish-eating populations in the Brazilian Amazon. J Expo Sci Environ Epidemiol. 2008;18:76–87. doi: 10.1038/sj.jes.7500599. [DOI] [PubMed] [Google Scholar]
- 48.Grotto D, Valentini J, Fillion M, Passos CJ, Garcia SC, Mergler D, et al. Mercury exposure and oxidative stress in communities of the Brazilian Amazon. Sci Total Environ. 2010;408:806–11. doi: 10.1016/j.scitotenv.2009.10.053. [DOI] [PubMed] [Google Scholar]
- 49.Fillion M, Lemire M, Philibert A, Frenette B, Weiler HA, Deguire JR, et al. Visual acuity in fish consumers of the Brazilian Amazon: risks and benefits from local diet. Public Health Nutr. 2011;14:2236–44. doi: 10.1017/S1368980011001765. [DOI] [PubMed] [Google Scholar]
- 50.Dórea JG, Marques RC, Isejima C. Neurodevelopment of Amazonian infants: antenatal and postnatal exposure to methyl- and ethylmercury. J Biomed Biotechnol. 2012;2012:132876. doi: 10.1155/2012/132876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barcelos GR, Grotto D, de Marco KC, Valentini J, Lengert AV, de Oliveira AA, et al. Polymorphisms in glutathione-related genes modify mercury concentrations and antioxidant status in subjects environmentally exposed to methylmercury. Sci Total Environ 20136319–2.5 10.1016/j.scitotenv.2013.06.029 [DOI] [PubMed] [Google Scholar]
- 52.Marques RC, Bernardi JVE, Dórea JG, Brandão KG, Bueno L, Leão RS, et al. Fish consumption during pregnancy, mercury transfer, and birth weight along the Madeira River Basin in Amazonia. Int J Environ Res Public Health. 2013;10:2150–63. doi: 10.3390/ijerph10062150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vieira SM, de Almeida R, Holanda IBB, Mussy MH, Galvão RCF, Crispim PTB, et al. Total and methyl-mercury in hair and milk of mothers living in the city of Porto Velho and in villages along the Rio Madeira, Amazon, Brazil. Int J Hyg Environ Health. 2013;216:682–9. doi: 10.1016/j.ijheh.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 54.Olivero-Verbel J, Caballero-Gallardo K, Marrugo Negrete J. Relationship between localization of gold mining areas and hair mercury levels in people from Bolivar, north of Colombia. Biol Trace Elem Res. 2011;144:118–32. doi: 10.1007/s12011-011-9046-5. [DOI] [PubMed] [Google Scholar]
- 55.Cordier S, Grasmick C, Paquier-Passelaigue M, Mandereau L, Weber J-P, Jouan M. Mercury exposure in French Guiana: levels and determinants. Arch Environ Health. 1998;53:299–303. doi: 10.1080/00039899809605712. [DOI] [PubMed] [Google Scholar]
- 56.Cordier S, Garel M, Mandereau L, Morcel HH, Doineau P, Gosme-Seguret S, et al. Neurodevelopmental investigations among methylmercury-exposed children in French Guiana. Environ Res. 2002;89:1–11. doi: 10.1006/enrs.2002.4349. [DOI] [PubMed] [Google Scholar]
- 57.Fujimura M, Matsuyama A, Harvard JP, Bourdineaud JP, Nakamura K. Mercury contamination in humans in Upper Maroni, French Guiana between 2004 and 2009. Bull Environ Contam Toxicol. 2012;88:135–9. doi: 10.1007/s00128-011-0497-3. [DOI] [PubMed] [Google Scholar]
- 58.Bose-O’Reilly S, Drasch G, Beinhoff C, Rodrigues-Filho S, Roider G, Lettmeier B, et al. Health assessment of artisanal gold miners in Indonesia. Sci Total Environ. 2010;408:713–25. doi: 10.1016/j.scitotenv.2009.10.070. [DOI] [PubMed] [Google Scholar]
- 59.Hacon S, Yokoo E, Valente J, Campos RC, da Silva VA, de Menezes ACC, et al. Exposure to mercury in pregnant women from Alta Floresta-Amazon basin, Brazil. Environ Res. 2000;84:204–10. doi: 10.1006/enrs.2000.4115. [DOI] [PubMed] [Google Scholar]
- 60.Marques RC, Garrofe Dórea J, Rodrigues Bastos W, de Freitas Rebelo M, de Freitas Fonseca M, Malm O. Maternal mercury exposure and neuro-motor development in breastfed infants from Porto Velho (Amazon), Brazil. Int J Hyg Environ Health. 2007;210:51–60. doi: 10.1016/j.ijheh.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Mohan S, Tiller M, van der Voet G, Kanhai H. Mercury exposure of mothers and newborns in Surinam: a pilot study. Clin Toxicol (Phila) 2005;43:101–4. [PubMed] [Google Scholar]
- 62.Dewailly E, Ayotte P, Bruneau S, Lebel G, Levallois P, Weber JP. Exposure of the Inuit population of Nunavik (Arctic Quebec) to lead and mercury. Arch Environ Health. 2001;56:350–7. doi: 10.1080/00039890109604467. [DOI] [PubMed] [Google Scholar]
- 63.Muckle G, Ayotte P, Dewailly E, Jacobson SW, Jacobson JL. Prenatal exposure of the northern Québec Inuit infants to environmental contaminants. Environ Health Perspect. 2001;109:1291–9. doi: 10.1289/ehp.011091291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lucas M, Dewailly E, Muckle G, Ayotte P, Bruneau S, Gingras S, et al. Gestational age and birth weight in relation to n-3 fatty acids among Inuit (Canada). Lipids. 2004;39:617–26. doi: 10.1007/s11745-004-1274-7. [DOI] [PubMed] [Google Scholar]
- 65.Butler Walker J, Houseman J, Seddon L, McMullen E, Tofflemire K, Mills C, et al. Maternal and umbilical cord blood levels of mercury, lead, cadmium, and essential trace elements in Arctic Canada. Environ Res. 2006;100:295–318. doi: 10.1016/j.envres.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 66.Fontaine J, Dewailly E, Benedetti J-L, Pereg D, Ayotte P, Déry S. Re-evaluation of blood mercury, lead and cadmium concentrations in the Inuit population of Nunavik (Québec): a cross-sectional study. Environ Health. 2008;7:25. doi: 10.1186/1476-069X-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grandjean P, Weihe P, Jørgensen PJ, Clarkson T, Cernichiari E, Viderø T. Impact of maternal seafood diet on fetal exposure to mercury, selenium, and lead. Arch Environ Health. 1992;47:185–95. doi: 10.1080/00039896.1992.9938348. [DOI] [PubMed] [Google Scholar]
- 68.Bjerregaard P, Hansen JC. Organochlorines and heavy metals in pregnant women from the Disko Bay area in Greenland. Sci Total Environ. 2000;245:195–202. doi: 10.1016/S0048-9697(99)00444-1. [DOI] [PubMed] [Google Scholar]
- 69.Nielsen ABS, Davidsen M, Bjerregaard P. The association between blood pressure and whole blood methylmercury in a cross-sectional study among Inuit in Greenland. Environ Health. 2012;11:44. doi: 10.1186/1476-069X-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Odland JO, Nieboer E, Romanova N, Thomassen Y, Brox J, Lund E. Self-reported ethnic status of delivering women, newborn body mass index, blood or urine concentrations of toxic metals, and essential elements in sera of Norwegian and Russian Arctic populations. Int J Circumpolar Health. 1999;58:4–13. [PubMed] [Google Scholar]
- 71.Hansen S, Nieboer E, Sandanger TM, Wilsgaard T, Thomassen Y, Veyhe AS, et al. Changes in maternal blood concentrations of selected essential and toxic elements during and after pregnancy. J Environ Monit. 2011;13:2143–52. doi: 10.1039/c1em10051c. [DOI] [PubMed] [Google Scholar]
- 72.Klopov VP. Levels of heavy metals in women residing in the Russian Arctic. Int J Circumpolar Health. 1998;57(Suppl 1):582–5. [PubMed] [Google Scholar]
- 73.Arnold SM, Lynn TV, Verbrugge LA, Middaugh JP. Human biomonitoring to optimize fish consumption advice: reducing uncertainty when evaluating benefits and risks. Am J Public Health. 2005;95:393–7. doi: 10.2105/AJPH.2004.042879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nilson SA, Jr, Costa M, Akagi H. Total and methylmercury levels of a coastal human population and of fish from the Brazilian northeast. Environ Sci Pollut Res Int. 2001;8:280–4. doi: 10.1007/BF02987408. [DOI] [PubMed] [Google Scholar]
- 75.Kuno R, Roquetti MH, Becker K, Seiwert M, Gouveia N. Reference values for lead, cadmium and mercury in blood of adults from the metropolitan area of Sao Paulo (Brazil). Toxicol Letters. 2010;196:1(S40). [Google Scholar]
- 76.Bruhn CG, Rodŕiguez AA, Barrios C, Jaramillo VH, Becerra J, Gonzáles U, et al. Determination of total mercury in scalp hair of pregnant and nursing women resident in fishing villages in the Eighth Region of Chile. J Trace Elem Electrolytes Health Dis. 1994;8:79–86. [PubMed] [Google Scholar]
- 77.Li Z, Wang Q, Luo Y. Exposure of the urban population to mercury in Changchun city, Northeast China. Environ Geochem Health. 2006;28:61–6. doi: 10.1007/s10653-005-9012-2. [DOI] [PubMed] [Google Scholar]
- 78.Zhang L, Wang Q. Preliminary study on health risk from mercury exposure to residents of Wujiazhan town on the Di’er Songhua river, Northeast China. Environ Geochem Health. 2006;28:67–71. doi: 10.1007/s10653-005-9013-1. [DOI] [PubMed] [Google Scholar]
- 79.Tang D, Li T-Y, Liu JJ, Zhou Z-J, Yuan T, Chen Y-H, et al. Effects of prenatal exposure to coal-burning pollutants on children’s development in China. Environ Health Perspect. 2008;116:674–9. doi: 10.1289/ehp.10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fang T, Aronson KJ, Campbell LM. Freshwater fish-consumption relations with total hair mercury and selenium among women in eastern China. Arch Environ Contam Toxicol. 2012;62:323–32. doi: 10.1007/s00244-011-9689-4. [DOI] [PubMed] [Google Scholar]
- 81.Pawlas N, Strömberg U, Carlberg B, Cerna M, Harari F, Harari R, et al. Cadmium, mercury and lead in the blood of urban women in Croatia, the Czech Republic, Poland, Slovakia, Slovenia, Sweden, China, Ecuador and Morocco. Int J Occup Med Environ Health. 2013;26:58–72. doi: 10.2478/S13382-013-0071-9. [DOI] [PubMed] [Google Scholar]
- 82.Olivero-Verbel J, Johnson-Restrepo B, Baldiris-Avila R, Güette-Fernández J, Magallanes-Carreazo E, Vanegas-Ramírez L, et al. Human and crab exposure to mercury in the Caribbean coastal shoreline of Colombia: impact from an abandoned chlor-alkali plant. Environ Int. 2008;34:476–82. doi: 10.1016/j.envint.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 83.Madeddu A, Sciacca S.Monitoraggio biologico sulla presenza di Hg, PCB e HCG in latte e capelli di donne residenti in un’area ad alta incidenza di nati malformati (Augusta). Ann Ig 200820Suppl 159–64.Italian [PubMed] [Google Scholar]
- 84.Deroma L, Parpinel M, Tognin V, Channoufi L, Tratnik J, Horvat M, et al. Neuropsychological assessment at school-age and prenatal low-level exposure to mercury through fish consumption in an Italian birth cohort living near a contaminated site. Int J Hyg Environ Health. 2013;216:486–93. doi: 10.1016/j.ijheh.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 85.Hsiao H-W, Ullrich SM, Tanton TW. Burdens of mercury in residents of Temirtau, Kazakhstan I: hair mercury concentrations and factors of elevated hair mercury levels. Sci Total Environ. 2011;409:2272–80. doi: 10.1016/j.scitotenv.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 86.Lim S, Chung H-U, Paek D. Low dose mercury and heart rate variability among community residents nearby to an industrial complex in Korea. Neurotoxicology. 2010;31:10–6. doi: 10.1016/j.neuro.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 87.Trasande L, Cortes JE, Landrigan PJ, Abercrombie MI, Bopp RF, Cifuentes E. Methylmercury exposure in a subsistence fishing community in Lake Chapala, Mexico: an ecological approach. Environ Health. 2010;9:1. doi: 10.1186/1476-069X-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Elhamri H, Idrissi L, Coquery M, Azemard S, El Abidi A, Benlemlih M, et al. Hair mercury levels in relation to fish consumption in a community of the Moroccan Mediterranean coast. Food Addit Contam. 2007;24:1236–46. doi: 10.1080/02652030701329611. [DOI] [PubMed] [Google Scholar]
- 89.Lacayo M, Cruz A, Lacayo J, Fomsgaard I. Mercury contamination in Lake Xolotlan (Managua). Hydrobiol Bull. 1991;25:173–6. doi: 10.1007/BF02291251. [DOI] [PubMed] [Google Scholar]
- 90.Bravo AG, Loizeau J-L, Bouchet S, Richard A, Rubin JF, Ungureanu V-G, et al. Mercury human exposure through fish consumption in a reservoir contaminated by a chlor-alkali plant: Babeni reservoir (Romania). Environ Sci Pollut Res. 2010;17:1422–32. doi: 10.1007/s11356-010-0328-9. [DOI] [PubMed] [Google Scholar]
- 91.Palkovicova L, Ursinyova M, Masanova V, Yu Z, Hertz-Picciotto I. Maternal amalgam dental fillings as the source of mercury exposure in developing fetus and newborn. J Expo Sci Environ Epidemiol. 2008;18:326–31. doi: 10.1038/sj.jes.7500606. [DOI] [PubMed] [Google Scholar]
- 92.Oskarsson A, Lagerkvist BJ, Ohlin B, Lundberg K. Mercury levels in the hair of pregnant women in a polluted area in Sweden. Sci Total Environ. 1994;151:29–35. doi: 10.1016/0048-9697(94)90483-9. [DOI] [PubMed] [Google Scholar]
- 93.Chang J-W, Pai M-C, Chen H-L, Guo H-R, Su H-J, Lee C-C. Cognitive function and blood methylmercury in adults living near a deserted chloralkali factory. Environ Res. 2008;108:334–9. doi: 10.1016/j.envres.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 94.Lincoln RA, Shine JP, Chesney EJ, Vorhees DJ, Grandjean P, Senn DB. Fish consumption and mercury exposure among Louisiana recreational anglers. Environ Health Perspect. 2011;119:245–51. doi: 10.1289/ehp.1002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rojas M, Nakamura K, Seijas D, Squiuante G, Pieters MA, Infante S. Mercury in hair as a biomarker of exposure in a coastal Venezuelan population. Invest Clin. 2007;48:305–15. [PubMed] [Google Scholar]
- 96.Black FJ, Bokhutlo T, Somoxa A, Maethamako M, Modisaemang O, Kemosedile T, et al. The tropical African mercury anomaly: lower than expected mercury concentrations in fish and human hair. Sci Total Environ. 2011;409:1967–75. doi: 10.1016/j.scitotenv.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 97.Girard M, Dumont C. Exposure of James Bay Cree to methylmercury during pregnancy for the years 1983–91. Water Air Soil Pollut. 1995;80:13–9. doi: 10.1007/BF01189648. [DOI] [Google Scholar]
- 98.Mahaffey KR, Mergler D. Blood levels of total and organic mercury in residents of the upper St. Lawrence River basin, Québec: association with age, gender, and fish consumption. Environ Res. 1998;77:104–14. doi: 10.1006/enrs.1998.3834. [DOI] [PubMed] [Google Scholar]
- 99.Belles-Isles M, Ayotte P, Dewailly E, Weber J-P, Roy R. Cord blood lymphocyte functions in newborns from a remote maritime population exposed to organochlorines and methylmercury. J Toxicol Environ Health A. 2002;65:165–82. doi: 10.1080/152873902753396794. [DOI] [PubMed] [Google Scholar]
- 100.Cole DC, Kearney J, Sanin LH, Leblanc A, Weber J-P. Blood mercury levels among Ontario anglers and sport-fish eaters. Environ Res. 2004;95:305–14. doi: 10.1016/j.envres.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 101.Morrissette J, Takser L, St-Amour G, Smargiassi A, Lafond J, Mergler D. Temporal variation of blood and hair mercury levels in pregnancy in relation to fish consumption history in a population living along the St. Lawrence River. Environ Res. 2004;95:363–74. doi: 10.1016/j.envres.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 102.Abdelouahab N, Mergler D, Takser L, Vanier C, St-Jean M, Baldwin M, et al. Gender differences in the effects of organochlorines, mercury, and lead on thyroid hormone levels in lakeside communities of Quebec (Canada). Environ Res. 2008;107:380–92. doi: 10.1016/j.envres.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 103.Jenssen MTS, Brantsæter AL, Haugen M, Meltzer HM, Larssen T, Kvalem HE, et al. Dietary mercury exposure in a population with a wide range of fish consumption–self-capture of fish and regional differences are important determinants of mercury in blood. Sci Total Environ. 2012;439:220–9. doi: 10.1016/j.scitotenv.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 104.Johnsson C, Sällsten G, Schütz A, Sjörs A, Barregård L. Hair mercury levels versus freshwater fish consumption in household members of Swedish angling societies. Environ Res. 2004;96:257–63. doi: 10.1016/j.envres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 105.Stewart P, Reihman J, Lonky E, Darvill T, Pagano J. Prenatal PCB exposure and neonatal behavioral assessment scale (NBAS) performance. Neurotoxicol Teratol. 2000;22:21–9. doi: 10.1016/S0892-0362(99)00056-2. [DOI] [PubMed] [Google Scholar]
- 106.Knobeloch L, Gliori G, Anderson H. Assessment of methylmercury exposure in Wisconsin. Environ Res. 2007;103:205–10. doi: 10.1016/j.envres.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 107.Schantz SL, Gardiner JC, Aguiar A, Tang X, Gasior DM, Sweeney AM, et al. Contaminant profiles in Southeast Asian immigrants consuming fish from polluted waters in northeastern Wisconsin. Environ Res. 2010;110:33–9. doi: 10.1016/j.envres.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Carneiro MFH, Moresco MB, Chagas GR, de Oliveira Souza VC, Rhoden CR, Barbosa F., Jr Assessment of trace elements in scalp hair of a young urban population in Brazil. Biol Trace Elem Res. 2011;143:815–24. doi: 10.1007/s12011-010-8947-z. [DOI] [PubMed] [Google Scholar]
- 109.Legrand M, Arp P, Ritchie C, Chan HM. Mercury exposure in two coastal communities of the Bay of Fundy, Canada. Environ Res. 2005;98:14–21. doi: 10.1016/j.envres.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 110.Albert I, Villeret G, Paris A, Verger P. Integrating variability in half-lives and dietary intakes to predict mercury concentration in hair. Regul Toxicol Pharmacol. 2010;58:482–9. doi: 10.1016/j.yrtph.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 111.Drouillet-Pinard P, Huel G, Slama R, Forhan A, Sahuquillo J, Goua V, et al. Prenatal mercury contamination: relationship with maternal seafood consumption during pregnancy and fetal growth in the ‘EDEN mother-child’ cohort. Br J Nutr. 2010;104:1096–100. doi: 10.1017/S0007114510001947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vahter M, Akesson A, Lind B, Björs U, Schütz A, Berglund M. Longitudinal study of methylmercury and inorganic mercury in blood and urine of pregnant and lactating women, as well as in umbilical cord blood. Environ Res. 2000;84:186–94. doi: 10.1006/enrs.2000.4098. [DOI] [PubMed] [Google Scholar]
- 113.Björnberg KA, Vahter M, Petersson-Grawé K, Glynn A, Cnattingius S, Darnerud PO, et al. Methyl mercury and inorganic mercury in Swedish pregnant women and in cord blood: influence of fish consumption. Environ Health Perspect. 2003;111:637–41. doi: 10.1289/ehp.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rosborg I, Nihlgård B, Gerhardsson L. Hair element concentrations in females in one acid and one alkaline area in southern Sweden. Ambio. 2003;32:440–6. doi: 10.1579/0044-7447-32.7.440. [DOI] [PubMed] [Google Scholar]
- 115.Brantsaeter AL, Haugen M, Thomassen Y, Ellingsen DG, Ydersbond TA, Hagve TA, et al. Exploration of biomarkers for total fish intake in pregnant Norwegian women. Public Health Nutr. 2010;13:54–62. doi: 10.1017/S1368980009005904. [DOI] [PubMed] [Google Scholar]
- 116.Gerhardsson L, Lundh T. Metal concentrations in blood and hair in pregnant females in southern Sweden. J Environ Health. 2010;72:37–41. [PubMed] [Google Scholar]
- 117.Renzoni A, Zino F, Franchi E. Mercury levels along the food chain and risk for exposed populations. Environ Res. 1998;77:68–72. doi: 10.1006/enrs.1998.3832. [DOI] [PubMed] [Google Scholar]
- 118.Ramon R, Murcia M, Aguinagalde X, Amurrio A, Llop S, Ibarluzea J, et al. Prenatal mercury exposure in a multicenter cohort study in Spain. Environ Int. 2011;37:597–604. doi: 10.1016/j.envint.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 119.Björnberg KA, Vahter M, Grawé KP, Berglund M. Methyl mercury exposure in Swedish women with high fish consumption. Sci Total Environ. 2005;341:45–52. doi: 10.1016/j.scitotenv.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 120.Bates CJ, Prentice A, Birch MC, Delves HT. Dependence of blood indices of selenium and mercury on estimated fish intake in a national survey of British adults. Public Health Nutr. 2007;10:508–17. doi: 10.1017/S1368980007246683. [DOI] [PubMed] [Google Scholar]
- 121.Dewailly E, Rouja P, Forde M, Peek-Ball C, Côté S, Smith E, et al. Evaluation of a public health intervention to lower mercury exposure from fish consumption in Bermuda. PLoS One. 2012;7:e47388. doi: 10.1371/journal.pone.0047388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stern AH, Gochfeld M, Weisel C, Burger J. Mercury and methylmercury exposure in the New Jersey pregnant population. Arch Environ Health. 2001;56:4–10. doi: 10.1080/00039890109604048. [DOI] [PubMed] [Google Scholar]
- 123.Ortiz-Roque C, López-Rivera Y. Mercury contamination in reproductive age women in a Caribbean island: Vieques. J Epidemiol Community Health. 2004;58:756–7. doi: 10.1136/jech.2003.019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Oken E, Wright RO, Kleinman KP, Bellinger D, Amarasiriwardena CJ, Hu H, et al. Maternal fish consumption, hair mercury, and infant cognition in a U.S. Cohort. Environ Health Perspect. 2005;113:1376–80. doi: 10.1289/ehp.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McKelvey W, Gwynn RC, Jeffery N, Kass D, Thorpe LE, Garg RK, et al. A biomonitoring study of lead, cadmium, and mercury in the blood of New York city adults. Environ Health Perspect. 2007;115:1435–41. doi: 10.1289/ehp.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Karouna-Renier NK, Ranga Rao K, Lanza JJ, Rivers SD, Wilson PA, Hodges DK, et al. Mercury levels and fish consumption practices in women of child-bearing age in the Florida Panhandle. Environ Res. 2008;108:320–6. doi: 10.1016/j.envres.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 127.Lederman SA, Jones RL, Caldwell KL, Rauh V, Sheets SE, Tang D, et al. Relation between cord blood mercury levels and early child development in a World Trade Center cohort. Environ Health Perspect. 2008;116:1085–91. doi: 10.1289/ehp.10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Caldwell KL, Mortensen ME, Jones RL, Caudill SP, Osterloh JD. Total blood mercury concentrations in the U.S. population: 1999–2006. Int J Hyg Environ Health. 2009;212:588–98. doi: 10.1016/j.ijheh.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 129.Wells EM, Jarrett JM, Lin YH, Caldwell KL, Hibbeln JR, Apelberg BJ, et al. Body burdens of mercury, lead, selenium and copper among Baltimore newborns. Environ Res. 2011;111:411–7. doi: 10.1016/j.envres.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.King E, Shih G, Ratnapradipa D, Quilliam DN, Morton J, Magee SR. Mercury, lead, and cadmium in umbilical cord blood. J Environ Health. 2013;75:38–43. [PubMed] [Google Scholar]
- 131.Traynor S, Kearney G, Olson D, Hilliard A, Palcic J, Pawlowicz M. Fish consumption patterns and mercury exposure levels among women of childbearing age in Duval County, Florida. J Environ Health. 2013;75:8–15. [PubMed] [Google Scholar]
- 132.Babi D, Vasjari M, Celo V, Koroveshi M. Some results on Hg content in hair in different populations in Albania. Sci Total Environ. 2000;259:55–60. doi: 10.1016/S0048-9697(00)00549-0. [DOI] [PubMed] [Google Scholar]
- 133.Miklavčič A, Casetta A, Snoj Tratnik J, Mazej D, Krsnik M, Mariuz M, et al. Mercury, arsenic and selenium exposure levels in relation to fish consumption in the Mediterranean area. Environ Res. 2013;120:7–17. doi: 10.1016/j.envres.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 134.Gibičar D, Horvat M, Nakou S, Sarafidou J, Yager J. Pilot study of intrauterine exposure to methylmercury in Eastern Aegean islands, Greece. Sci Total Environ. 2006;367:586–95. doi: 10.1016/j.scitotenv.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 135.Vardavas CI, Patelarou E, Grandér M, Chatzi L, Palm B, Fthenou E, et al. The association between active/passive smoking and toxic metals among pregnant women in Greece. Xenobiotica. 2011;41:456–63. doi: 10.3109/00498254.2011.559294. [DOI] [PubMed] [Google Scholar]
- 136.Fakour H, Esmaili-Sari A, Zayeri F. Mercury exposure assessment in Iranian women’s hair of a port town with respect to fish consumption and amalgam fillings. Sci Total Environ. 2010;408:1538–43. doi: 10.1016/j.scitotenv.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 137.Salehi Z, Esmaili-Sari A. Hair mercury levels in pregnant women in Mahshahr, Iran: fish consumption as a determinant of exposure. Sci Total Environ. 2010;408:4848–54. doi: 10.1016/j.scitotenv.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 138.Barghi M, Behrooz RD, Esmaili-Sari A, Ghasempouri SM. Mercury exposure assessment in Iranian pregnant women’s hair with respect to diet, amalgam filling, and lactation. Biol Trace Elem Res. 2012;148:292–301. doi: 10.1007/s12011-012-9384-y. [DOI] [PubMed] [Google Scholar]
- 139.Okati N, Sari AE, Ghasempouri SM. Hair mercury concentrations of lactating mothers and breastfed infants in Iran (fish consumption and mercury exposure). Biol Trace Elem Res. 2012;149:155–62. doi: 10.1007/s12011-012-9424-7. [DOI] [PubMed] [Google Scholar]
- 140.Díez S, Montuori P, Pagano A, Sarnacchiaro P, Bayona JM, Triassi M. Hair mercury levels in an urban population from southern Italy: fish consumption as a determinant of exposure. Environ Int. 2008;34:162–7. doi: 10.1016/j.envint.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 141.Bou-Olayan AH, Al-Yakoob SN. Mercury in human hair: a study of residents in Kuwait. J Environ Sci Health A Tox Hazard Subst Environ Eng. 1994;A29:1541–51. [Google Scholar]
- 142.Khassouani CD, Soulaymani R, Jana M, Mauras Y, Allain P. Blood mercury concentrations in the population of Rabat area, Morocco. Bull Environ Contam Toxicol. 2001;66:439–42. doi: 10.1007/s00128-001-0025-y. [DOI] [PubMed] [Google Scholar]
- 143.Myers GJ, Marsh DO, Davidson PW, Cox C, Shamlaye CF, Tanner M, et al. Main neurodevelopmental study of Seychellois children following in utero exposure to methylmercury from a maternal fish diet: outcome at six months. Neurotoxicology. 1995;16:653–64. [PubMed] [Google Scholar]
- 144.Channa K, Odland JØ, Kootbodien T, Theodorou P, Naik I, Sandanger TM, et al. Differences in prenatal exposure to mercury in South African communities residing along the Indian Ocean. Sci Total Environ. 2013;463-464:11–9. doi: 10.1016/j.scitotenv.2013.05.055. [DOI] [PubMed] [Google Scholar]
- 145.Rudge CV, Röllin HB, Nogueira CM, Thomassen Y, Rudge MC, Odland JO. The placenta as a barrier for toxic and essential elements in paired maternal and cord blood samples of South African delivering women. J Environ Monit. 2009;11:1322–30. doi: 10.1039/b903805a. [DOI] [PubMed] [Google Scholar]
- 146.Soria ML, Sanz P, Martínez D, López-Artíguez M, Garrido R, Grilo A, et al. Total mercury and methylmercury in hair, maternal and umbilical blood, and placenta from women in the Seville area. Bull Environ Contam Toxicol. 1992;48:494–501. doi: 10.1007/BF00199063. [DOI] [PubMed] [Google Scholar]
- 147.Unuvar E, Ahmadov H, Kiziler AR, Aydemir B, Toprak S, Ulker V, et al. Mercury levels in cord blood and meconium of healthy newborns and venous blood of their mothers: clinical, prospective cohort study. Sci Total Environ. 2007;374:60–70. doi: 10.1016/j.scitotenv.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 148.Choy CMY, Lam CWK, Cheung LTF, Briton-Jones CM, Cheung LP, Haines CJ. Infertility, blood mercury concentrations and dietary seafood consumption: a case-control study. BJOG. 2002;109:1121–5. doi: 10.1111/j.1471-0528.2002.02084.x. [DOI] [PubMed] [Google Scholar]
- 149.Fok TF, Lam HS, Ng PC, Yip AS, Sin NC, Chan IH, et al. Fetal methylmercury exposure as measured by cord blood mercury concentrations in a mother-infant cohort in Hong Kong. Environ Int. 2007;33:84–92. doi: 10.1016/j.envint.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 150.Gao Y, Yan CH, Tian Y, Wang Y, Xie HF, Zhou X, et al. Prenatal exposure to mercury and neurobehavioral development of neonates in Zhoushan City, China. Environ Res. 2007;105:390–9. doi: 10.1016/j.envres.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 151.Liu X, Cheng J, Song Y, Honda S, Wang L, Liu Z, et al. Mercury concentration in hair samples from Chinese people in coastal cities. J Environ Sci (China) 2008;20:1258–62. doi: 10.1016/S1001-0742(08)62218-4. [DOI] [PubMed] [Google Scholar]
- 152.Dewailly E, Suhas E, Mou Y, Dallaire R, Chateau-Degat L, Chansin R. High fish consumption in French Polynesia and prenatal exposure to metals and nutrients. Asia Pac J Clin Nutr. 2008;17:461–70. [PubMed] [Google Scholar]
- 153.Nakagawa R. Concentration of mercury in hair of Japanese people. Chemosphere. 1995;30:127–33. doi: 10.1016/0045-6535(94)00382-5. [DOI] [PubMed] [Google Scholar]
- 154.Iwasaki Y, Sakamoto M, Nakai K, Oka T, Dakeishi M, Iwata T, et al. Estimation of daily mercury intake from seafood in Japanese women: Akita cross-sectional study. Tohoku J Exp Med. 2003;200:67–73. doi: 10.1620/tjem.200.67. [DOI] [PubMed] [Google Scholar]
- 155.Yasutake A, Matsumoto M, Yamaguchi M, Hachiya N. Current hair mercury levels in Japanese: survey in five districts. Tohoku J Exp Med. 2003;199:161–9. doi: 10.1620/tjem.199.161. [DOI] [PubMed] [Google Scholar]
- 156.Arakawa C, Yoshinaga J, Okamura K, Nakai K, Satoh H. Fish consumption and time to pregnancy in Japanese women. Int J Hyg Environ Health. 2006;209:337–44. doi: 10.1016/j.ijheh.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 157.Ohno T, Sakamoto M, Kurosawa T, Dakeishi M, Iwata T, Murata K. Total mercury levels in hair, toenail, and urine among women free from occupational exposure and their relations to renal tubular function. Environ Res. 2007;103:191–7. doi: 10.1016/j.envres.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 158.Sakamoto M, Kaneoka T, Murata K, Nakai K, Satoh H, Akagi H. Correlations between mercury concentrations in umbilical cord tissue and other biomarkers of fetal exposure to methylmercury in the Japanese population. Environ Res. 2007;103:106–11. doi: 10.1016/j.envres.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 159.Sakamoto M, Kubota M, Murata K, Nakai K, Sonoda I, Satoh H. Changes in mercury concentrations of segmental maternal hair during gestation and their correlations with other biomarkers of fetal exposure to methylmercury in the Japanese population. Environ Res. 2008;106:270–6. doi: 10.1016/j.envres.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 160.Miyake Y, Tanaka K, Yasutake A, Sasaki S, Hirota Y. Lack of association of mercury with risk of wheeze and eczema in Japanese children: the Osaka Maternal and Child Health Study. Environ Res. 2011;111:1180–4. doi: 10.1016/j.envres.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 161.Kim EH, Kim IK, Kwon JY, Kim SW, Park YW. The effect of fish consumption on blood mercury levels of pregnant women. Yonsei Med J. 2006;47:626–33. doi: 10.3349/ymj.2006.47.5.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kim SA, Jeon CK, Paek DM. Hair mercury concentrations of children and mothers in Korea: implication for exposure and evaluation. Sci Total Environ. 2008;402:36–42. doi: 10.1016/j.scitotenv.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 163.Jo E-M, Kim B-G, Kim Y-M, Yu S-D, You C-H, Kim J-Y, et al. Blood mercury concentration and related factors in an urban coastal area in Korea. J Prev Med Public Health. 2010;43:377–86. doi: 10.3961/jpmph.2010.43.5.377. [DOI] [PubMed] [Google Scholar]
- 164.Kim NS, Lee BK. Blood total mercury and fish consumption in the Korean general population in KNHANES III, 2005. Sci Total Environ. 2010;408:4841–7. doi: 10.1016/j.scitotenv.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 165.Lee B-E, Hong Y-C, Park H, Ha M, Koo BS, Chang N, et al. Interaction between GSTM1/GSTT1 polymorphism and blood mercury on birth weight. Environ Health Perspect. 2010;118:437–43. doi: 10.1289/0900731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kim BM, Lee BE, Hong YC, Park H, Ha M, Kim YJ, et al. Mercury levels in maternal and cord blood and attained weight through the 24 months of life. Sci Total Environ. 2011;410-411:26–33. doi: 10.1016/j.scitotenv.2011.08.060. [DOI] [PubMed] [Google Scholar]
- 167.Kim Y, Lee BK. Associations of blood lead, cadmium, and mercury with estimated glomerular filtration rate in the Korean general population: analysis of 2008–2010 Korean National Health and Nutrition Examination Survey data. Environ Res. 2012;118:124–9. doi: 10.1016/j.envres.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 168.You C-H, Kim B-G, Jo E-M, Kim G-Y, Yu B-C, Hong M-G, et al. The relationship between the fish consumption and blood total/methyl-mercury concentration of costal area in Korea. NeuroToxicol. 2012;33:676–82. doi: 10.1016/j.neuro.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 169.Eom SY, Choi SH, Ahn SJ, Kim DK, Kim DW, Lim JA, et al. Reference levels of blood mercury and association with metabolic syndrome in Korean adults. Int Arch Occup Environ Health 2013. [Epub]. 10.1007/s00420-013-0891-8 [DOI] [PubMed]
- 170.Hong D, Cho SH, Park SJ, Kim SY, Park SB. Hair mercury level in smokers and its influence on blood pressure and lipid metabolism. Environ Toxicol Pharmacol. 2013;36:103–7. doi: 10.1016/j.etap.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 171.Kim N-Y, Ahn S-J, Ryu D-Y, Choi B-S, Kim H, Yu I-J, et al. Effect of lifestyles on the blood mercury level in Korean adults. Hum Exp Toxicol. 2013;32:591–9. doi: 10.1177/0960327112467041. [DOI] [PubMed] [Google Scholar]
- 172.Marsh DO, Turner MD, Smith JC, Allen P, Richdale N. Fetal methylmercury study in a Peruvian fish-eating population. Neurotoxicology. 1995;16:717–26. [PubMed] [Google Scholar]
- 173.Hsu C-S, Liu P-L, Chien L-C, Chou S-Y, Han B-C. Mercury concentration and fish consumption in Taiwanese pregnant women. BJOG. 2007;114:81–5. doi: 10.1111/j.1471-0528.2006.01142.x. [DOI] [PubMed] [Google Scholar]
- 174.Chien L-C, Gao C-S, Lin H-H. Hair mercury concentration and fish consumption: risk and perceptions of risk among women of childbearing age. Environ Res. 2010;110:123–9. doi: 10.1016/j.envres.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 175.Sato RL, Li GG, Shaha S. Antepartum seafood consumption and mercury levels in newborn cord blood. Am J Obstet Gynecol. 2006;194:1683–8. doi: 10.1016/j.ajog.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 176.Tsuchiya A, Hinners TA, Krogstad F, White JW, Burbacher TM, Faustman EM, et al. Longitudinal mercury monitoring within the Japanese and Korean communities (United States): implications for exposure determination and public health protection. Environ Health Perspect. 2009;117:1760–6. doi: 10.1289/ehp.0900801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gundacker C, Komarnicki G, Zödl B, Forster C, Schuster E, Wittmann K. Whole blood mercury and selenium concentrations in a selected Austrian population: does gender matter? Sci Total Environ. 2006;372:76–86. doi: 10.1016/j.scitotenv.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 178.Rudge CVC, Calderon IMP, Rudge MVC, Volpato G, Silva JLP, Duarte G, et al. Toxic and essential elements in blood from delivering women in selected areas of São Paulo State, Brazil. J Environ Monit. 2011;13:563–71. doi: 10.1039/c0em00570c. [DOI] [PubMed] [Google Scholar]
- 179.Rhainds M, Levallois P, Dewailly E, Ayotte P. Lead, mercury, and organochlorine compound levels in cord blood in Québec, Canada. Arch Environ Health. 1999;54:40–7. doi: 10.1080/00039899909602235. [DOI] [PubMed] [Google Scholar]
- 180.Puklová V, Krsková A, Cerná M, Cejchanová M, Rehůrková I, Ruprich J, et al. The mercury burden of the Czech population: An integrated approach. Int J Hyg Environ Health. 2010;213:243–51. doi: 10.1016/j.ijheh.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 181.Cerná M, Krsková A, Cejchanová M, Spěváčková V. Human biomonitoring in the Czech Republic: an overview. Int J Hyg Environ Health. 2012;215:109–19. doi: 10.1016/j.ijheh.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 182.Huel G, Sahuquillo J, Debotte G, Oury J-F, Takser L. Hair mercury negatively correlates with calcium pump activity in human term newborns and their mothers at delivery. Environ Health Perspect. 2008;116:263–7. doi: 10.1289/ehp.10381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Anwar M, Ando T, Maaz A, Ghani S, Munir M, Qureshi IU, et al. Scalp hair mercury concentrations in Pakistan. Environ Sci. 2007;14:167–75. [PubMed] [Google Scholar]
- 184.Jędrychowski W, Perera F, Rauh V, Flak E, Mróz E, Pac A, et al. Fish intake during pregnancy and mercury level in cord and maternal blood at delivery: an environmental study in Poland. Int J Occup Med Environ Health. 2007;20:31–7. doi: 10.2478/v10001-007-0002-8. [DOI] [PubMed] [Google Scholar]
- 185.Al-Saleh I, Shinwari N, Mashhour A, Mohamed Gel-D, Ghosh MA, Shammasi Z, et al. Cadmium and mercury levels in Saudi women and its possible relationship with hypertension. Biol Trace Elem Res. 2006;112:13–30. doi: 10.1385/BTER:112:1:13. [DOI] [PubMed] [Google Scholar]
- 186.Al-Saleh I, Coskun S, Mashhour A, Shinwari N, El-Doush I, Billedo G, et al. Exposure to heavy metals (lead, cadmium and mercury) and its effect on the outcome of in-vitro fertilization treatment. Int J Hyg Environ Health. 2008;211:560–79. doi: 10.1016/j.ijheh.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 187.Al-Saleh I, Shinwari N, Mashhour A, Mohamed GelD, Rabah A. Heavy metals (lead, cadmium and mercury) in maternal, cord blood and placenta of healthy women. Int J Hyg Environ Health. 2011;214:79–101. doi: 10.1016/j.ijheh.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 188.Al-Saleh I, Abduljabbar M, Al-Rouqi R, Elkhatib R, Alshabbaheen A, Shinwari N. Mercury (Hg) exposure in breast-fed infants and their mothers and the evidence of oxidative stress. Biol Trace Elem Res. 2013;153:145–54. doi: 10.1007/s12011-013-9687-7. [DOI] [PubMed] [Google Scholar]
- 189.Miklavčič A, Cuderman P, Mazej D, Snoj Tratnik J, Krsnik M, Planinšek P, et al. Biomarkers of low-level mercury exposure through fish consumption in pregnant and lactating Slovenian women. Environ Res. 2011;111:1201–7. doi: 10.1016/j.envres.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 190.Díez S, Delgado S, Aguilera I, Astray J, Pérez-Gómez B, Torrent M, et al. Prenatal and early childhood exposure to mercury and methylmercury in Spain, a high-fish-consumer country. Arch Environ Contam Toxicol. 2009;56:615–22. doi: 10.1007/s00244-008-9213-7. [DOI] [PubMed] [Google Scholar]
- 191.Díez S, Esbrí JM, Tobias A, Higueras P, Martínez-Coronado A. Determinants of exposure to mercury in hair from inhabitants of the largest mercury mine in the world. Chemosphere. 2011;84:571–7. doi: 10.1016/j.chemosphere.2011.03.065. [DOI] [PubMed] [Google Scholar]
- 192.Bjermo H, Sand S, Nälsén C, Lundh T, Enghardt Barbieri H, Pearson M, et al. Lead, mercury, and cadmium in blood and their relation to diet among Swedish adults. Food Chem Toxicol. 2013;57:161–9. doi: 10.1016/j.fct.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 193.Knobeloch L, Anderson HA, Imm P, Peters D, Smith A. Fish consumption, advisory awareness, and hair mercury levels among women of childbearing age. Environ Res. 2005;97:220–7. doi: 10.1016/j.envres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 194.Xue F, Holzman C, Rahbar MH, Trosko K, Fischer L. Maternal fish consumption, mercury levels, and risk of preterm delivery. Environ Health Perspect. 2007;115:42–7. doi: 10.1289/ehp.9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pollack AZ, Schisterman EF, Goldman LR, Mumford SL, Albert PS, Jones RL, et al. Cadmium, lead, and mercury in relation to reproductive hormones and anovulation in premenopausal women. Environ Health Perspect. 2011;119:1156–61. doi: 10.1289/ehp.1003284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pollack AZ, Schisterman EF, Goldman LR, Mumford SL, Perkins NJ, Bloom MS, et al. Relation of blood cadmium, lead, and mercury levels to biomarkers of lipid peroxidation in premenopausal women. Am J Epidemiol. 2012;175:645–52. doi: 10.1093/aje/kwr375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Brune D, Nordberg GF, Vesterberg O, Gerhardsson L, Wester PO. A review of normal concentrations of mercury in human blood. Sci Total Environ. 1991;100:235–82. doi: 10.1016/0048-9697(91)90380-W. [DOI] [PubMed] [Google Scholar]
- 198.Sioen I, De Henauw S, Van Camp J, Volatier J-L, Leblanc J-C. Comparison of the nutritional-toxicological conflict related to seafood consumption in different regions worldwide. Regul Toxicol Pharmacol. 2009;55:219–28. doi: 10.1016/j.yrtph.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 199.Bellanger M, Pichery C, Aerts D, Berglund M, Castaño A, Cejchanová M, et al. DEMO/COPHES Economic benefits of methylmercury exposure control in Europe: monetary value of neurotoxicity prevention. Environ Health. 2013;12:3. doi: 10.1186/1476-069X-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Stern AH, Smith AE. An assessment of the cord blood:maternal blood methylmercury ratio: implications for risk assessment. Environ Health Perspect. 2003;111:1465–70. doi: 10.1289/ehp.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Dye BA, Schober SE, Dillon CF, Jones RL, Fryar C, McDowell M, et al. Urinary mercury concentrations associated with dental restorations in adult women aged 16–49 years: United States, 1999–2000. Occup Environ Med. 2005;62:368–75. doi: 10.1136/oem.2004.016832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Axelrad DA, Bellinger DC, Ryan LM, Woodruff TJ. Dose-response relationship of prenatal mercury exposure and IQ: an integrative analysis of epidemiologic data. Environ Health Perspect. 2007;115:609–15. doi: 10.1289/ehp.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Griffiths C, McGartland A, Miller M. A comparison of the monetized impact of IQ decrements from mercury emissions. Environ Health Perspect. 2007;115:841–7. doi: 10.1289/ehp.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Trasande L, Landrigan PJ, Schechter C. Public health and economic consequences of methyl mercury toxicity to the developing brain. Environ Health Perspect. 2005;113:590–6. doi: 10.1289/ehp.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Poulin J, Gibb H. Mercury: assessing the environmental burden of disease at national and local levels (Environmental burden of disease series no. 16). Geneva: World Health Organization; 2008. [Google Scholar]
- 206.Yokoo EM, Valente JG, Grattan L, Schmidt SL, Platt I, Silbergeld EK. Low level methylmercury exposure affects neuropsychological function in adults. Environ Health. 2003;2:8. doi: 10.1186/1476-069X-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Alloway TP. Working memory and executive function profiles of individuals with borderline intellectual functioning. J Intellect Disabil Res. 2010;54:448–56. doi: 10.1111/j.1365-2788.2010.01281.x. [DOI] [PubMed] [Google Scholar]
- 208.Fewtrell L, Kaufmann R, Pruss-Ustun A. Lead: assessing the environmental burden of disease at national and local levels (Environmental burden of disease series no. 2). Geneva: World Health Organization; 2003. [Google Scholar]
- 209.Roman HA, Walsh TL, Coull BA, Dewailly É, Guallar E, Hattis D, et al. Evaluation of the cardiovascular effects of methylmercury exposures: current evidence supports development of a dose-response function for regulatory benefits analysis. Environ Health Perspect. 2011;119:607–14. doi: 10.1289/ehp.1003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mercury use in artisanal and small scale gold mining (module 3) Geneva: United Nations Environment Programme; 2008. Available from: http://www.chem.unep.ch/mercury/awareness_raising_package/E_01-16_BD.pdf [accessed 13 October 2013].
- 211.Streets DG, Zhang Q, Wu Y. Projections of global mercury emissions in 2050. Environ Sci Technol. 2009;43:2983–8. doi: 10.1021/es802474j. [DOI] [PubMed] [Google Scholar]
- 212.Sunderland EM, Selin NE. Future trends in environmental mercury concentrations: implications for prevention strategies. Environ Health. 2013;12:2. doi: 10.1186/1476-069X-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Draft Minamata Convention on Mercury. Geneva: United Nations Environment Programme; 2013. Available from: http://www.unep.org/hazardoussubstances/Portals/9/Mercury/Documents/INC5/INC5_7asterisk_final%20report_26%2008_e.pdf [accessed 9 December 2013].