Abstract
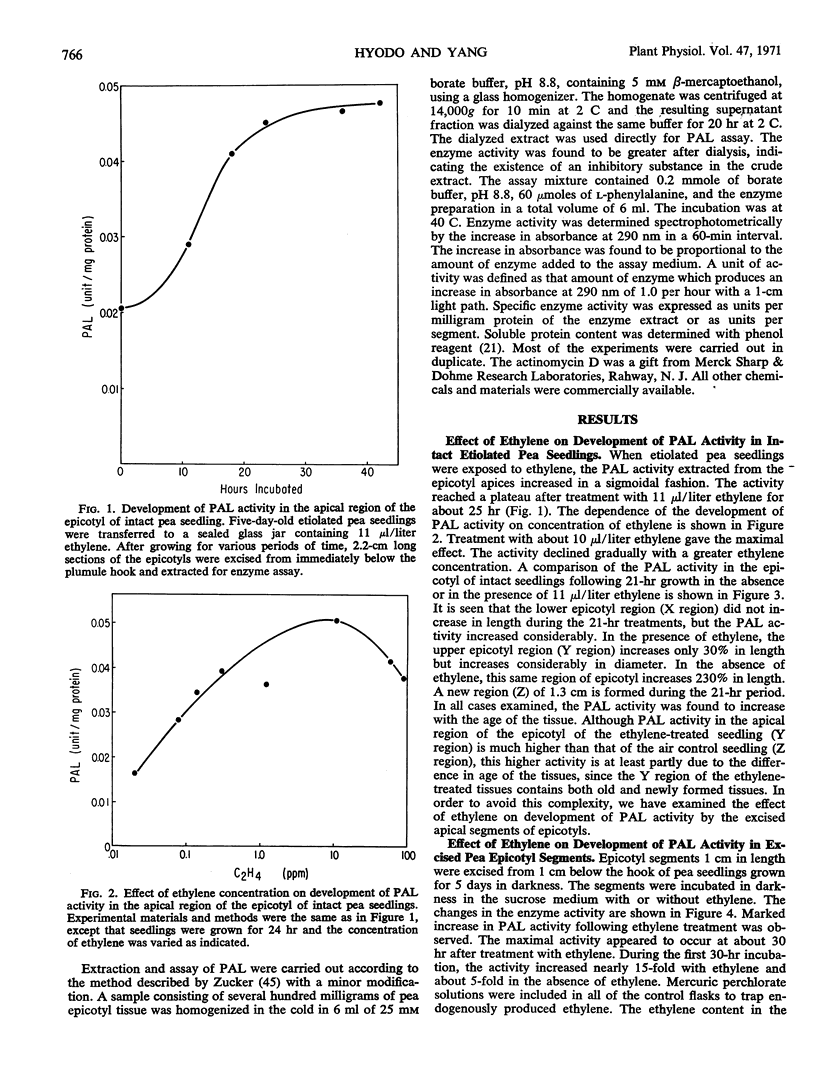
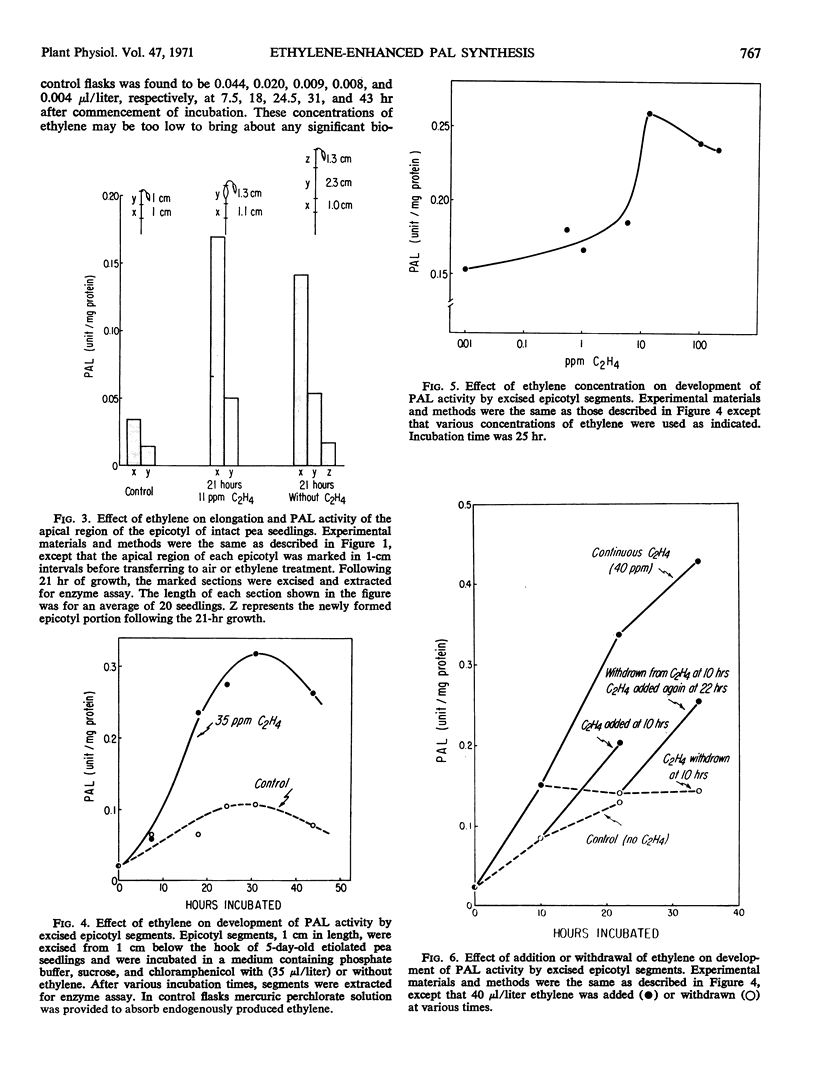
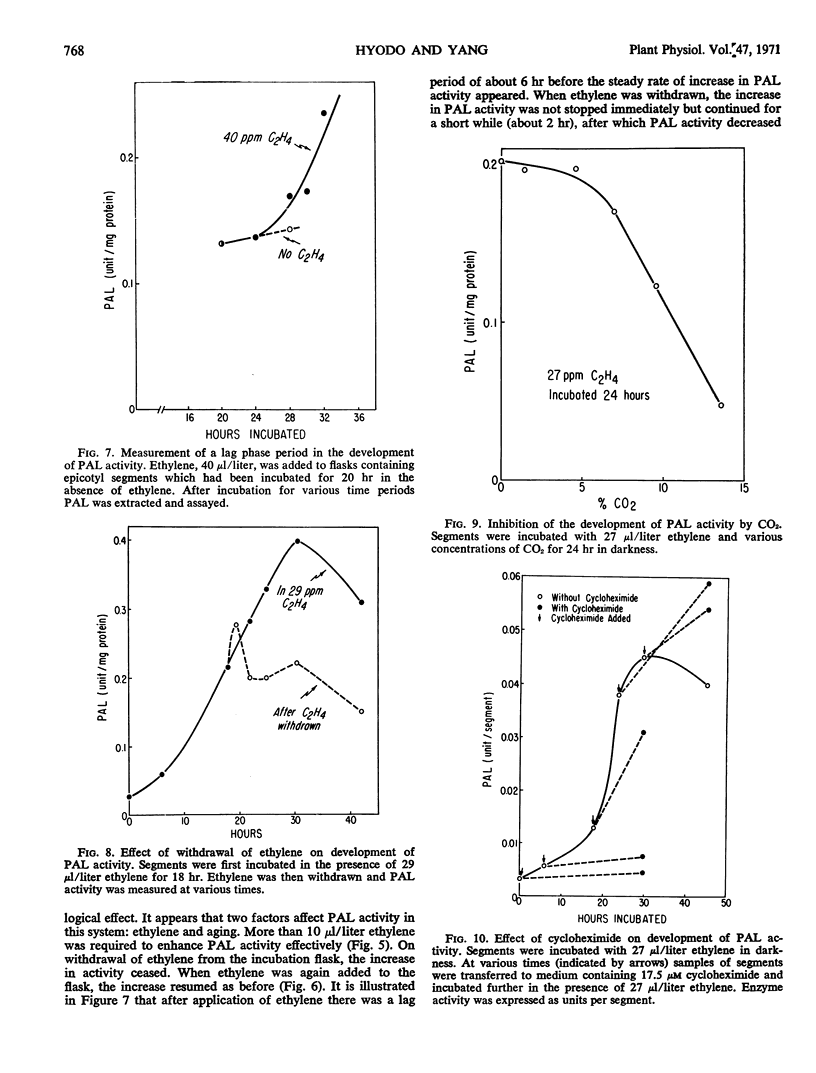
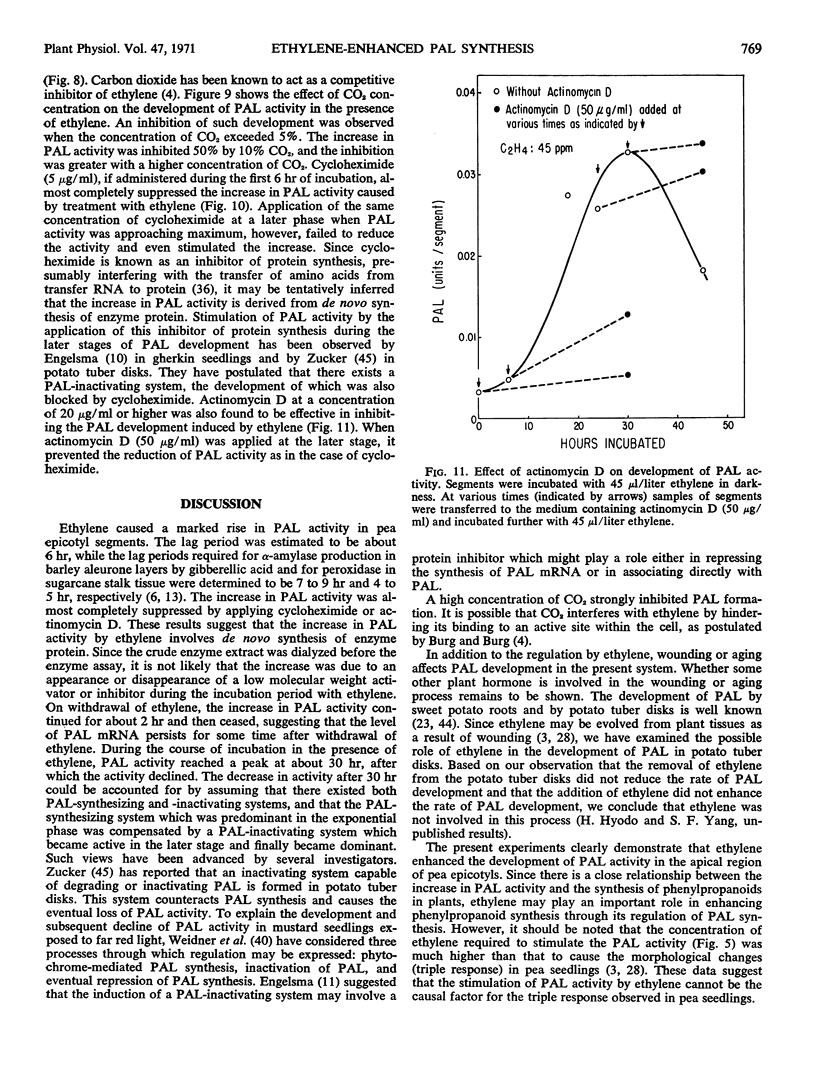
The effect of ethylene on the development of phenylalanine ammonia-lyase activity in segments excised from the epicotyl apex of pea seedling was studied. Although there was some increase in phenylalanine ammonia-lyase activity in segments not treated with ethylene, a marked increase in phenylalanine ammonia-lyase activity occurred in ethylene-treated tissues during the incubation. The induction period was estimated to be about 6 hours. The activity reached a maxmum at 30 hours and then declined. On withdrawal of ethylene, the increase was sustained for a short period and then stopped. After retreatment with ethylene, the increase was resumed. Addition of CO2 reduced the effect of ethylene. Administration of cycloheximide or actinomycin D at an early period almost completely suppressed the increase in phenylalanine ammonia-lyase activity. However, if these inhibitors were administered at a later period, while phenylalanine ammonia-lyase activity was approaching a maximum, they not only failed to reduce but rather stimulated the activity. These results are consistent with the view that there exist both phenylalanine ammonia-lyase-synthesizing and -inactivating systems, and that the development of both systems may involve de novo synthesis of protein.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles F. B. Role of RNA and protein synthesis in abscission. Plant Physiol. 1968 Sep;43(9 Pt B):1577–1586. [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Molecular requirements for the biological activity of ethylene. Plant Physiol. 1967 Jan;42(1):144–152. doi: 10.1104/pp.42.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Varner J. E. Hormonal control of enzyme synthesis: on the mode of action of gibberellic Acid and abscisin in aleurone layers of barley. Plant Physiol. 1967 Jul;42(7):1008–1016. doi: 10.1104/pp.42.7.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durst F., Mohr H. Phytochrome-mediated induction of enzyme synthesis in mustard seedlings(Sinapis alba L.). Naturwissenschaften. 1966 Oct;53(20):531–532. doi: 10.1007/BF00600655. [DOI] [PubMed] [Google Scholar]
- Engelsma G. Effect of cycloheximide on the inactivation of phenylalanine deaminase in gherkin seedlings. Naturwissenschaften. 1967 Jun;54(12):319–320. doi: 10.1007/BF00640619. [DOI] [PubMed] [Google Scholar]
- Hadwiger L. A., Schwochau M. E. Induction of phenylalanine ammonia lyase and pisatin in pea pods by poly-lysine, spermidine or histone fractions. Biochem Biophys Res Commun. 1970 Feb 20;38(4):683–691. doi: 10.1016/0006-291x(70)90635-2. [DOI] [PubMed] [Google Scholar]
- Holm R. E., O'brien T. J., Key J. L., Cherry J. H. The Influence of Auxin and Ethylene on Chromatin-directed Ribonucleic Acid Synthesis in Soybean Hypocotyl. Plant Physiol. 1970 Jan;45(1):41–45. doi: 10.1104/pp.45.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ogawa M., Uritani I. Metabolic changes in sweet potato roots induced by gamma raation in response to cutting. Radiat Res. 1969 Jul;39(1):117–125. [PubMed] [Google Scholar]
- Powell R. D., Morgan P. W. Factors involved in the opening of the hypocotyl hook of cotton and beans. Plant Physiol. 1970 May;45(5):548–552. doi: 10.1104/pp.45.5.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riov J., Monselise S. P., Kahan R. S. Ethylene-controlled Induction of Phenylalanine Ammonia-lyase in Citrus Fruit Peel. Plant Physiol. 1969 May;44(5):631–635. doi: 10.1104/pp.44.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riov J., Monselise S. P., Kahan R. S. Ethylene-controlled Induction of Phenylalanine Ammonia-lyase in Citrus Fruit Peel. Plant Physiol. 1969 May;44(5):631–635. doi: 10.1104/pp.44.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. W., Galston A. W. Blockage by gibberellic Acid of phytochrome effects on growth, auxin responses, and flavonoid synthesis in etiolated pea internodes. Plant Physiol. 1969 Sep;44(9):1211–1216. doi: 10.1104/pp.44.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J. A., Salminen S. O. Comparative studies of effect of auxin and ethylene on permeability and synthesis of RNA and protein. Plant Physiol. 1969 Oct;44(10):1371–1377. doi: 10.1104/pp.44.10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M. R., Sisler H. D. Site of action of cycloheximide in cells of Saccharomyces pastorianus. 3. Further studies on the mechanism of action and the mechanism of resistance in saccharomyces species. Biochim Biophys Acta. 1965 Aug 10;103(4):558–567. [PubMed] [Google Scholar]
- Stahmann M. A., Clare B. G., Woodbury W. Increased disease resistance and enzyme activity induced by ethylene and ethylene production of black rot infected sweet potato tissue. Plant Physiol. 1966 Nov;41(9):1505–1512. doi: 10.1104/pp.41.9.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton D. C., Sondheimer E. Effects of Abscisin II on Phenylalanine Ammonia-Lyase Activity in Excised Bean Axes. Plant Physiol. 1968 Mar;43(3):467–469. doi: 10.1104/pp.43.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster B. D. Anatomical aspects of abscission. Plant Physiol. 1968 Sep;43(9 Pt B):1512–1544. [PMC free article] [PubMed] [Google Scholar]
- Zucker M. Induction of Phenylalanine Deaminase by Light and its Relation to Chlorogenic Acid Synthesis in Potato Tuber Tissue. Plant Physiol. 1965 Sep;40(5):779–784. doi: 10.1104/pp.40.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker M. Induction of phenylalanine ammonia-lyase in Xanthium leaf disks. Photosynthetic requirement and effect of daylength. Plant Physiol. 1969 Jun;44(6):912–922. doi: 10.1104/pp.44.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker M. Sequential Induction of Phenylalanine Ammonia-lyase and a Lyase-inactivating System in Potato Tuber Disks. Plant Physiol. 1968 Mar;43(3):365–374. doi: 10.1104/pp.43.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]


