Abstract
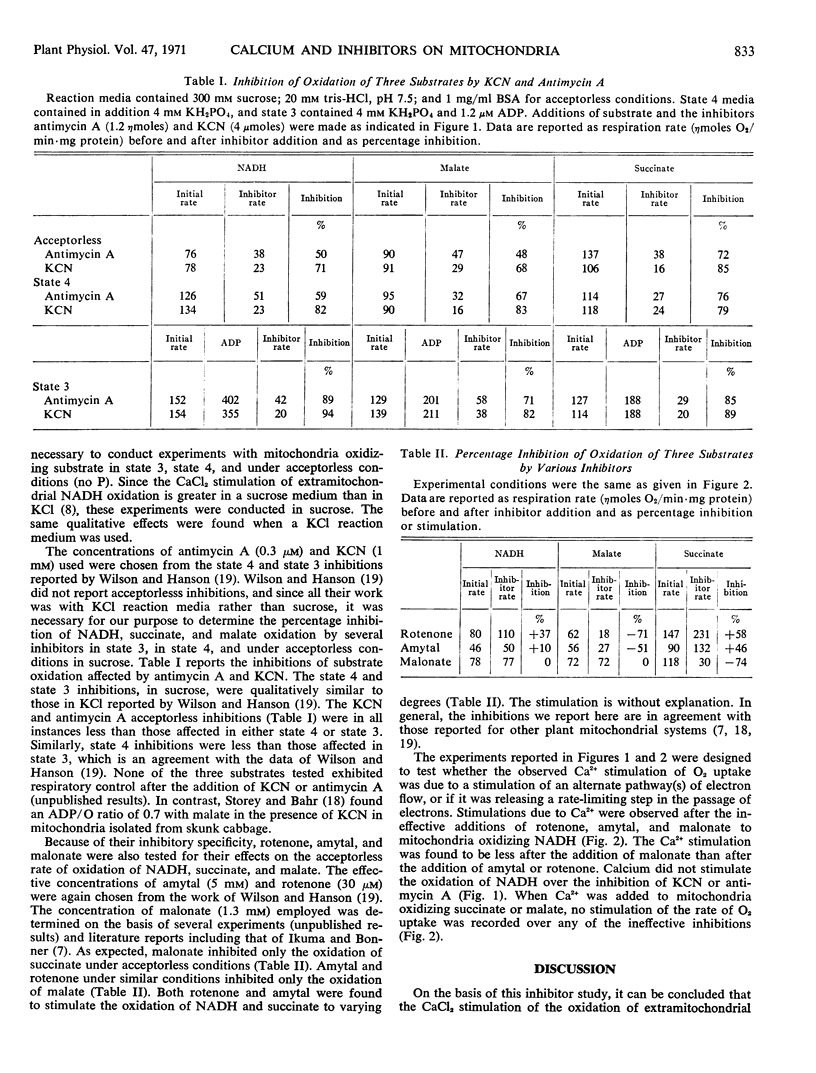
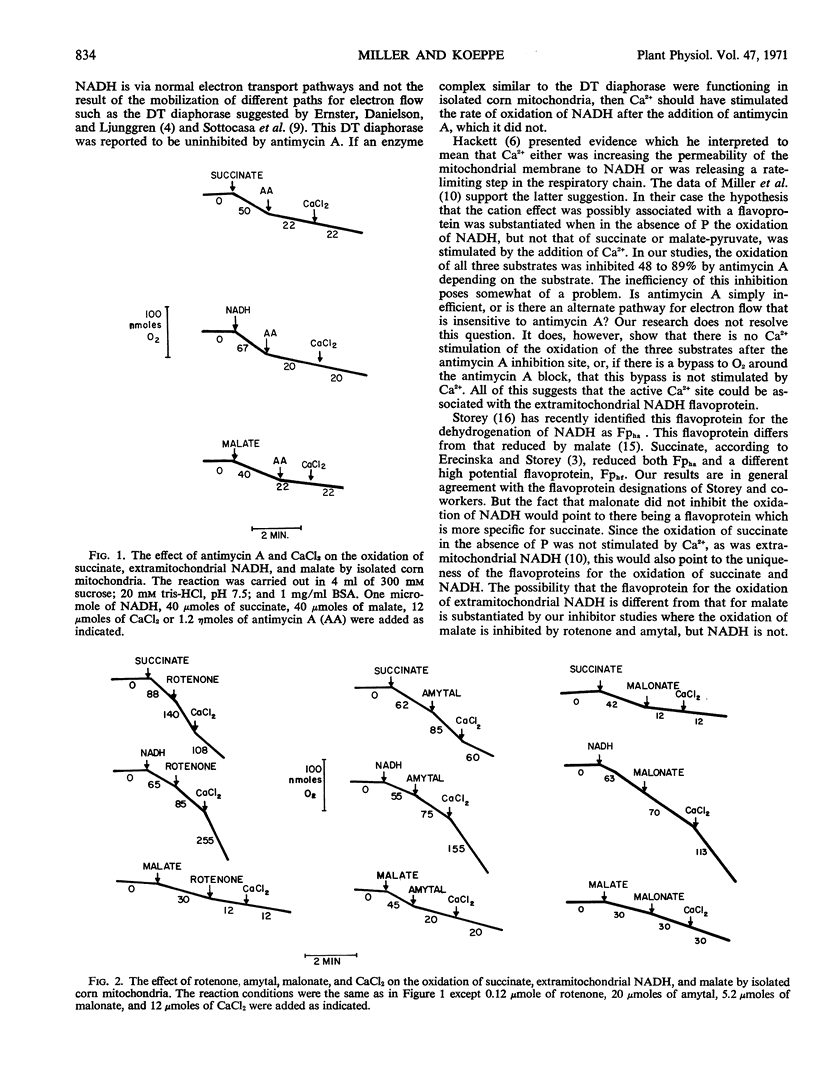
The effects of KCN, antimycin A, malonate, rotenone, and amytal on the oxidation of malate, succinate, and extramitochondrial reduced nicotinamide adenosine dinucleotide (NADH) by corn mitochondria were studied. Potassium cyanide and antimycin A inhibited the oxidation of all three substrates. Rotenone and amytal inhibited only the oxidation of malate, and malonate inhibited only the oxidation of succinate. Rotenone, amytal, and malonate did not inhibit the oxidation of extramitochondrial NADH. The calcium stimulation of the oxidation of extramitochondrial NADH was prevented by KCN and antimycin A but not by amytal, rotenone, or malonate. It is suggested that corn mitochondria possess a flavoprotein specific for extramitochondrial NADH and that this flavoprotein is sensitive to divalent cations.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ERNSTER L., DANIELSON L., LJUNGGREN M. DT diaphorase. I. Purification from the soluble fraction of rat-liver cytoplasm, and properties. Biochim Biophys Acta. 1962 Apr 9;58:171–188. doi: 10.1016/0006-3002(62)90997-6. [DOI] [PubMed] [Google Scholar]
- Erecinska M., Storey B. T. The Respiratory Chain of Plant Mitochondria: VII. Kinetics of Flavoprotein Oxidation in Skunk Cabbage Mitochondria. Plant Physiol. 1970 Oct;46(4):618–624. doi: 10.1104/pp.46.4.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland P. B., Chance B., Ernster L., Lee C. P., Wong D. Flavoproteins of mitochondrial fatty acid oxidation. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1696–1702. doi: 10.1073/pnas.58.4.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett D. P. Effects of salts on DPNH oxidase activity & structure of sweet potato mitochondria. Plant Physiol. 1961 Jul;36(4):445–452. doi: 10.1104/pp.36.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. III. Effects of Respiratory Inhibitors. Plant Physiol. 1967 Nov;42(11):1535–1544. doi: 10.1104/pp.42.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller R. J., Dumford S. W., Koeppe D. E., Hanson J. B. Divalent cation stimulation of substrate oxidation by corn mitochondria. Plant Physiol. 1970 Jun;45(6):649–653. doi: 10.1104/pp.45.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The respiratory chain of plant mitochondria. I. Electron transport between succinate and oxygen in skunk cabbage mitochondria. Plant Physiol. 1969 Jan;44(1):115–125. doi: 10.1104/pp.44.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The respiratory chain of plant mitochondria. II. Oxidative phosphorylation in skunk cabbage mitochondria. Plant Physiol. 1969 Jan;44(1):126–134. doi: 10.1104/pp.44.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The Respiratory Chain of Plant Mitochondria. III. Oxidation Rates of the Cytochromes c and b in Mung Bean Mitochondria Reduced With Succinate. Plant Physiol. 1969 Mar;44(3):413–421. doi: 10.1104/pp.44.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The Respiratory Chain of Plant Mitochondria: VI. Flavoprotein Components of the Respiratory Chain of Mung Bean Mitochondria. Plant Physiol. 1970 Jul;46(1):13–20. doi: 10.1104/pp.46.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The Respiratory Chain of Plant Mitochondria: VIII. Reduction Kinetics of the Respiratory Chain Carriers of Mung Bean Mitochondria with Reduced Nicotinamide Adenine Dinucleotide. Plant Physiol. 1970 Oct;46(4):625–630. doi: 10.1104/pp.46.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The respiratory chain of plant mitochondria. IV. Oxidation rates of the respiratory carriers of mung bean mitochondria in the presence of cyanide. Plant Physiol. 1970 Apr;45(4):447–454. doi: 10.1104/pp.45.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The respiratory chain of plant mitochondria. V. Reaction of reduced cytochromes a and a3 in mung bean mitochondria with oxygen in the presence of cyanide. Plant Physiol. 1970 Apr;45(4):455–460. doi: 10.1104/pp.45.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. H., Hanson J. B. The effect of respiratory inhibitors on NADH, succinate and malate oxidation in corn mitochondria. Plant Physiol. 1969 Sep;44(9):1335–1341. doi: 10.1104/pp.44.9.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]


