Abstract
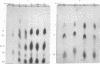
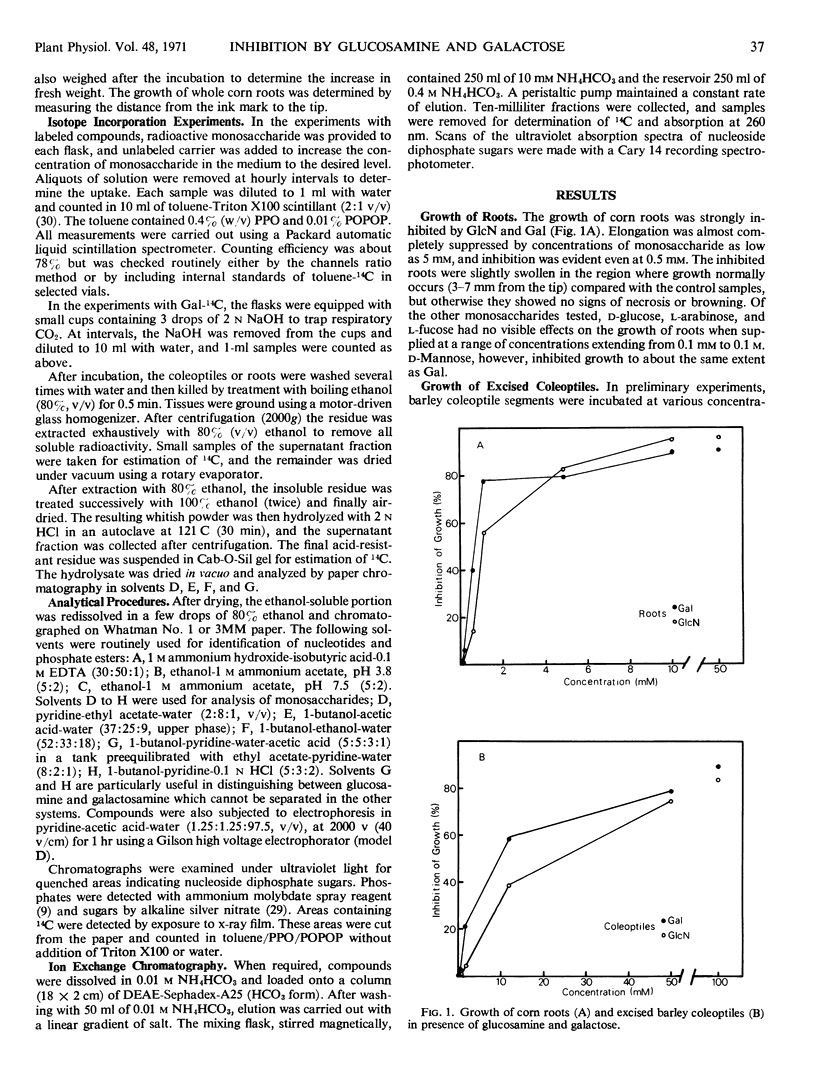
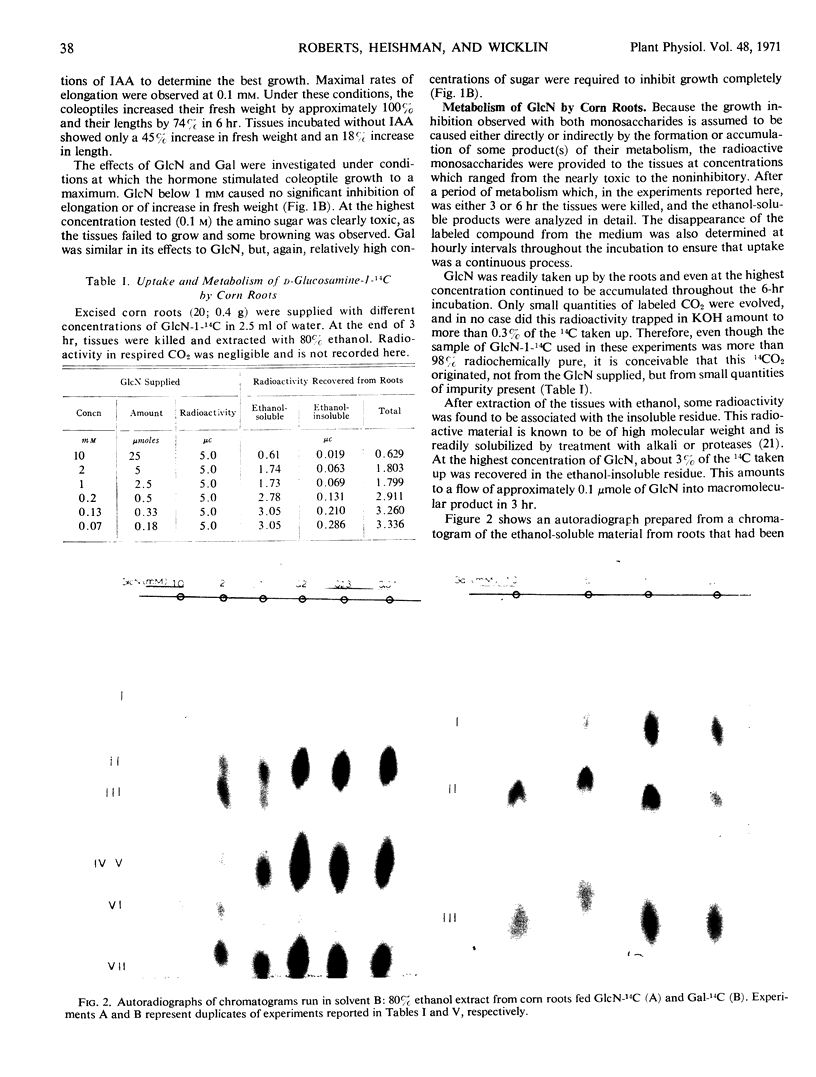
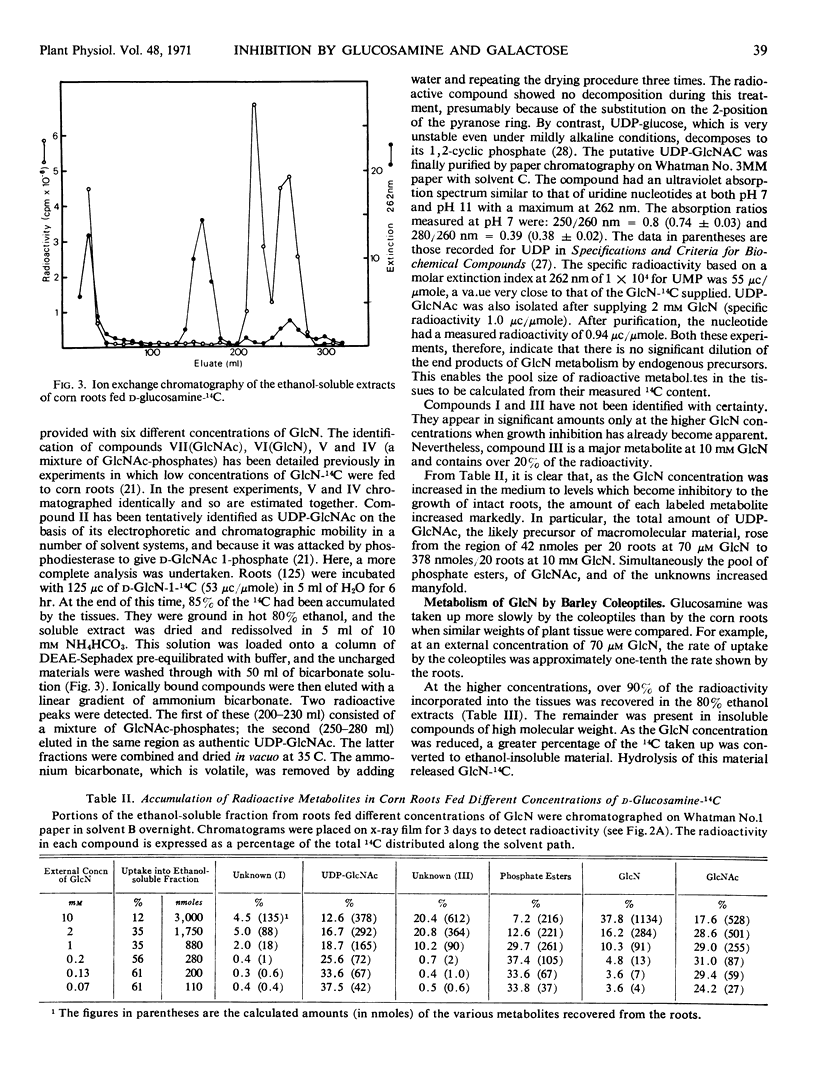
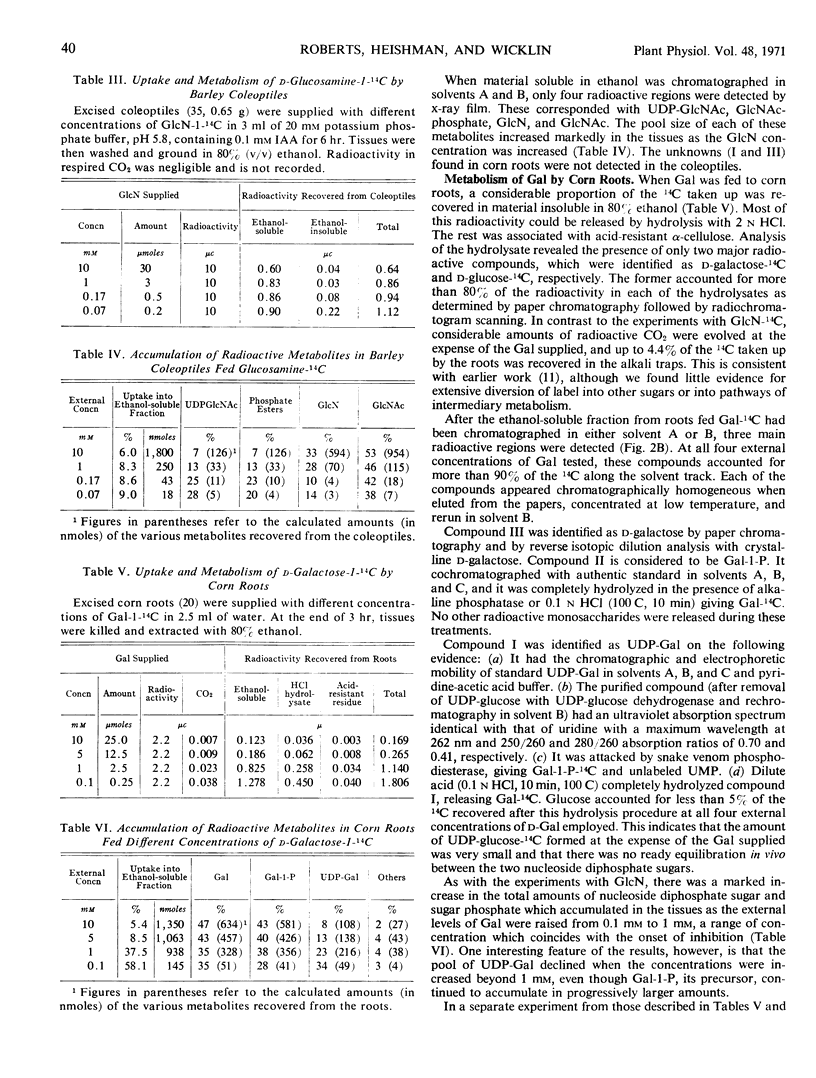
The growth of corn (Zea mays) roots and barley (Hordeum vulgare) coleoptiles is sensitive to the presence of external d-glucosamine and d-galactose. In order to investigate this effect, tissues were fed the radioactive monosaccharides at concentrations that ranged from those that were strongly inhibitory to those that had little influence on growth. At low concentrations, d-glucosamine is converted to uridine diphosphate-N-acetyl-d-glucosamine, phosphate esters of N-acetylglucosamine, and free N-acetylglucosamine. As the external concentrations were increased, the pool levels of each of these metabolites rose several fold; and, in corn roots, two unidentified compounds, which had not been detected previously, began to accumulate in the tissues. The major products of d-galactose metabolism were uridine diphosphate-d-galactose and d-galactose 1-phosphate at all the concentrations tested. Both these compounds showed a marked increase as the external galactose concentrations were raised to inhibitory levels. The experiments indicate that efficient pathways exist in plants for the metabolism of d-glucosamine and d-galactose. These pathways, however, do not appear to be under strict control, so that metabolites accumulate in unusually high amounts and presumably interfere competitively with normal carbohydrate metabolism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal K. M., Bahl O. P. Glycosidases of Phaseolus vulgaris. II. Isolation and general properties. J Biol Chem. 1968 Jan 10;243(1):103–111. [PubMed] [Google Scholar]
- Bahl O. P., Agrawal K. M. Glycosidases of Phaseolus vulgaris. I. Isolation and characterization of beta-N-acetylglucosaminidase. J Biol Chem. 1968 Jan 10;243(1):98–102. [PubMed] [Google Scholar]
- Baker D. B., Ray P. M. Relation between Effects of Auxin on Cell Wall Synthesis and Cell Elongation. Plant Physiol. 1965 Mar;40(2):360–368. doi: 10.1104/pp.40.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekesi J. G., Winzler R. J. The effect of D-glucosamine on the adenine and uridine nucleotides of sarcoma 180 ascites tumor cells. J Biol Chem. 1969 Oct 25;244(20):5663–5668. [PubMed] [Google Scholar]
- Bernheim N. J., Dobrogosz W. J. Amino sugar sensitivity in Escherichia coli mutants unable to grow on N-acetylglucosamine. J Bacteriol. 1970 Feb;101(2):384–391. doi: 10.1128/jb.101.2.384-391.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRENCH D. The raffinose family of oligosaccharides. Adv Carbohydr Chem. 1954;9:149–184. doi: 10.1016/s0096-5332(08)60375-6. [DOI] [PubMed] [Google Scholar]
- GINSBURG V., HASSID W. Z., STUMPF P. K. The isolation of uridine diphosphate derivatives of D-glucose, D-galactose, D-xylose, and L-arabinose from mung bean seedlings. J Biol Chem. 1956 Dec;223(2):977–983. [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- HASSID W. Z., PUTMAN E. W., GINSBURG V. Metabolism of galactose in Canna leaves and wheat seedlings. Biochim Biophys Acta. 1956 Apr;20(1):17–22. doi: 10.1016/0006-3002(56)90256-6. [DOI] [PubMed] [Google Scholar]
- Hassid W. Z., Neufeld E. F., Feingold D. S. SUGAR NUCLEOTIDES IN THE INTERCONVERSION OF CARBOHYDRATES IN HIGHER PLANTS. Proc Natl Acad Sci U S A. 1959 Jul;45(7):905–915. doi: 10.1073/pnas.45.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNFELD S., KORNFELD R., NEUFELD E. F., O'BRIEN P. J. THE FEEDBACK CONTROL OF SUGAR NUCLEOTIDE BIOSYNTHESIS IN LIVER. Proc Natl Acad Sci U S A. 1964 Aug;52:371–379. doi: 10.1073/pnas.52.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOEWUS F. A., JANG R. The conversion of C14-labeled sugars to L-ascorbic acid in ripening strawberries. III. Labeling patterns from berries administered pentose-1-C14. J Biol Chem. 1958 May;232(1):521–532. [PubMed] [Google Scholar]
- Mayer F. C., Bikel I., Hassid W. Z. Pathway of Uridine Diphosphate N-Acetyl-d-Glucosamine Biosynthesis in Phaseolus aureus. Plant Physiol. 1968 Jul;43(7):1097–1107. doi: 10.1104/pp.43.7.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordin L., Bonner J. Effect of Galactose on Growth and Metabolism of Avena Coleoptile Sections. Plant Physiol. 1957 May;32(3):212–215. doi: 10.1104/pp.32.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. M. The incorporation of D-glucosamine-14C into root tissues of higher plants. Plant Physiol. 1970 Mar;45(3):263–267. doi: 10.1104/pp.45.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. M. The metabolism of L-fucose by sycamore (Acer pseudoplatanus L.) cell cultures. Arch Biochem Biophys. 1968 Dec;128(3):818–820. doi: 10.1016/0003-9861(68)90092-1. [DOI] [PubMed] [Google Scholar]
- SCHWARZ V., GOLBERG L., KOMROWER G. M., HOLZEL A. Some disturbances of erythrocyte metabolism in galactosaemia. Biochem J. 1956 Jan;62(1):34–40. doi: 10.1042/bj0620034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLMS J., HASSID W. Z. Isolation of uridine diphosphate N-acetylglucosamine and uridine diphosphate glucuronic acid from mung bean seedlings. J Biol Chem. 1957 Sep;228(1):357–364. [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Tovey K. C., Roberts R. M. An artefact in the chromatography of sugar nucleotides using solvents containing ammonium acetate. J Chromatogr. 1970 Mar 4;47(2):287–290. doi: 10.1016/0021-9673(70)80043-7. [DOI] [PubMed] [Google Scholar]
- Turner J. C. Triton X-100 scintillant for carbon-14 labelled materials. Int J Appl Radiat Isot. 1968 Jul;19(7):557–563. doi: 10.1016/0020-708x(68)90065-3. [DOI] [PubMed] [Google Scholar]