Abstract
The photoreduction of protochlorophyllide a to chlorophyllide a in intact 6-day-old seedlings of etiolated barley (Hordeum vulgare) exhibits a small initial phase, followed by an induction period of about 1 hour before a rapid phase of additional chlorophyll formation begins. Cycloheximide, an inhibitor of protein synthesis, has no effect on the initial phase of conversion of preformed protochlorophyllide, but it either abolishes or severely inhibits the subsequent phase of rapid chlorophyll synthesis within 45 minutes of its application to the seedlings. An analysis of the biphasic inhibition process suggests that the lifetime of the enzyme controlling protochlorophyllide synthesis (probably δ-amino-levulinic acid synthetase) is not longer than 10 minutes.
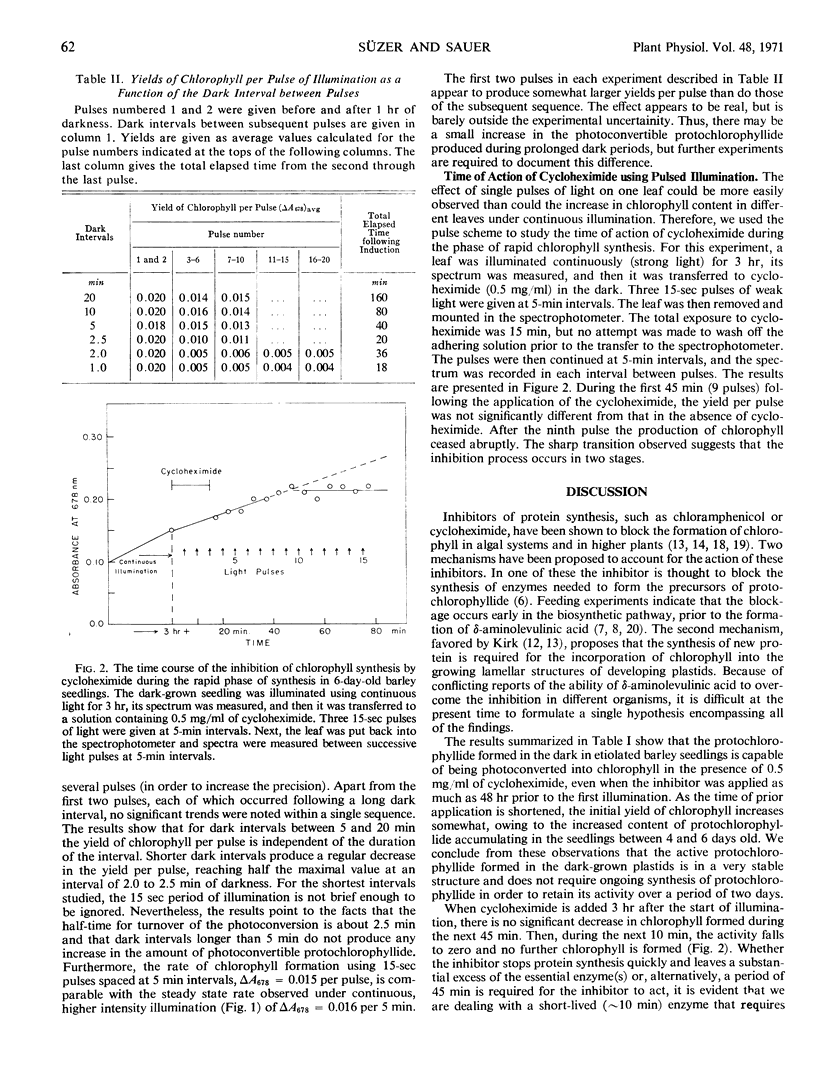
The rapid phase of chlorophyll formation can be effected by a series of brief (15 second) pulses of light spaced at least 5 minutes apart. When longer dark intervals are used, no increase is observed in the yield of chlorophyll per pulse. We interpret the findings to indicate that the photoconversion takes place at distinct enzymatic sites whose concentration does not increase during a period of 4 hours following the initial illumination. The sites can be used repeatedly with a turnover time determined by the removal of the product chlorophyllide and the synthesis and placement of a new protochlorophyllide molecule.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLAAUW-JANSEN G., KOMEN J. G., THOMAS J. B. On the relation between the formation of assimilatory pigments and the rate of photosynthesis in etiolated oat seedlings. Biochim Biophys Acta. 1950 Apr;5(2):179–185. [PubMed] [Google Scholar]
- BOARDMAN N. K. Studies on a protochlorophyll-protein complex. I. Purification and molecular-weight determination. Biochim Biophys Acta. 1962 Jul 30;62:63–79. doi: 10.1016/0006-3002(62)90492-4. [DOI] [PubMed] [Google Scholar]
- Butler W. L., Briggs W. R. The relation between structure and pigments during the first stages of proplastid greening. Biochim Biophys Acta. 1966 Jan 4;112(1):45–53. doi: 10.1016/s0926-6585(96)90006-0. [DOI] [PubMed] [Google Scholar]
- GOEDHEER J. C. Effect of changes in chlorophyll concentration on photosynthetic properties. I. Fluorescence and absorption of greening bean leaves. Biochim Biophys Acta. 1961 Aug 19;51:494–504. doi: 10.1016/0006-3002(61)90605-9. [DOI] [PubMed] [Google Scholar]
- Gassman M., Bogorad L. Control of chlorophyll production in rapidly greening bean leaves. Plant Physiol. 1967 Jun;42(6):774–780. doi: 10.1104/pp.42.6.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassman M., Bogorad L. Studies on the regeneration of protochlorophyllide after brief illumination of etiolated bean leaves. Plant Physiol. 1967 Jun;42(6):781–784. doi: 10.1104/pp.42.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedheer J. C., Verhülsdonk C. A. Fluorescence and phototransformation of protochlorophyll with etiolated bean leaves from minus 196 to +20 degrees C. Biochem Biophys Res Commun. 1970 Apr 24;39(2):260–266. doi: 10.1016/0006-291x(70)90787-4. [DOI] [PubMed] [Google Scholar]
- Granick S., Gassman M. Rapid regeneration of protochlorophyllide(650). Plant Physiol. 1970 Feb;45(2):201–205. doi: 10.1104/pp.45.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk J. T., Allen R. L. Dependence of chloroplast pigment synthesis on protein synthesis: effect of actidione. Biochem Biophys Res Commun. 1965 Dec 21;21(6):523–530. doi: 10.1016/0006-291x(65)90516-4. [DOI] [PubMed] [Google Scholar]
- Margulies M. M. Effect of Chloramphenicol on Light Dependent Development of Seedlings of Phaseolus vulgaris var. Black Valentine, With Particular Reference to Development of Photosynthetic Activity. Plant Physiol. 1962 Jul;37(4):473–480. doi: 10.1104/pp.37.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies M. M. Effect of Chloramphenicol on Light-Dependent Synthesis of Proteins and Enzymes of Leaves and Chloroplasts of Phaseolus vulgaris. Plant Physiol. 1964 Jul;39(4):579–585. doi: 10.1104/pp.39.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler K., Granick S. Controls on chlorophyll synthesis in barley. Plant Physiol. 1970 Aug;46(2):240–246. doi: 10.1104/pp.46.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIRONVAL C., MICHEL-WOLWERTZ M. R., MADSEN A. ON THE NATURE AND POSSIBLE FUNCTIONS OF THE 673- AND 684-MU FORMS IN VIVO OF CHLOROPHYLL. Biochim Biophys Acta. 1965 Mar 29;94:344–354. doi: 10.1016/0926-6585(65)90043-9. [DOI] [PubMed] [Google Scholar]
- Schopfer P., Siegelman H. W. Purification of protochlorophyllide holochrome. Plant Physiol. 1968 Jun;43(6):990–996. doi: 10.1104/pp.43.6.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFF J. B., PRICE L. Terminal steps of chlorophyll A biosynthesis in higher plants. Arch Biochem Biophys. 1957 Dec;72(2):293–301. doi: 10.1016/0003-9861(57)90205-9. [DOI] [PubMed] [Google Scholar]



