Abstract
The aim of this study is to review of our knowledge about distribution of recently known species of vent shrimps and to analyze factors influencing distribution patterns. Analyses are based upon (1) original material taken during eight cruises in the Atlantic Ocean (a total of 5861 individuals) and (2) available literature data from the Atlantic, Pacific, and Indian Oceans. Vent shrimps have two patterns of the species ranges: local (single vent site) and regional (three - six vent sites). Pacific species ranges are mainly of the local type and the Atlantic species ranges are of the regional type. The regional type of species ranges may be associated with channels providing easy larval dispersal (rift valleys, trenches), while the local type is characteristic for other areas. Specialization of a shrimp genus to extreme vent habitats leads to two effects: (1) an increase in the number of vent fields inhabited by the genus and (2) a decrease of species number within the genus.
Introduction
The discovery of hydrothermal vents along the Galápagos Ridge in 1977 [1] has stimulated an increasing research effort examining the diversity, ecology, physiology, and biogeography of vent organisms, as well as new avenues of research into the origins of life on Earth [2]. Unusual characteristics of deep-sea vents compared with other deep-sea habitats, coupled with the ephemeral nature of hydrothermal circulation, may have important implications for deep-sea biology [3]. Decades of exploration have revealed numerous vent sites and faunal assemblages at many mid-ocean ridges and back-arc basins. As the global biogeography of vent organisms has been elucidated, separate biogeographic provinces have been erected for the shallow and deep Atlantic, the East Pacific, the North East Pacific, West Pacific back-arc basins, and the Indian Ocean [4]. Recent studies have modified this general scheme, proving existence of a single province for the Atlantic, a single province for the North West Pacific, a single province for the South West Pacific and Indian Ocean, and a separation of the North East Pacific, North East Pacific Rise, and South East Pacific Rise [5]. However, there are some shortcomings in the methodology of Bachraty et al. [5], some of which have been addressed by Rogers et al. [3].
At the same time, several attempts have been made to understand which factors may affect the observed distributional patterns of vent biota. The effects of spreading rate and geomorphology of the mid-ocean ridge axis have been proposed to be among these factors [6]. We assume that some of patterns will become clearer and much more visible if we revise and analyze the global distribution of a selected taxonomic group, including species with similar morphological, physiological, biochemical, and reproductive features. This group should be widely distributed and have numerous species to provide statistically significant conclusions. One potential group for such study is the shrimp family Alvinocarididae.
Alvinocaridid shrimps (Caridea: Alvinocarididae) represent the key elements of hydrothermal communities of several vent fields on the Mid-Atlantic Ridge (MAR), and they are members of the hydrothermal communities in other areas of the oceans [7]. We know that numerous species occur in the Atlantic, Pacific, and Indian Oceans, with new species described during the last 10 years [8]–[18]. Despite the importance and visibility of the group, no recent review of its composition, distribution, and spatial/ecological biogeography is available.
Even among the severe conditions of the deep sea (elevated pressure, complete absence of light), the environments of hydrothermal vents may be considered extreme, with unique physical and chemical properties such as high and rapidly changing temperature (from 2–4 °C to 400 °C), acidic pH, toxic heavy metals, an hydrogen sulfide [19]–[20]. Vent shrimp genera show numerous adaptations to vent habitats. These adaptations are fewer and less conspicuous in the genus Alvinocaris, which is similar to usual deep-sea shrimps, and numerous and prominent in the genus Rimicaris [21]–[23]. Specialization to vent habitats implies morphological adaptations (highly specialized mouthparts covered with soft setae, enlarged branchial chambers formed by the carapace, appearance of dorsal organ – [21]–[23] and adaptations reflected in life cycles [24]. Specialization to hydrothermal conditions increases in the string Alvinocaris - Opaepele - Chorocaris - Mirocaris - Rimicaris [23]–[24]. Recently the family is ripe for review of its internal phylogeny.
In this paper we summarize original data about composition and distribution of Alvinocaridid vent shrimps and put them in the context of relevant general literature information. Further we try to (1) reveal general patterns of vent shrimp distribution, (2) understand factors determining the species distribution and (3) estimate role of specialization to extreme biotopes in the composition and distribution of vent shrimp genera.
Material and Methods
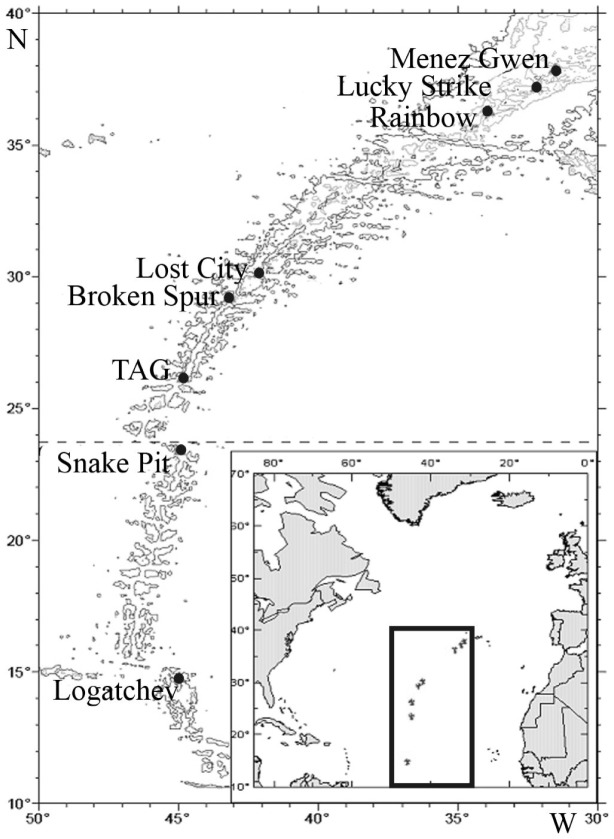
Original material was taken along the Mid-Atlantic Ridge during eight cruises of R/V “Akademik Mstislav Keldysh” with use of two deep-sea manned submersibles "Mir–1" and "Mir–2" (Fig. 1, Table 1). Seven vent fields were investigated during 1994-2005, including Menez Gwen (37.8417 N, 31.525 W), Lucky Strike (37.2933 N, 32.2733 W), Rainbow (36.23 N, 33.902 W), Broken Spur (29.17 N, 43.1717 W), TAG (26.1367 N, 44.8267 W), Snake Pit (23.3683 N, 44.95 W) and Logatchev (14.752 N, 44.9785 W). No specific permissions were required for field studies for all locations. The field studies did not involve endangered or protected species.
Figure 1. Map of the Atlantic hydrothermal vent fields visited during collection of the original material.
Isobaths 500 m, 1000 m, 2000 m, and 3000 m are shown.
Table 1. Vent shrimps, original collections during 34–41 Cruises of R/V “Akademik Mstislav Keldysh”, submersibles “Mir”, Atlantic Ocean.
| Hydrothermal field | Rimicaris exoculata | Alvinocaris markensis | ||
| Hydrothermal field | Date | No of individuals | Date | No of individuals |
| Menez Gwen | - | 01.03.1997 | 1 | |
| Lucky Strike | - | 11.06.2002 | 4 | |
| Rainbow | 25.10.1998 | 138 | 25.10.1998 | 21 |
| 17.07.2002 | 40 | 18.07.2002 | 12 | |
| 17.07.2002 | 27 | 03.09.2005 | 110 | |
| 18.07.2002 | 47 | - | - | |
| 19.07.2002 | 61 | - | - | |
| 02.09.2005 | 79 | - | - | |
| 03.09.2005 | 68 | - | - | |
| Broken Spur | 03.09.1996 | 75 | 6–8.09.1996 | 4 |
| 04.09.1996 | 527 | 01.06.2002 | 8 | |
| 08.09.1996 | 49 | 25.08.2005 | 98 | |
| 08.09.1996 | 299 | - | - | |
| 01.07.2002 | 330 | - | - | |
| 01.07.2002 | 35 | - | - | |
| 29.09–01.10.1994 | 13 | - | - | |
| TAG | 24.09.1994 | 330 | 26–27.06.2002 | 1 |
| 24.09.1994 | 199 | - | - | |
| 25.06.2002 | 401 | - | - | |
| 26.06.2002 | 45 | - | - | |
| 27.06.2002 | 474 | - | - | |
| 17.09–22.09.1994 | 21 | - | - | |
| Snake Pit | 20.06.2002 | 500 | 22.06.2002 | 22.06.2002 |
| 20.06.2002 | 190 | 12.08.2003 | 12.08.2003 | |
| 21.06.02 | 100 | - | - | |
| 22.06.2002 | 82 | - | - | |
| 12.08.2003 | 147 | - | - | |
| 12.08.2003 | 211 | - | - | |
| 12.08.2003 | 232 | - | - | |
| 13.08.2003 | 65 | - | - | |
| Logachev | 18.11.1998 | 461 | 01.02.1995 | 1 |
| Logachev | - | - | 27.07.1998 | 8 |
Faunal composition, micro-scale distribution, behavior and population structure of shrimps in various vent microbiotopes were thoroughly investigated. Shrimps were collected using baited traps and submersible suction samplers. Immediately after retrieval all specimens were sorted, measured, and fixed in 80% alcohol. Measurements follow established methods for shrimp morphological description [25]. Type material is deposited in the Zoological Museum, Moscow, and the Oxford University Museum of Natural History (OUMNH). A total of 5861 individuals of vent shrimps were analyzed.
Analysis of shrimp distribution was made with use of original and all available literature data (see references in Table 2) including original descriptions. Detailed description of material and discussion of the species status may be found in [17], [26]–[27].
Table 2. Recently known hydrothermal vent and seep shrimp species.
| Species | Region | Habitat | Coordinates | Depth | Authors |
| Alvinocaridinides formosa | Taiwan | Gueishandao, Yilan County | 24°51.231′N, 121°59.204′E | 252–275 m | [16] |
| Alvinocaris alexander | New Zealand, Kermadec Ridge | Rumble V Seamount | 36°08.27–35 S 178°11.74 E; | 730–415 m | [14] |
| Brothers Caldera | 34°52.89 S 179°03.76 E | 1346–1196 m | |||
| Alvinocaris brevitelsonis | Okinawa Trough | Minami-Ensei Knoll | 28°23.35'N, 127°38.38'E | 705 m | [50] |
| Alvinocaris chelis | Taiwan | Gueishandao, Yilan County | 24°49–51N 121°59–122°0′E | 300–252 m | [16] |
| Alvinocaris dissimilis | Okinawa Trough | Minami-Ensei Knoll | 28°23.35'N, 127°38.38'E | 705 m | [10] |
| Alvinocaris komaii | South-West Pacific: Lau | Kilo Moana | 20°9′S, 176°12′E | 2620 m | [15] |
| Tow Cam | 20°19′S, 176°8′E | 2700 m | |||
| ABE | 20°45′S, 176°11′E | 2145 m | |||
| Alvinocaris longirostris | Sagami Bay | Off Hatsuchima site (cold seep) | 35°00′ N; 139°14′E | ∼1100 m | [28]–[29] |
| Okinawa Trough | Iheya Ridge | 27°32.70′N, 126°58.20′E | 1360 m | [51] | |
| Hatoma Knoll | 24°51′N; 123°50.4′E | ∼1950 m | [52] | ||
| Miname-Ensei Knoll | 28°23.4′ N; 127°38.4′E | ∼700 m | |||
| New Zealand, Kermadec Ridge | Brothers Caldera | 34° 51-53′S 179° 3-4′E | 1850–1196 m | [9] | |
| Alvinocaris lusca | Galapagos Rift: | Rose Garden area | 00°48.15′N, 86°13.29′W | 2450 m | [53] |
| East Pacific Ridge | 9°N site, | 09°50.3′N, 104°17.4′W | 2520 m | [54]–[55] | |
| Alvinocaris markensis | Mid-Atlantic Ridge | Lucky Strike | 37° 17.598'N 32° 16.398'W | 1600–1740 m | [56] |
| Rainbow | 36° 13.800'N33° 54.120'W | 2270–2320 m | |||
| Broken Spur | 29° 10.200'N 43° 10.302'W | 3100 m | [57] | ||
| TAG | 26° 8.202'N 44° 49.602'W | 3436–3670 m | [21] | ||
| Snake Pit | 23° 22.098'N 44° 57.000'W | 3450–3500 m | [55] | ||
| Logatchev | 14° 45.120'N 44° 58.710'W | 2925–3050 m | [54] | ||
| Alvinocaris niwa | New Zealand: Kermadec Ridge | Brothers Caldera | 34°52.89–52.87′S 179°3.76–3.21′E; | 1346–1196 m | [9] |
| Rumble V Seamount | 36°8.63–8.57′S, 178°11.77–11.50′E; | 877–655 m | |||
| Alvinocaris williamsi | Mid-Atlantic Ridge | Menez Gwen | 37° 50.502'N 31° 31.500'W | 840–865 m | [58] |
| Chorocaris chacei | Mid-Atlantic Ridge | Moytirra | 45° 28.998'N 27° 51.000'W | 3000 m | [59] |
| Lucky Strike | 37° 17.598'N 32° 16.398'W | 1600–1740 m | [60] | ||
| Broken Spur | 29° 10.200'N 43° 10.302'W | 3100 m | |||
| TAG | 26° 8.202'N 44° 49.602'W | 3436–3670 m | |||
| Snake Pit | 23° 22.098'N 44° 57.000'W | 3450–3500 m | |||
| Logatchev | 14° 45.120'N 44° 58.710'W | 2925–3050 m | |||
| Ashadze | 12° 58.398'N 44° 51.798' W | 4080 m | [61] | ||
| Chorocaris paulexa | East Pacific Rise | 17 37'S, EPR, Homer Vent Site | 17°37.220′S, 113°15.123′W | 2596 m | [11] |
| Rapa Nui vent field, Brandon vents | 21°33.7′S 114°17.9′W | 3640 m | |||
| Chorocaris vandoverae | Western Pacific Ocean,Mariana Back-Arc Spreading Center | Alice Springs vent field; | 18°12.599′N, 144°42.431′E; | 3660 m | [55]–[56], [62] |
| Nautilocaris saintlaurentae | North Fiji Basin | White Lady site | 16°59.50′S, 173°55.47′E, | 2000 m | [63] |
| Lau Basin | Vaï-Lili site | 22°13′S, 176°38′E | 1750 m | ||
| Opaepele loihi | Pacific Ocean, Hawaii | Loihi Seamount; | 18°55′N, 155°16′W | 980 m | [64] |
| Mariana Arc | NW Rota-1 Volcano | 14°36.0′N, 144°46.5′E | 530 m | [65] | |
| Philippine Sea Plate | Nikko Seamount | 23° 4.856′ N 142° 19.512′ E | 456 m | [66] | |
| Opaepele susannae | South Mid-Atlantic Ridge | Semenov | 13° 30.822'N 44° 57.780'W | 2440 m | [67] |
| Mephisto | 04°47.834S, 12°22.593W | 3045 m | |||
| Turtle Pits | 04°48.57 S, 12°22.41 W | 2998 m | [13] | ||
| Sisters Peak | 04°48.188S, 12°22.301W | 2986 m | |||
| Lilliput | 9° 33.000' S 13° 10.800'W | 1500 m | |||
| Opaepele vavilovi | Mid-Atlantic Ridge | Broken Spur | 29.1700 N 43.1717 E | 3100 m | [17] |
| Rimicaris exoculata | Mid-Atlantic Ridge | Moytirra | 45° 28.998'N 27° 51.000'W | 3000 m | [59] |
| Lucky Strike (very low abundance) | 37° 17.598'N32° 16.398'W | 1600–1740 m | [10] | ||
| Rainbow | 36° 13.800'N 33° 54.120'W | 2270–2320 m | [60] | ||
| Broken Spur | 29° 10.200'N 43° 10.302'W | 3100 m | |||
| TAG | 26° 8.202'N 44° 49.602'W | 3436–3670 m | |||
| Snake Pit | 23° 22.098'N 44° 57.000'W | 3450–3500 m | |||
| Logatchev | 14° 45.120'N 44° 58.710'W | 2925–3050 m | |||
| Ashadze | 12° 58.398'N 44° 51.798' W | 4080 m | [61] | ||
| South MAR | Mephisto | 04°47.834S, 12°22.593W | 3045 m | ||
| Rimicaris kairei | Central Indian Ridge, Rodriguez Triple Junction | Kairei Field; | 25°19.16′S, 70°02.40′E; | 2454 m | [8] |
| Edmond vent field | 23°52.68′S, 69°35.80′E | 3290–3320 m | [68] | ||
| Dodo hydrothermal field | 18°20.19S, 65°17.99E; | 2745 m | [69] | ||
| Solitaire hydrothermal field | 19°33.413S, 65°50.888E | 2606 m | |||
| SW Indian Ridge | Dragon | 37° 46.998'S 49° 39.000'W | 2785 m | Copley J., pers. comm. | |
| Rimicaris hybisae | Mid-Cayman Spreading Centre | Beebe | 18° 32.688'N 81° 43.170'W | 4960 m | [18] |
| Von Damm | 18° 22.596'N 81° 47.832'W | 2300 m | |||
| Shinkaicaris leurokolos | Okinawa Trough | Minami-Ensei Knoll | 28°23.35'N, 127°38.38'E | 705 m | [50] |
| Mirocaris fortunata | Mid-Atlantic Ridge | Moytirra | 45° 28.998'N 27° 51.000'W | 3000 m | [59] |
| Menez Gwen | 37° 50.502'N 31° 31.500'W | 840–865 m | [70] | ||
| Lucky Strike | 37° 17.598'N32° 16.398'W | 1600–1740 m | |||
| Rainbow | 36° 13.800'N 33° 54.120'W | 2270–2320 m | |||
| Broken Spur | 29° 10.200'N 43° 10.302'W | 3100 m | |||
| TAG | 26° 8.202'N 44° 49.602'W | 3436–3670 m | |||
| Snake Pit | 23° 22.098'N 44° 57.000'W | 3450–3500 m | |||
| Logatchev | 14° 45.120'N 44° 58.710'W | 2925–3050 m | |||
| Ashadze | 12° 58.398'N 44° 51.798' W | 4080 m | [61] | ||
| South MAR | Turtle Pits | 04°48.57 S, 12°22.41 W | 2998 m | ||
| Mirocaris indica | Central Indian Ridge, Rodriguez Triple Junction: | Kairei Field | 25°19.2′S, 70°02.4′E | 2422 m | [12] |
| Edmond Field | 23°52.7 ‘S, 69°35.8′E, | 3300 m | |||
| Solitaire hydrothermal field | 19°33.413S, 65°50.888E | 2606 m | [69] | ||
| SW Indian Ridge | Dragon | 37° 46.998'S 49° 39.000'W | 2785 m | Copley J., pers. comm. |
As differences in sampling efforts may affect current records of species' distributions, we analyzed the InterRidge database including all recently recorded active vent sites (http://irvents-new3.whoi.edu). Table 3 illustrates the number of explored active vent sites within major geographic regions and the number of sites within those areas where Alvinocaridid shrimps have been recorded. To examine the possible correlation between these two numbers, we used the Spearman correlation coefficient. For a sample of size n and difference d coefficient ρ is computed from these: ρ = 1−(6Σd2/n(n2−1)). All maps were created with use of the CorelDraw (styled CorelDRAW) vector graphics editor, version X6, graphics were made with use of Microsoft Excel spreadsheet application, version 14.0.
Table 3.
| Region | Number of sites explored | Number of sites with shrimp records | Share of active vents inhabited by shrimps | Depth range, m | Sites explored |
| Southern Ocean | 3 | 0 | 0.00 | 45–270 | ESR; E2, Adventure Caldera, Kemp Caldera |
| Northwest Atlantic | 7 | 2 | 0.29 | 1–4960 | Champagne Hot Springs, Kick'em Jenny submarine volcano, Montserrat Volcano, Beebe, Europa, Von Damm, Don Joao de Castro Bank |
| Mid-Atlantic Ridge | 22 | 11 | 0.50 | 350–4200 | Ashadze, Ashadze 2, Broken Spur, Bubbylon, Evan, Logatchev, Logatchev 2, Lost City, Lucky Strike, Menez Gwen, Menez Hom, Moytirra, Rainbow, Saldanha, Semyenov, Snake Pit, TAG, Steinaholl Vent Field, Baily's Beads, Lilliput, MAR; 4 48'S, Nibelungen |
| Indian Ocean | 6 | 5 | 0.83 | 1600–3320 | Aden, Dodo Field, Edmond Field, Kairei Field, Solitaire Field, SWIR Area A |
| Central Pacific | 5 | 2 | 0.40 | 150–4800 | Loihi Seamount, Bounty Seamount, Macdonald Seamount, Teahitia vents, Vailulu'u Seamount |
| Galapagos Rift | 6 | 1 | 0.17 | 1640–2700 | Calyfield, Galapagos Mounds, Iguanas-Pinguinos, Navidad, Precious Stone Mountain, Rose Garden |
| Juan de Fuca Ridge | 20 | 0 | 0.00 | 1540–3460 | Axial Volcano; ASHES, Axial Volcano; CASM, Axial Volcano; South Rift Zone, Baby Bare Seamount, Central Cleft; off-axis, East Blanco Depression, Floc, Flow, High-Rise Field, Main Endeavour Field, Middle Valley; Dead Dog Vent Field, Middle Valley; ODP Mound, Mothra Field, North Cleft; high temperature, North Cleft; low temperature, Not Dead Yet, Salty Dawg Field, Sasquatch Field, Source, South Cleft |
| Kermadec Arc | 9 | 2 | 0.22 | 130–1800 | Brothers volcano, Clark volcano, Giggenbach volcano, Healy volcano, Macauley Caldera, Rumble III volcano, Rumble V volcano, Vulkanolog, Wright volcanic center |
| Lau Basin | 17 | 4 | 0.24 | 1200–2700 | ABE, CDE, CLSC; A3, Hine Hina, Kilo Moana, Kulo Lasi, Maka, Mariner, Misiteli, Si'iSi'i, Tahi Moana 2, TELVE, Tow Cam, Tu'i Malila, Vai Lili, Volcano O, White Church |
| Manus Basin | 8 | 0 | 0.00 | 535–2500 | DESMOS Cauldron, PACMANUS field, Solwara 11, Solwara 13, Solwara 17, SuSu Knolls, Vienna Woods, Vienna Woods; Hydrothermal Field 4 |
| Mariana Arc and Trough | 21 | 2 | 0.10 | 55–3676 | Daikoku volcano, East Diamante volcano, Esmeralda Bank, Forecast, Kasuga 2 Seamount, Kasuga 3 Seamount, Maug Caldera, Minami-Hiyoshi submarine volcano, Nikko volcano, Northwest Eifuku, Northwest Rota-1 volcano, Ruby, Seamount X, TOTO Caldera, West Rota volcano, 13 N Ridge Site, Alice Springs Field, Mariana Mounds, Mariana Trough; unnamed, Pika, Snail |
| North East-Pacific Rise | 22 | 1 | 0.05 | 2000–2950 | AHA Field, EPR; 10 02'N, EPR; 10 44.6'N, EPR; 11 17'N, EPR; 11 24'N, EPR; 11 42'N, EPR; 13 N, EPR; 13 N; Marginal High, EPR; 21 N, EPR; 3.9 N offset, EPR; 8 38'N, EPR; 9 17'N, EPR; 9 30'N, EPR; 9 33'N, EPR; 9 40'N, EPR; 9 47'N, EPR; 9 50'N, Feather Duster, Medusa, Mounds and Microbes, Red Seamount, Teotihuacan |
| Okinawa Trough | 11 | 5 | 0.45 | 30–1850 | Iheya Ridge, Irabu Knoll, Izena Cauldron, Kueishan Island, Kueishan Island; offshore, Minami-Ensei Knoll, Natsushima 84-1 Knoll, North Knoll; Iheya Ridge, SPOT; Hatoma Knoll, SPOT; Yonaguni Knoll IV, Yoron Hole |
| South East-Pacific Rise | 27 | 2 | 0.07 | 2064–3050 | Animal Farm, EPR; 1.4 S; off-axis, EPR; 11 18'S, EPR; 14 S, EPR; 17 12'S, EPR; 17 34'S, EPR; 17 44'S, EPR; 18 10'S, EPR; 18 15'S, EPR; 18 26'S, EPR; 18 32'S, EPR; 2 S, EPR; 20 06'S, EPR; 21 25'S, EPR; 23 30'S, EPR; 23 50'S, EPR; 26 10'S, EPR; 26.5 S, EPR; 7 25'S, EPR; Ridge 1; 20 40'S propaging rift, EPR; Ridge 3; 20 40'S propaging rift, Nolan's Nook, Pito Seamount, Rapa Nui, Rehu-Marka, Saguaro Field, Stealth |
| Tonga Arc | 7 | 0 | 0.00 | 210–2600 | Mata Fitu, Mata Tolu, Monowai Caldera, Tonga Arc; Volcano 1, Tonga Arc; Volcano 19, Tonga Arc; Volcano P, West Mata submarine volcano |
Results and Discussion
A list of all recently known species of Alvinocaridid vent shrimps is presented in Table 2. The shrimps represent eight genera and 26 species. Most of the Alvinocaridid species inhabit the Pacific (54% species) and Atlantic (38%) Oceans. Only two species (8%) are recorded from the Indian Ocean, which may be a result of less exploration of this area.
Most species listed in Table 2 (22 of 26) were reported exclusively from hydrothermal vents. A single species, A. longirostris, was found both in hot vents (Kermadec fault, [9]) and cold seeps (Sagami Bay, Japan, [28]–[29]. Three species (Alvinocaris methanophyla, A. muricola, and A. stactophyla) were reported from cold seeps only. These species may be found in hot vents in the future (as occurred with A. longirostris), but for now they are excluded from our analyses.
While analyzing the species ranges for the obligate vent fauna, Mironov et al. [30] recognized three types of species ranges: (1) local, (2) regional, and (3) transoceanic ranges. A range is local type if the species has been recorded so far from a single hydrothermal field. A regional type of distribution represents cases where a species has been reported from more than one hydrothermal vent field within a large geographic region (e.g. Eastern Pacific, Western Pacific and the Mid-Atlantic Ridge). Species inhabiting at least two large geographic regions are classified as transoceanic in range type.
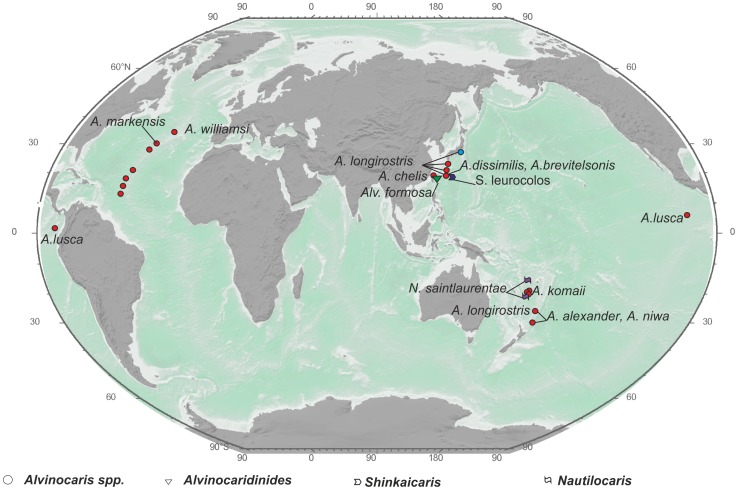
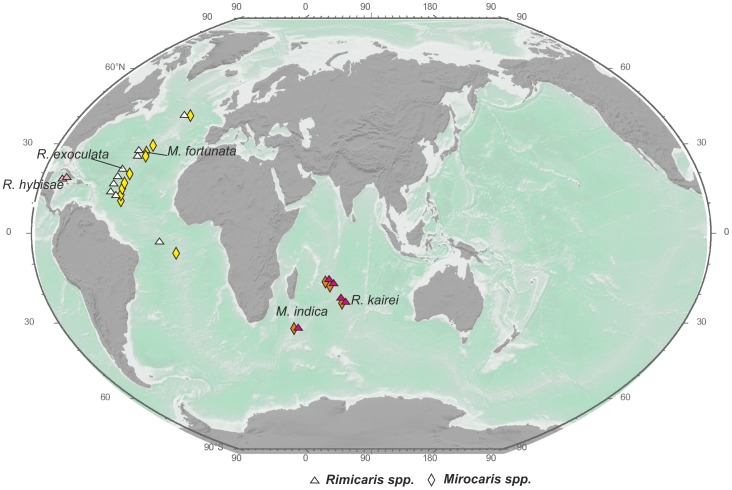
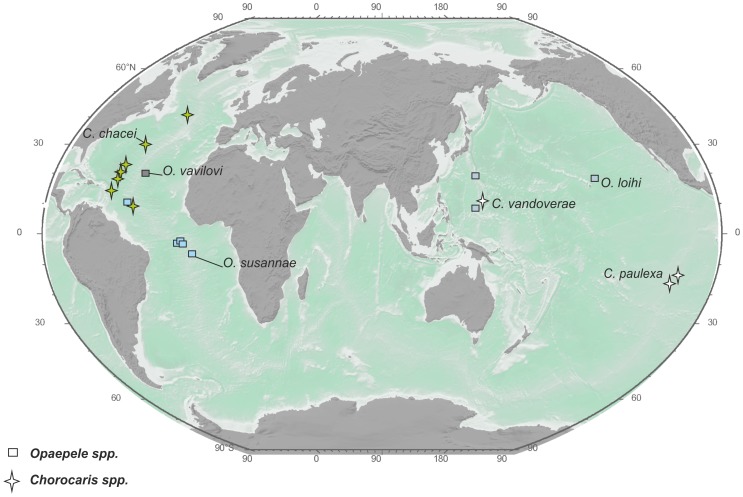
For vent shrimps we observe two types of species ranges. Most species of the genera Alvinocaris, Opaepele, and Chorocaris along with the monotypic genera Nautilocaris, Shinkaicaris, and Alvinocaridinides exhibit local ranges (Fig. 2–4).
Figure 2. Distribution of the genera Alvinocaris, Alvinocaridinides, Shinkaicaris, and Nautilocaris.
The same symbol shape indicates the same genus, and the same symbol color indicates the same species.
Figure 4. Distribution of the genera Chorocaris and Opaepele.
The same symbol shape indicates the same genus, and the same symbol color indicates the same species.
Figure 3. Distribution of the genera Rimicaris and Mirocaris.
The same symbol shape indicates the same genus, and the same symbol color indicates the same species.
Regional-type species ranges (Fig. 2–4) are typical for Atlantic vent shrimps and for Alvinocaris longirostris. Ranges in this category include 3–6 hydrothermal sites mostly along the Mid-Atlantic Ridge.
As difference in sampling efforts may contribute to species distributions being classified as "local", we tried to compare sampling efforts using data in the Atlantic, Pacific, and Indian Oceans. Table 3 shows that Alvinocaridid vent shrimps occur in most geographic areas with active vents. The proportion of active vents in each region inhabited by Alvinocaridid shrimps varies from 0 (South Ocean, Juan de Fuca Ridge, Manus Basin, Tonga Arc) to 0.4–0.5 (Mid-Atlantic Ridge, Central Pacific, Okinawa Trough) and even to 0.8 (Indian Ocean). The average value is 0.22±0.06 (n = 15).
The correlation between the number of sites explored and the number of sites with Alvinocaridid shrimp records in the same area were analyzed. The Spearman correlation coefficient is 0,25 and P-level is 0,36, indicating a low relation between these parameters. We therefore suggest that differences in sampling efforts within various geographic regions do not significantly affect our conclusions.
Table 3 also indicates that all regions with vent shrimp records include vent sites with similar depth ranges, from shelf to ca. 3–5 thousand meters (except Kermadec Arc and Okinawa Trough where maximal depths are slightly less than 2 thousand meters). That means that the depth factor may not significantly control the types of ranges exhibited by species.
This is remarkable that the local type of species ranges is characteristic for the Pacific Ocean, while the regional-type species ranges are usual for the Atlantic. Most of the Atlantic vent sites occur within a long narrow rift valleys, which is absent in the eastern Pacific Ocean, and we suggest that the global biogeographic difference between eastern Pacific and Atlantic Oceans might be influenced by such geomorphological differences. Indeed, the presence of a large number of species with regional species ranges is characteristic for the rift valleys in the Atlantic Ocean and the Okinawa Trench in the western Pacific. Both areas are similar in having long, narrow, and deep bottom channels. Regional species ranges were also reported for other Atlantic vent animals: on average, the local type of distribution is reported for 75% of the obligate vent species in all oceans [19], while for the Atlantic Ocean this value is 43% [31].
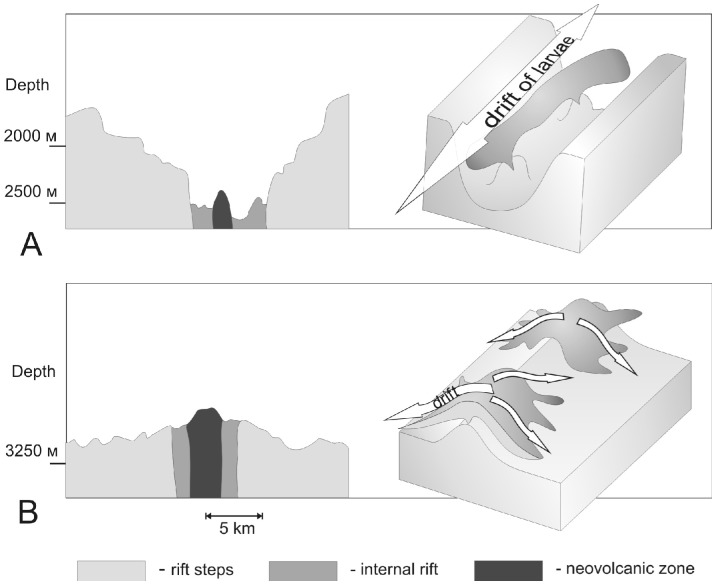
The Mid-Atlantic Ridge has a low spreading rate (ca 5 cm per year). The central part of rift valley is characterized by tectonic and volcanic activity (Fig. 5 A, B); it is the area where the vent communities develop. On average, the rift valley is about 12.5 km wide and 2.8 km deep [32]. The buoyant plume of hydrothermal vent fields cannot go beyond the internal rift and spread along the ridge axis [33]. The buoyant plume carries dispersal stages of shrimps from vent sites to the water column at the level of buoyant plume, approximately 200–300 m above the hydrothermal source [34]. Thus, species spread along the MAR and may colonize a number of hydrothermal fields. The rift valley may therefore serve as a corridor channeling the dispersal of a vent animal's larvae along the valley (Fig. 5A) without considerable loss of individuals.
Figure 5. A - Mid-Atlantic Ridge, spreading rate 2.5 cm/year; B – East-Pacific Rise, full spreading rate 15 cm/year.
The spreading rate of the East Pacific Rise is much greater (15 cm per year) than that of the Mid-Atlantic Ridge. The valley is the apical part of the ridge and the hydrothermal plumes rise above the flanking edges [35]–[36]. Under these circumstances, deep water flows take the dispersal stages out of the rift valley, thus reducing the chance to settle at the neighboring vent fields (Fig. 6B).
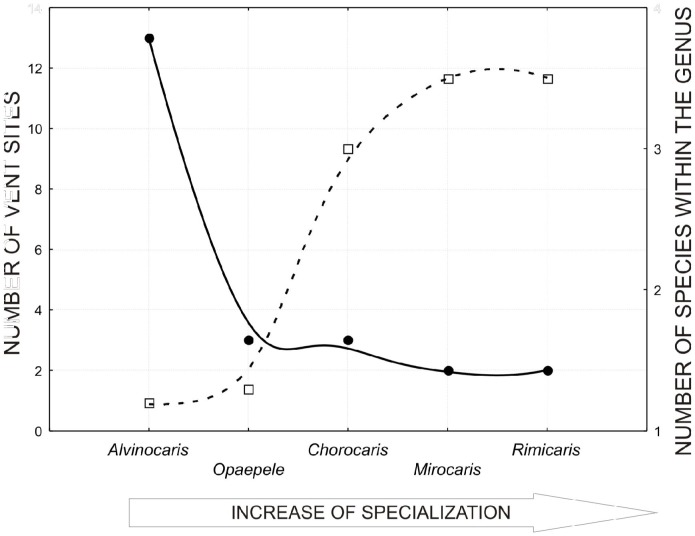
Figure 6. Relation between specialization, speciation and average number of sites inhabited by the species within the genus.
Trend lines are made with use of distance weighted least squares analyses. These are just trends, not statistically significant effects. Specialization grows from left to right. Solid line and closed circles correspond to left y-axis. Dotted line and open squares correspond to right y-axis.
The type of recruitment is reflected in the type of the species ranges. The presence of the dispersal corridor in the Atlantic Ocean may provide extensive gene exchange between populations inhabiting neighboring vent and prevent geographic isolation and speciation. In this case, we observe elongated species ranges of regional type along the Mid-Atlantic Ridge. Conversely, the absence of such a dispersal corridor in the eastern Pacific Ocean may lead to the significant loss larvae, considerable geographic isolation and higher speciation. In this case, we observe local species ranges.
Here we are faced with 2 problems:
There are vent taxa such as the siboglinid polychaetes that show a regional distribution along the EPR, in contrast to Alvinocaridid shrimp. A possible explanation for this pattern may be related to differences in the fecundity of these taxa and their levels of gene flow between neighbouring vent fields. At present, we do not have estimates of lifetime fecundity for any siboglinid [37]. Observations on spawning by a group of 155 females showed that a large numbers of oocytes were being spawned each day, but it was impossible to state whether any particular female spawned each day or how much. However, the average spawning rate of oocytes per female per day (over a 7-day period) was 335 (±130) [38]. Given that spawning lasts at least hundreds/thousands of days, lifetime fecundity for siboglinid could be of order of magnitude 104–5 propagules per female.
Total fecundity of Alvinocaris muricola is related to female size and ranges between 1432 to 5798 embryos [39], e.g. 1–2 orders of magnitude less than that suggested for siboglinid polychaetes. Total abundances of alvinocaridid shrimp and siboglinid polychaetes at vent fields are difficult to estimate, but abundances of siboglinid species may be at least as high as that of alvinocaridid species. If so, the gene flow between neighbouring fields for siboglinid species could be 1–2 orders of magnitude higher than that of alvinocaridid species, if we assume comparable larval duration and mortality. This difference in potential gene flow could prevent isolation and maintain the regional type of species range for the siboglinids in the eastern Pacific. New data about the fecundity, population densities, larval mortality, and gene flow, for example from the use of molecular methods along with modeling of water mass advection, may test some of the assumptions in this overall hypothesis.
Fundamentally, can a "local" distribution be "real": how can a species endemic to an ephemeral environment such as hydrothermal vents only be present at only one vent field? When venting inevitably ceases at a vent field, offspring of that population must have colonised neighbouring vent fields for the species to persist. Along the MAR during monitoring of vent fields between 1994 and 2005 [26], we recorded different morphs of Alvinocaris (identified as species 1–6). However, final examination of hundreds of specimens (based on morphology) and statistical analysis have proven that all individuals represented a single highly diverse species [26]. At any time we observed different populations moving along the MAR. The channeling effect of the MAR rift valley may have promoted panmixia of the population through the region.
Conversely, at the EPR, lack of the channeling effect does not prevent speciation and may lead to appearance of similar related species each inhabiting 1–2 neighboring vent fields. We also acknowledge the additional possibility that a perceived "local" pattern may just be a result of uneven sampling effort so far.
With new information about composition and distribution of vent shrimps, we can consider whether these parameters are related to specialization to vent habitats demonstrated by different Alvinocaridid genera. Specialization to the vent environment in adult forms increases in the string Alvinocaris - Opaepele - Chorocaris - Mirocaris – Rimicaris [23]. This appears to lead to two effects: (1) increase of average number of vent fields inhabited by the one species of the genus and (2) decrease of species number within the genus (Fig. 6). The less specialized genus (Alvinocaris), with least number of adaptations to vent environments in adult form, was found to be much more speciose than the specialized genera including 2–3 species each (Mirocaris, Rimicaris). The leap in specialization occurs between Alvinocaris and all other genera of Alvinocarididae both morphologically (significantly modified characters) and ecologically (harbouring exosymbionts). It is here that we find difference in number of species within the genus (>10 in Alvinocaris and 2–3 each in the other genera). Each species within less specialized genera (Opaepele, Chorocaris) inhabits one or two vent fields, whilst each species of the most specialized species occurs in numerous vent sites. Genera with intermediate specialization demonstrate intermediate patterns.
The analyses of palaeontological data and living marine mollusks indicate that proportion of monotypic genera may provide an index of the genus origination rate [40]. Since most of vent shrimp genera are nearly monotypic and including two or three very similar species, we may suggests that the genus origination rate (and thus the speciation rate) within the group is high.
Analysis of the global biogeography of vent shrimps provides the clues to understand what factors drive and shape the distribution of animals under extreme environmental conditions. One possible factor, as explored here, is the influence of ridge axis geomorphology on larval dispersal. A second possible factor is the specialization to extreme biotopes that may leads to (1) extension of the species range and (2) reducing of species number within the genus, to the limit of monophyly.
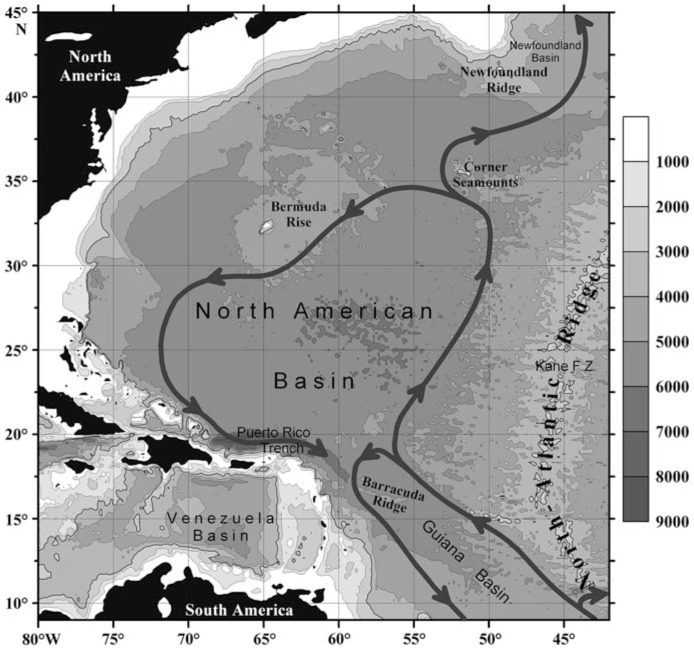
Finally, a third possible factor may be global circulation patterns acting over long periods of time: for example, near-bottom circulation in the deep Atlantic. Information about this circulation is scant, but available data indicate that it is dominated by Antarctic Bottom Water, flowing to the North American Basin after passing the Equatorial Channel and Guiana Basin [41]. Antarctic Bottom Water propagates mainly near the western slope of the Mid-Atlantic Ridge [42]–[43] and the circulation in the basin is cyclonic [43]–[45] (Fig. 7). Near-bottom meridional transport along with channeling effect is a unique feature of the Atlantic ocean that could also contribute to long regional species ranges within this area. Morphological analyses [26], [46] revealed fast population changes during 1994–2005, gene flow (measured by morphology) being apparently directed northward, coaxially with the main stream of Antarctic Bottom Water.
Figure 7. Circulation of Antarctic Bottom Water (Lower Circumpolar Water) in the Central and North Atlantic [41].
We may expect that species ranges from active vents in the South Atlantic are also of regional type and that any further discovered sites will appear to be populated by shrimp fauna similar to those of the North and Central Atlantic.
The Antarctic Circumpolar Current may be another factor influencing global shrimp distribution and preventing their occurrence in the Southern Ocean. According to mitochondrial cytochrome oxidase subunit analyses, vent shrimps radiated in the Miocene (less than ∼20 Myr; [47]) and since then have been distributed worldwide except the Southern Ocean. Indeed, recent description of fauna associated with high-temperature hydrothermal vents on the East Scotia Ridge in the Southern Ocean indicate an absence of Alvinocaridid shrimps [3]. But it is not just Alvinocaridid shrimp that are excluded from the Southern Ocean vents seen so far: also Bathymodiolid mussels, and indeed any vent taxa with planktotrophic larvae. This is more likely to be a result of "Thorsen's Rule", given the productivity regime of polar regions, than it is to be a result of ACC as a hydrographic barrier. Other non-planktotrophic vent taxa have managed to colonise the region arguably within the period that the ACC has been active (e.g. Kiwidae crabs; [48]). In addition, planktotrophic larvae appear to be rare among the taxa present at Arctic vents (e.g. [49]), also consistent with "Thorsen's Rule".
Acknowledgments
The authors are grateful to A.V. Gebruk, A.N. Mironov, and J. Copley for help and fruitful discussions.
Funding Statement
The studies were supported by the Ministry of Education and Science of the Russian Federation, by the Russian Foundation for Basic Research, and by the Program for a Basic Research of the Presidium of the Russian Academy of Sciences. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Corliss JB, Dymond J, Gordon LI, Edmond JM, von Herzen RP, et al. (1979) Submarine thermal springs on the Galapagos Rift. Science 203: 1073–1083. [DOI] [PubMed] [Google Scholar]
- 2. Martin W, Baross J, Kelley D, Russell MJ (2008) Hydrothermal vents and the origin of life. Nat. Rev. Microbiol 6: 805–814. [DOI] [PubMed] [Google Scholar]
- 3. Rogers AD, Tyler PA, Connelly DP, Copley JT, James R, et al. (2012) The Discovery of New Deep-Sea Hydrothermal Vent Communities in the Southern Ocean and Implications for Biogeography. PLoS Biol 10(1): e1001234 doi:10.1371/journal.pbio.1001234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Dover CL, German CR, Speer KL, Parson LM, Vrijenhoek RC (2002) Evolution and biogeography of deep-sea vent and seep invertebrates. Science 295: 1253–1257. [DOI] [PubMed] [Google Scholar]
- 5. Bachraty C, Legrende P, Desbruyeres D (2009) Biogeographic relationships among hydrothermal vent faunas on a global scale. Deep Sea Res Part 1 Oceanogr Res Pap 56: 1371–1378. [Google Scholar]
- 6. Ramirez-Llodra E, Shank TM, German CR (2007) Biodiversity and biogeography of hydrothermal vent species: Thirty years of discovery and investigations. Oceanography 20(1): 30–41. [Google Scholar]
- 7. Martin JW, Haney TA (2005) Decapod crustaceans from hydrothermal vents and cold seeps: a review through 2005. Zool J Linn Soc 145: 445–522. [Google Scholar]
- 8. Watabe H, Hashimoto J (2002) A new species of the genus Rimicaris (Alvinocarididae: Caridea: Decapoda) from the active hydrothermal vent field, ‘Kairei field’, on the Central Indian Ridge, the Indian Ocean. Zoolog Sci 19: 1167–1174. [DOI] [PubMed] [Google Scholar]
- 9. Webber R (2004) A new species of Alvinocaris (Crustacea:Decapoda: Alvinocarididae) and new records of alvinocaridids from hydrothermal vents north of New Zealand. Zootaxa 444: 1–26. [Google Scholar]
- 10.Komai T, Segonzac M (2006) Decapoda, Caridea. In: Desbruyères DI, Segonzac M, Bright M, editors. Handbook of Deep-Sea Hydrothermal Vent Fauna. Denisia 18 : pp. 410–454. [Google Scholar]
- 11. Martin J, Shank T (2005) A new species of the shrimp genus Chorocaris (Decapoda, Caridea, Alvinocarididae) from hydrothermal vents in the eastern Pacific. Proc. Biol. Soc. Wash 118: 183–198. [Google Scholar]
- 12. Komai T, Martin J, Zala K, Tsuchida S, Hashimoto J (2006) A new species of Mirocaris (Crustacea, Decapoda, Caridea, Alvinocarididae) associated with hydrothermal vents on the Central Indian Ridge, Indian Ocean. Si Mar 70: 109–119. [Google Scholar]
- 13. Komai T, Giere O, Segonzac M (2007) New record of alvinocaridid shrimps (Crustacea: Decapoda: Caridea) from hydrothermal vent fields on the Southern Mid-Atlantic Ridge, including a new species of the genus Opaepele . Species Diversity 12: 237–253. [Google Scholar]
- 14. Ahyong S (2009) New Species and New Records of Hydrothermal Vent Shrimps from New Zealand (Caridea: Alvinocarididae, Hippolytidae). Crustaceana 82: 775–794. [Google Scholar]
- 15. Zelnio K, Hourdes S (2009) A new species of Alvinocaris (rustacea: Decapoda: Caridea: Alvinocarididae) from hydrothermal vents at the Lau Basin, southwest Pacific, and a key to the species of Alvinocarididae. Proc. Entomol. Soc. Wash 122: 52–71. [Google Scholar]
- 16. Komai T, Chan T-Y (2010) A new genus and two new species of alvinocaridid shrimps (Crustacea:Decapoda: Caridea) from a hydrothermal vent field off northeastern Taiwan. Zootaxa 2372: 15–32. [Google Scholar]
- 17. Lunina A, Vereshchaka A (2010) A new vent shrimp (Crustacea: Decapoda: Alvinocarididae) from the Mid-Atlantic Ridge. Zootaxa 2372: 69–74. [Google Scholar]
- 18. Nye V, Copley J, Plouviez S (2011) A new species of Rimicaris (Crustacea: Decapoda: Caridea: Alvinocarididae) from hydrothermal vent fields on the Mid-Cayman Spreading Centre, Caribbean. J Mar Biol Assoc U.K 92: 1–16. [Google Scholar]
- 19. Tunnicliffe V, McArthur G, McHugh D (1998) A biogeographical perspective of the deep-sea hydrothermal vent fauna. Adv Mar Biol 34: 355–442. [Google Scholar]
- 20. Zierenberg RA, Adams MW, Arp AJ (2000) Life in extreme environments: Hydrothermal vents. Proc Natl Acad Sci USA 97: 12961–12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gebruk A, Galkin S, Vereshchaka A, Moskalev L, Southward AJ (1997) Ecology and biogeography of the hydrothermal vent fauna of the Mid-Atlantic Ridge. Adv Mar Biol 32: 93–144. [Google Scholar]
- 22. Gebruk A, Southward E, Kennedy H, Southward A (2000) Food sources, behavior, and distribution of hydrothermal vent shrimps at the Mid-Atlantic Ridge. J Mar Biol Assoc U.K. 80: 485–499. [Google Scholar]
- 23.Vereshchaka A, Gebruk A (2002) Shrimps (Decapoda Macrura Natantia) In: Gebruk A, editor. Biology of hydrothermal systems. Moscow, KMK. pp. 185–197.
- 24. Vereshchaka A, Vinogradov G, Ivanenko V (1998) Common features of reproductive biology of some hydrothermal crustaceans (amphipods, copepods, shrimps). Dokl Biol Sci 360: 269–270. [Google Scholar]
- 25.Vereshchaka A (2000) Revision of the genus Sergia (Decapoda: Dendrobranchiata: Sergestidae): Taxonomy and distribution. Galathea Report 18: , 69–207. [Google Scholar]
- 26. Lunina A, Vereshchaka A (2008) Hydrothermal vent shrimps Alvinocaris markensis: interpopulation variation. Dokl Biol Sci 421: 266–268. [DOI] [PubMed] [Google Scholar]
- 27.Lunina A (2011) Vent shrimps of the Mid-Atlantic Ridge. PhD Thesis, P.P. Shirshov Institute of Oceanology of RAS, Russia, Moscow [in Russian].
- 28.Fujikura K, Hashimoto J, Fujiwara Y, Okutani T (1995) Community ecology of the chemosynthetic community at Off Hatsushima site, Sagami Bay, Japan. JMSTC Journal of Deep Sea Research 11: : 227–241 [in Japanese with English summary]. [Google Scholar]
- 29.Fujikura K, Hashimoto J, Fujiwara Y, Okutani T (1996) Community ecology of the chemosynthetic community at Off Hatsushima site, Sagami Bay, Japan-II: comparisons of faunal similarity. JMSTC Journal of Deep Sea Research 12: : 133–153 [in Japanese with English summary]. [Google Scholar]
- 30.Mironov A, Gebruk A, Moskalev L (2002) In: Gebruk A, editor. Biology of hydrothermal systems. Moscow, KMK. pp. 410–455.
- 31.Gebruk A, Mironov A (2006) Biogeography of the Atlantic hydrothermal vents. In: Vinogradov ME, Vereshchaka AL, editors. Ecosystems of the Atlantic hydrothermal vents. Moscow, Nauka. pp. 119–162. [in Russian].
- 32. Smith D, Cann J (1993) Building the crust at the Mid-Atlantic Ridge. Nature 365: 707–715. [Google Scholar]
- 33.Bogdanov Y, Sagalevich A (2002) Geological deep-sea studies by manned submersibles "Mir". Moscow, Nauchny mir [in Russian].
- 34.Gurvich E (1998) Metalliferous sediments of World Ocean. Nauchny Mir, Moscow.
- 35. Tyler P, Young C (2003) Dispersal at hydrothermal vents: a summary of recent progress. Hydrobiologia 503: 9–19. [Google Scholar]
- 36.Zonnenshine L, Kuzmin M, Baranov B, Shilovskii P, Poroshina I (1992) Hydrothermal formation of the Mid-Atlantic Ridge, pp.12–44. Moscow, Nauka [in Russian].
- 37. Hilário A, Capa M, Dahlgren TG, Halanych KM, Little CTS, et al. (2011) New perspectives on the ecology and evolution of siboglinid tubeworms. PLoS ONE 6(2): e16309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rouse GW, Wilson NG, Goffredi SK, Johnson SB, Smart T, et al. (2009) Spawning and development in Osedax boneworms (Siboglinidae, Annelida). Mar. Biol 156: 395–405. [Google Scholar]
- 39. Ramirez-Llodra E, Segonzac M (2006) Reproductive biology of Alvinocaris muricola (Decapoda: Caridea: Alvinocarididae) from cold seeps in the Congo Basin. Journal J Mar Biol Assoc U.K. 86: 1347–1356. [Google Scholar]
- 40. Foote M (2012) Evolutionary dynamics of taxonomic structure. Biol Lett 8: 135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morozov E, Demidov A, Tarakanov R (2010) Abyssal channels in the Atlantic Ocean: Water structure and flows. Springer. 266 p. [Google Scholar]
- 42. Wunsch C (1984) An eclectic Atlantic Ocean circulation model. Part I: The meridional flux of heat. J Phys Oceanogr 14(11): 1712–1733. [Google Scholar]
- 43. Stephens JC, Marshall DP (2000) Dynamical pathways of Antarctic Bottom Water in the Atlantic. J Phys Oceanogr 30(3): 622–640. [Google Scholar]
- 44. Weatherly GL, Kelley EA (1982) ‘Too cold’ bottom layers at the base of the Scotian Rise. J Mar Res 40(4): 985–1012. [Google Scholar]
- 45. Lavin AM, Bryden HL, Parrilla G (2003) Mechanisms of heat, freshwater, oxygen and nutrient transports and budgets at 24.5° N in the subtropical North Atlantic. Deep Sea Res I 50: 1099–1128. [Google Scholar]
- 46.Vereshchaka A (1997) Comparative morphological studies on four populations of the shrimp Rimicaris exoculata from the Mid-Atlantic ridge. Deep-Sea Research I, V. 44 (11) , p. 1905–1921. [Google Scholar]
- 47. Shank TM, Black MB, Halanych KM, Luts RA, Vrijenhoek RC (1999) Miocene radiation of deep-sea hydrothermal vent shrimp (Caridea: Bresiliidae): evidence from mitochondrial cytochrome oxidase subunit I Mol. Phylogenet. Evol 13: 244–254. [DOI] [PubMed] [Google Scholar]
- 48.Roterman CN, Copley JT, Linse KT, Tyler PA, Rogers AD (2013) The biogeography of the yeti crabs (Kiwaidae) with notes on the phylogeny of the Chirostyloidea (Decapoda: Anomura). Proc Biol Sci 280 (1764).. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pedersen RB, Rapp HT, Thorseth IH, Lilley MD, Barriga FJ, et al. (2010) Discovery of a black smoker vent field and vent fauna at the Arctic Mid-Ocean Ridge. Nat Commun 1: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kikuchi T, Hashimoto J (2000) Two new caridean shrimps of the family Alvinocarididae (Crustacea, Decapoda) from a hydrothermal vent field at the Minami-Ensei Knoll in the Mid-Okinawa Trough, Japan. Species Diversity 5: 135–148. [Google Scholar]
- 51.Watabe H, Miyake H (2000) Decapod fauna of the hydrothermally active and adjacent fields on the Hatoma Knoll, southern Japan. JAMSTEC Journal of Deep Sea Research 17: : 29–34 [in Japanese with English summary]. [Google Scholar]
- 52. Kikuchi T, Ohta S (1995) Two caridean shrimps of the families Bresiliidae and Hippolytidae from a hydrothermal field on the Iheya Ridge, off the Ryukyu Islands, Japan. Journal of Crustacean Biology 15: 771–785. [Google Scholar]
- 53. Williams A, Chace F Jr (1982) A new caridean shrimp of the family Bresiliidae from thermal vents of the Galapagos Rift. Journal of Crustacean Biology 2: 136–147. [Google Scholar]
- 54. Shank TM, Black MB, Halanych KM, Lutz RA, Vrijenhoek RC (1999) Miocene radiation of deep-sea hydrothermal vent shrimp (Caridea: Bresiliidae): Evidence from mitochondrial cytochrome oxidase subunit I. Mol Phylogenet Evol 13: 244–254. [DOI] [PubMed] [Google Scholar]
- 55.Shank TM (1997) Alvinocaris lusca Williams, Chace Jr., 1982, Alvinocaris markensis Williams, 1988, Chorocaris chacei (Williams and Rona, 1986), Chorocaris vandoverae Martin, Hessler, 1990. In: Desbruyères D, Segonzac M, editors. Handbook of deep-sea hydrothermal vent fauna. Brest: IFREMER. pp. 191–194.
- 56. Williams A (1988) New marine decapod crustaceans from waters influenced by hydrothermal discharge, brine, and hydrocarbon seepage. Fish. Bull. (Wash.D. C.) 86: 263–287. [Google Scholar]
- 57. Segonzac M, de Saint Laurent M, Casanova B (1993) L'énigme du comportement trophique des crevettes Alvinocarididae des sites hydrothermaux de la dorsale médioatlantique. Cah. Biol. Mar. 34: 535–571. [Google Scholar]
- 58. Shank T, Martin J (2003) A new caridean shrimp of the family Alvinocarididae from thermal vents at Menez Gwen on the Mid-Atlantic Ridge. Proc. Biol. Soc. Wash. 116: 158–167. [Google Scholar]
- 59.Wheeler AJ, Murton B, Copley J, Lim A, Carlsson J, et al. (2013) Moytirra: Discovery of the first known deep-sea hydrothermal vent field on the slow-spreading Mid-Atlantic Ridge north of the Azores. Geochemistry, Geophysics, Geosystems, 14(00): , n/a–n/a. doi:10.1002/ggge.20243. [Google Scholar]
- 60. Williams A, Rona P (1986) Two new caridean shrimps (Bresiliidae) from a hydrothermal field on the Mid-Atlantic Ridge. J Crustacean Biol 6: 446–462. [Google Scholar]
- 61. Fabri M-C, Bargain A, Briand P, Gebruk A, Fouquet Y, et al. (2010) The hydrothermal vent community of a new deep-sea field, Ashadze-1, 12°58′N on the Mid-Atlantic Ridge. J Mar Biol Assoc U.K. 91: 1–13. [Google Scholar]
- 62. Martin J, Hessler R (1990) Chorocaris vandoverae, a new genus and species of hydrothermal vent shrimp (Crustacea, Decapoda, Bresiliidae) from the western Pacific. Contributions in Science, Natural History Museum of Los Angeles 417: 1–11. [Google Scholar]
- 63. Komai T, Segonzac M (2004) A new genus and species of alvinocarid shrimp (Crustacea: Decapoda: Caridea) from hydrothermal vents on the North Fiji and Lau Basins, southwestern Pacific. J Mar Biol Assoc U.K. 84: 1179–1188. [Google Scholar]
- 64. Williams A, Dobbs F (1995) A new genus and species of caridean shrimp (Crustacea, Decapoda, Bresiliidae) from hydrothermal vents on Loihi Seamount, Hawaii. Proc. Entomol. Soc. Wash 108: 228–237. [Google Scholar]
- 65. Limen H, Juniper SK, Tunnicliffe V, Clement M (2006) Benthic community structure on two peaks of an erupting seamount: Northwest Rota-1 Volcano, Mariana Arc, western Pacific. Cah. Biol. Mar 47: 457–463. [Google Scholar]
- 66. Yang J-S, Lu B, Chen D-F, Yu Y-Q, Yang F, et al. (2013) When did decapods invade hydrothermal vents? Clues from the Western Pacific and Indian Oceans. Mol Biol Evol 30: 305–9. [DOI] [PubMed] [Google Scholar]
- 67. Beltenev V, Ivanov V, Rozhdestvenskaya I, Cherkashov G, Stepanova T, et al. (2009) New data about hydrothermal fields on the Mid-Atlantic Ridge between 11°–14°N: 32nd Cruise of R/V Professor Logatchev. InterRidgeNews 18: 13–17. [Google Scholar]
- 68. Van Dover CL, Humphris SE, Fornari D, Cavanaugh C, Collier R, et al. (2001) Biogeography and ecological setting of Indian Ocean hydrothermal vents. Science 294: 818–823. [DOI] [PubMed] [Google Scholar]
- 69. Nakamura K, Watanabe H, Miyazaki J, Takai K, Kawagucci S, et al. (2012) Discovery of new hydrothermal activity and chemosynthetic fauna on the Central Indian Ridge at 18°–20° S. PloS One 7: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Martin J, Christiansen J (1995) A new species of the shrimp genus Chorocaris Martin and Hessler, 1990 (Crustacea, Decapoda, Bresiliidae) from hydrothermal vent fields along the Mid-Atlantic Ridge. Proc. Biol. Soc. Wash 108: 220–227. [Google Scholar]