Abstract
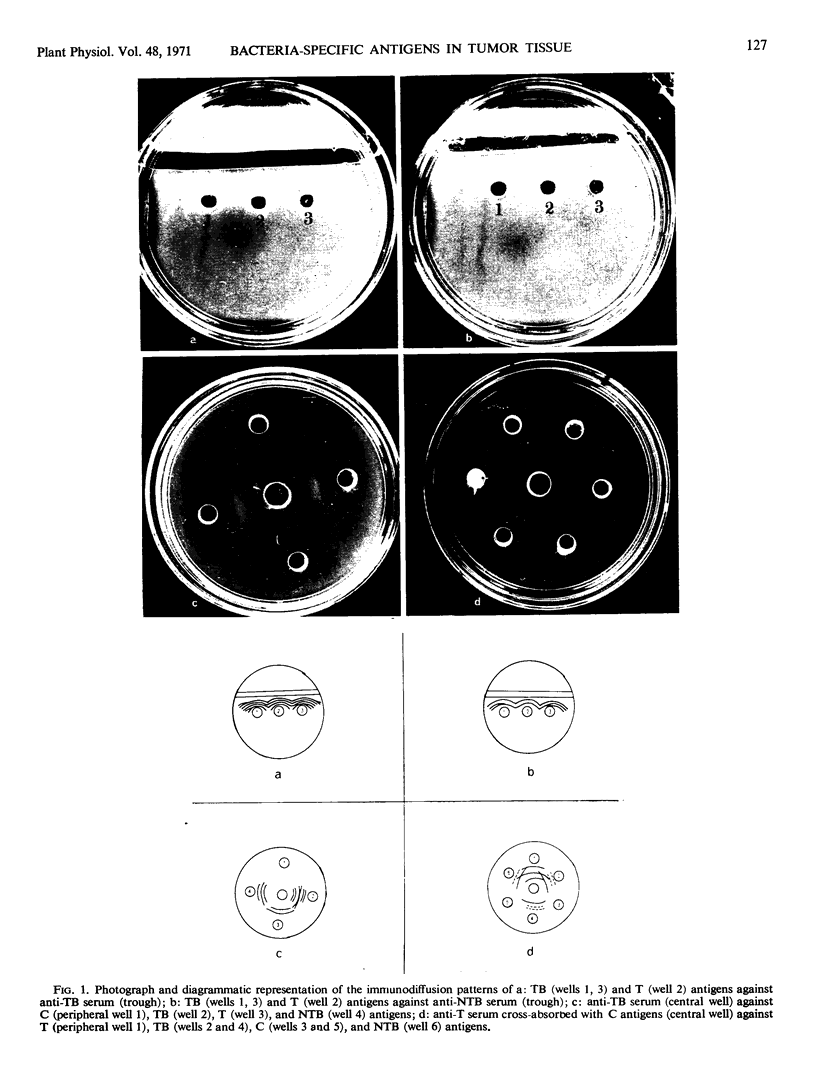
Cross-reacting antigens were found in bacteria-free crown gall tumor tissue tested with serum prepared against Agrobacterium tumefaciens (Smith and Towns.) Conn., but no such antigens were detected in callus tissue. Soluble proteins from tumor tissue, callus tissue, and the crown gall bacteria were fractionated on a DEAE-Sephadex (A-50) column. The diethylaminoethyl-Sephadex elution profile for tumor tissue showed three protein fractions that were not detected in the callus tissue. Two of these protein fractions were shown to be exclusively bacteria specific. Besides these qualitative differences between the two tissues, significant quantitative differences in the amount of protein fractions were also observed. The diethylaminoethyl-Sephadex column fractions from tumorigenic strain of A. tumefaciens corresponding in position to the three additional peaks in the tumor tissue also showed cross-reacting antigens when tested with serum prepared against sterile tumor tissue. It is suggested that tumor formation by A. tumefaciens involves integration of the bacterial genome into the host-cell genome.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CROWLE A. J. A simplified micro double-diffusion agar precipitin technique. J Lab Clin Med. 1958 Nov;52(5):784–787. [PubMed] [Google Scholar]
- McCown B. H., Beck G. E., Hall T. C. Plant leaf and stem proteins. I. Extraction and electrophoretic separation of the basic, water-soluble fraction. Plant Physiol. 1968 Apr;43(4):578–582. doi: 10.1104/pp.43.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milo G. E., Srivastava B. I. RNA-DNA hybridization studies with the crown gall bacteria and the tobacco tumor tissue. Biochem Biophys Res Commun. 1969 Jan 27;34(2):196–199. doi: 10.1016/0006-291x(69)90631-7. [DOI] [PubMed] [Google Scholar]
- Quétier F., Huguet T., Guillé E. Induction of Crown-gall: partial homology between tumor-cell DNA, bacterial DNA and the G+C--rich DNA of stressed normal cells. Biochem Biophys Res Commun. 1969 Jan 6;34(1):128–133. doi: 10.1016/0006-291x(69)90538-5. [DOI] [PubMed] [Google Scholar]
- Reddi K. K. Ribonuclease induction in cells transformed by Agrobacterium tumefaciens. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1207–1214. doi: 10.1073/pnas.56.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilperoort R. A., Meijs W. H., Pippel G. M.W., Veldstra H. Agrobacterium tumefaciens cross-reacting antigens in sterile crown-gall tumors. FEBS Lett. 1969 May;3(3):173–176. doi: 10.1016/0014-5793(69)80127-4. [DOI] [PubMed] [Google Scholar]
- Srivastava B. I., Chadha K. C. Liberation of Agrobacterium tumefaciens DNA from the crown gall tumor cell DNA by shearing. Biochem Biophys Res Commun. 1970 Aug 24;40(4):968–972. doi: 10.1016/0006-291x(70)90998-8. [DOI] [PubMed] [Google Scholar]
- Srivastava B. I. Patterns of nucleic acids synthesis in normal and crown gall tumor tissue cultures of tobacco. Arch Biochem Biophys. 1968 Jun;125(3):817–823. doi: 10.1016/0003-9861(68)90519-5. [DOI] [PubMed] [Google Scholar]
- Srivastava B. I. Studies on nucleic acids from healthy and tumorous tomato stem tissue. Biochim Biophys Acta. 1965 Jun 8;103(2):349–352. doi: 10.1016/0005-2787(65)90178-4. [DOI] [PubMed] [Google Scholar]