Abstract
Maternal adiponectin (ADN) levels are inversely correlated with birth weight, and ADN infusion in pregnant mice down-regulates placental nutrient transporters and decreases fetal growth. In contrast to the insulin-sensitizing effects in adipose tissue and muscle, ADN inhibits insulin signaling in the placenta. However, the molecular mechanisms involved are unknown. We hypothesized that ADN inhibits insulin signaling and insulin-stimulated amino acid transport in primary human trophoblasts by peroxisome proliferator-activated receptor-α (PPARα)-mediated ceramide synthesis. Primary human term trophoblast cells were treated with ADN and/or insulin. ADN increased the phosphorylation of p38 MAPK and PPARα. ADN inhibited insulin signaling and insulin-stimulated amino acid transport. This effect was dependent on PPARα, because activation of PPARα with an agonist (GW7647) inhibited insulin signaling and function, whereas PPARα-small interfering RNA reversed the effects of ADN on the insulin response. ADN increased ceramide synthase expression and stimulated ceramide production. C2-ceramide inhibited insulin signaling and function, whereas inhibition of ceramide synthase (with Fumonisin B1) reversed the effects of ADN on insulin signaling and amino acid transport. These findings are consistent with the model that maternal ADN limits fetal growth mediated by activation of placental PPARα and ceramide synthesis, which inhibits placental insulin signaling and amino acid transport, resulting in reduced fetal nutrient availability.
Maternal obesity and gestational diabetes mellitus (GDM) increase the risk of giving birth to a large-for-gestational-age (highest 10th percentile) or macrosomic (>4000 g) infant (1, 2). These infants, in turn, are likely to be insulin resistant (3) and are susceptible to develop metabolic syndrome in childhood (4). Fetal growth is highly dependent on maternal nutrient availability and placental transport of nutrients and oxygen. Studies in recent years have highlighted the critical role of both the maternal and fetal hormonal milieu in regulating the functional capacity of the placenta to transport nutrients to the fetus (5–8). Hormones such as insulin and leptin, which are elevated in the maternal circulation in obesity or GDM (9), stimulate amino acid uptake in cultured primary human trophoblasts (PHTs) (10) and placental villous explants (11). Insulin is recognized as a major growth factor in a number of tissues and regulates both the growth and metabolic functions of the placenta (12). As such, modulation of placental insulin sensitivity and signaling may have profound effects on placental function and, consequently, fetal growth. In accordance with this hypothesis, placental insulin signaling has been reported to be activated in obese women giving birth to large babies (9) and reduced in intrauterine growth restriction (13).
Adiponectin (ADN) is the most abundantly secreted hormone from adipose tissue (14). The cellular effects of ADN are mediated by binding to adiponectin receptor 1 (AdipoR1) or 2 (AdipoR2) in the plasma membrane (15) and the subsequent activation of the AMP-activated protein kinase (AMPK), p38 MAPK, and/or peroxisome proliferator-activated receptor α (PPARα) signaling pathways (15, 16). ADN decreases plasma glucose and triglycerides through increased insulin sensitivity in the liver, skeletal muscle, and adipose, predominantly mediated by activation of AMPK (15).
Activation of both AdipoR1 and AdipoR2 has recently been associated with improved glucose homeostasis through ceramidase-mediated conversion of ceramide to sphingosine-1-phosphate (S1P), independent of AMPK (17). Ceramide and S1P are bioactive sphingolipid metabolites that exhibit potent and often opposing biological effects in many tissues including the placenta (18, 19). Increased levels of ceramide promote insulin resistance whereas S1P is associated with increased insulin sensitivity (20). Recent studies also indicate that elevated levels of ceramide decrease the plasma membrane abundance of glucose and amino acid transporters (21). Interestingly, activation of PPARα promotes ceramide biosynthesis in the liver (22), heart (23), and skin (24). However, a link between ADN and PPARα-mediated regulation of sphingolipid metabolism has not been explored in any detail.
Both AdipoR1 and AdipoR2 are expressed in the placenta (25); ADN exerts pleiotropic effects in this tissue, which include regulation of endocrine activity (26), inflammation (27), trophoblast differentiation (25), and proliferation (28). Maternal plasma ADN levels are reduced in pregnancies complicated by obesity or GDM (29). Moreover, early gestation maternal ADN levels are inversely correlated with birth weight in healthy pregnant women across the spectrum of body mass indices (9). Given that maternal ADN does not cross the placenta (30), we originally hypothesized that maternal ADN regulates fetal growth by modulating placental nutrient transport, possibly via its effects on placental insulin signaling. Indeed, ADN has been shown to inhibit insulin signaling and prevent insulin-stimulated amino acid transport in PHTs (10). In addition, maternal ADN infusion in pregnant mice inhibits placental insulin signaling, down-regulates placental nutrient transporters, and decreases fetal growth (31). However the mechanisms responsible for ADN-mediated placental insulin resistance remain largely unknown.
In this study, we test the hypothesis that ADN inhibits insulin signaling and insulin-stimulated amino acid transport in primary human trophoblasts by PPARα-mediated ceramide synthesis.
Materials and Methods
Isolation and culture of primary human trophoblasts from term placentas
Healthy pregnant women at term were recruited following written informed consent, according to approval by the Institutional Review Board UTHSCSA IRB (HSC20100262H). Placentas were coded and relevant medical information was obtained. Placental tissue was transported to the laboratory within 10 minutes of delivery, and PHT cells were isolated by trypsin digestion and Percoll purification, as previously described (32, 33). Cells were cultured in DMEM (Sigma-Aldrich) and Ham's F-12 nutrient mixture (Life Technologies) containing 10% fetal calf serum (FCS, Atlanta Biological), 50 μg/mL gentamicin, 60 μg/mL benzyl penicillin, and 100 μg/mL streptomycin (Sigma-Aldrich). Cells were plated in 35-mm dishes at a density of 2.75 × 106 for subsequent protein analyses, 10 × 106 in 100-mm dishes for lipid extraction, 0.1 × 106 per well in 96-well plates for 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays, or 0.6 × 106 per well in 12-well plates for uptake assays, and incubated in a 5% CO2 humidified atmosphere at 37°C. After 18 hours, attached PHT cells were washed twice in warm Dulbecco's PBS, and subsequently culture media were changed daily. PHT cell purity was confirmed by high protein expression of Cytokeratin-7 (epithelial cell marker) and absence of Vimentin (fibroblast cell marker) expression as previously described (33).
Small interfering RNA (siRNA) transfection and cell culture treatments
Following 18 hours of culture, PHTs were transfected with 100 nM of siRNAs targeting p38 MAPK or PPARα using Dharmafect2 transfection reagent (ThermoScientific) according to the manufacturer's protocol. For both p38 MAPK and PPARα siRNA transfections, silencing efficiency was initially tested using 2 different siRNA sequences (Supplemental Figure A published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org) (catalog nos: p38MAPK, SASI_Hs01_00018465, and SASI_Hs01_00018467; PPARα, SASI_Hs02_00304005, and SASI_Hs02_00304006; Sigma-Aldrich). Based on these pilot experiments, p38 MAPK (SASI_Hs01_00018467) and PPARα (SASI_Hs02_00304006) were chosen for experiments described in this study. An equal concentration of a nontargeting scrambled (Scr) siRNA sequence (SIC001, Sigma-Aldrich) was used as a control. siRNA transfections did not influence cell viability as measured by MTT assay (Supplemental Figure B) or trophoblast differentiation as measured by human chorionic gonadotropin (hCG) secretion (Supplemental Figure Ci).
For pharmacologic targeting of p38 MAPK and PPARα, PHTs were treated at 65.5 hours of culture with the p38 MAPK inhibitor SB203580, 20 μM (SB, Cell Signaling Technology), or PPARα agonist GW7647, 0.1 μM (GW, Sigma-Aldrich), reconstituted in dimethylsulfoxide. For vehicle controls, PHTs were incubated with an equal concentration of dimethylsulfoxide (0.1% vol/vol). None of these pharmacologic agents affected cell viability or apoptosis (Supplemental Figure D, i and ii).
PHTs were also treated at 65.5 hours with ceramide synthase (CerS) inhibitor Fumonisin B1 (FB1, 50 μM; Enzo Life Sciences) or C2-Ceramide (C2-Cer, 10 μM; Enzo Life Sciences) conjugated to fatty acid-free BSA according to manufacturer's instructions. An equal concentration of fatty acid-free BSA was used as vehicle control (0.1% vol/vol). The concentrations of C2-Cer or FB1 used in this study did not significantly alter cell viability or apoptosis (Supplemental Figure D, i and ii).
The effects of ADN on cellular signaling and function were investigated by treating PHTs with physiological concentrations (5 μg/mL) of full-length recombinant ADN (R&D Systems) at 66 hours. The timing of insulin treatment (5.8 ng/mL) was dependent on the experimental outcome under investigation, as previously described (33). Briefly, to investigate the effects of insulin on amino acid transport, insulin was added to media at 66 hours of culture; for measuring changes in Akt signaling, insulin stimulation was at 87 hours of culture; and for changes in insulin receptor substrate (IRS)-1 signaling, cells were treated with insulin at 89.5 hours of culture. All experiments were terminated at 90 hours of culture. At this time, protein lysates were collected for immunoblotting analyses, lipids were isolated for mass spectrometry, and amino acid uptake or cell viability assays were performed. ADN and insulin concentrations used in this study did not influence PHT viability or apoptosis (Supplemental Figure D, i and ii) or hCG secretion (Supplemental Figure Cii).
MTT cell viability assay
PHTs plated in 96-well plates were incubated with 1 mg/mL of MTT reagent [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] for 4 hours at 37°C, lysed with 10% sodium dodecyl sulfate overnight at room temperature, and absorbance read at 570 nm. Cell viability measurements were assessed as a percentage of controls treated with vehicle or transfected with scramble siRNA.
hCG ELISA
Culture media was collected at 18, 42, 66, and 90 hours following cell plating. PHT cells were either transfected with siRNAs or treated with insulin and ADN as described above. hCG secretion into media was measured by sandwich ELISA method according to the manufacturer's protocol (Immuno-Biological Laboratories). hCG secretion was normalized to vehicle control, or scrambled siRNA at 18 hours and expressed as fold change.
Amino acid transport
Systems A and L transport activities were determined by measuring Na+-dependent uptake of [14C]methyl-aminoisobutyric acid and 2-amino-2-norbornane-carboxylic acid-inhibitable uptake of [3H]leucine (Leu), respectively, as previously described (11, 33). Following the treatments described above, PHTs were washed and then incubated in Tyrode's salt solution (with Na+, or without Na+ with addition of 1 mM 2-amino-2-norbornane-carboxylic acid) containing [14C]methyl-aminoisobutyric acid (20 μM at 1.19 μCi/mL) and [3H]Leu (12.5 nM at 0.68 μCi/mL) for 8 minutes. After washing in cold Tyrode's solution without Na+, cells were lysed in distilled water, and radioactivity in the water was counted in a liquid scintillation counter. Protein content was determined using the Lowry method (34). Transport activity per mg of protein was initially calculated, and the activity was expressed as fold change from control.
Western blot analyses
Cell protein lysates were harvested in radioimmunoprecipitation buffer containing protease inhibitors and phosphatase inhibitor cocktail 1 and 2 (1:100, Sigma-Aldrich). Western blot analyses were carried out as previously described (33). Antibodies were diluted as follows: rabbit antiphospho -IRS-1 (Tyr612), -Akt (Thr308), -AMPK (Thr172), -p38 MAPK (Thr180/Tyr182), PPARα (Ser21), IRS-1, Akt, AMPK, caspase 3, poly ADP ribose polymerase, p38 MAPK, and PPARα at 1 μg/mL, mouse anti-β-actin at 0.2 μg/mL, peroxidase-conjugated antirabbit at 0.5 μg/mL and -mouse at 0.2 μg/mL. Phospho-PPARα (Ser21) and PPARα antibodies were from Abcam, anti-β-actin antibody was obtained from Sigma; all other antibodies were purchased from Cell Signaling Technology.
Reverse transcription and quantitative-PCR (Q-PCR)
Total RNA was extracted from cells and DNAse treated using the RNEasy Mini Kit (QIAGEN), cDNA synthesis with random primers was carried out using Superscript III Reverse Transcriptase (Life Technologies) according to the manufacturer's protocols. Q-PCR for CerS-1, -2, -3, -4; succinate dehydrogenase complex subunit A and TATA-binding protein (see Supplemental Figure E for primer sequences) was performed in triplicate on 1 μg of cDNA using 1:2 diluted SYBR Green PCR Master Mix and 0.5 μM primers. PCR amplification and detection were performed on Applied Biosystems Step One Plus Real-Time PCR System (Life Technologies) using the initial denaturation condition of 95°C for 10 minutes, followed by 40 cycles at 95°C, 58, or 60°C and 60°C for 30 seconds each. Exponential amplification of all PCRs ranged from 1.95–2.00 across 7 serial log dilutions of template. Amplification of a single product was confirmed by melting curve analysis and visualization of the product by gel electrophoresis on a 2% agarose gel. Expression of target mRNA was quantified using the comparative threshold cycle (Ct) method for relative quantification (2-ΔΔCt), normalized to the geometric mean of reference genes succinate dehydrogenase complex subunit A and TATA-binding protein according to a previously published algorithm (35).
Lipid analysis by HPLC-electrospray ionization tandem mass spectrometry (HPLC-ESI-MS/MS)
PHT cells plated in 100-mm dishes were treated with ADN (5 μg/mL) from 66–90 hours as described above. At 90 hours, PHTs were scraped into glass tubes and lipids extracted using 2:1 chloroform/methanol (−20°C) and maintained on ice for 30 minutes, as previously described (36). After centrifugation at 13 800 × g for 10 minutes, the chloroform layers were removed, dried in vacuo, and reconstituted in isopropanol before HPLC-ESI-MS/MS.
HPLC-ESI-MS/MS analyses were conducted on a Thermo Fisher Q Exactive mass spectrometer with on-line separation using a Thermo Fisher/Dionex RSLC nano HPLC. HPLC conditions were: Atlantis dC18, 3 μm, 300 μm × 150 mm column (Waters Corp); mobile phase A, acetonitrile/water (40:60) containing 10 mM ammonium acetate; mobile phase B, acetonitrile/isopropanol (10:90) containing 10 mM ammonium acetate; flow rate, 6 μL/min; gradient, 10% B to 60% B over 5 minutes, 60% B to 99% B over 35 minutes and held at 99% B for 10 minutes. Data-dependent tandem-MS scans were performed using one full MS scan (m/z 200–2000; 70 000 resolution [m/z 300]) followed by fragmentation in the higher-energy collisional dissociation cell of the 6 most abundant ions in the precursor scan using a normalized collision energy (NCE) of 35 arbitrary units and mass analysis in the orbitrap at 17 500 resolution. Separate analyses were conducted using positive and negative ion detections.
Progenesis CoMet (Nonlinear Dynamics Limited) was used to process the raw data files. Peak alignment and integration were performed, and the relative abundance was generated for each lipid among the different experimental groups. Sphingolipid species were identified by searching the following databases: METLIN (http://metlin.scripps.edu/index.php); lipid maps (http://www.lipidmaps.org/data/structure/); HMDB (Human Metabolome Database; http://www.hmdb.ca/) using a 5-ppm mass tolerance. The putative lipid identifications were manually verified through examination of the tandem mass spectra and in comparison with the retention times from commercially available standards (Avanti Polar Lipids). All identified sphingolipids were detected with < 2 ppm mass error and exhibited the expected fragmentation. The mass values and HPLC retention times of the quantified sphigolipids are provided in Supplemental Table A.
Data presentation and statistical analysis
All studies were repeated in cultures from 4–6 different placentas. Data are presented as mean + SEM. Statistical significance was determined by one-way ANOVA followed by Bonferroni's post hoc test or Student's paired two-tailed t test. P < .05 was considered significant. Statistical analysis and graph plotting were performed using Prism 5 software (GraphPad).
Results
ADN inhibits insulin signaling and prevents insulin-stimulated amino acid transport
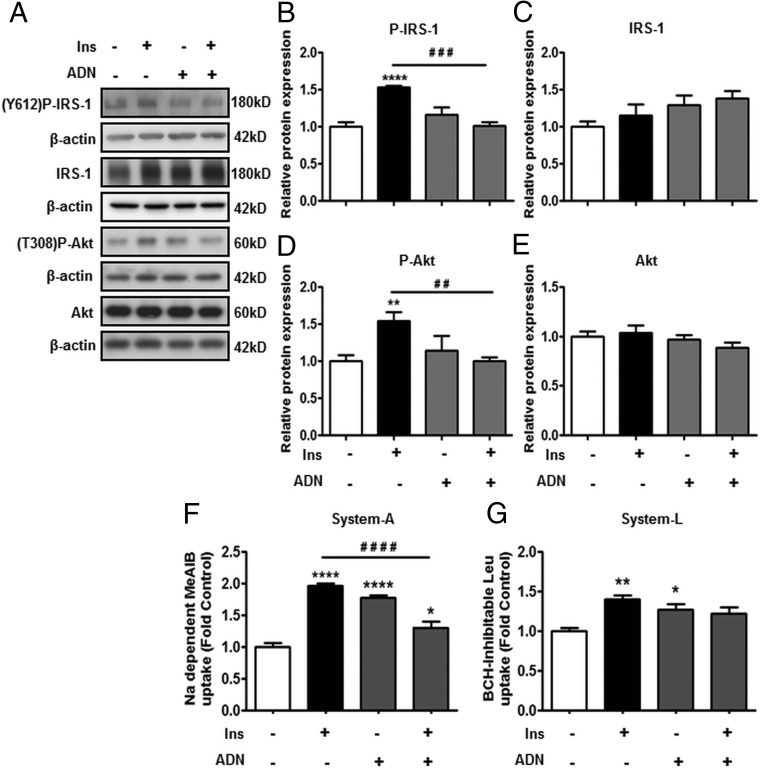
Physiological concentrations of insulin increased the phosphorylation (P-) of IRS-1 (Y612) (Figure 1B) and Akt (T308) (Figure 1D) in PHTs. ADN treatment by itself did not influence the phosphorylation of these proteins, but ADN inhibited the insulin-stimulated increase in P-IRS-1 (Y612) (Figure 1B) and P-Akt (T308) expression (Figure 1D). Total expression of IRS-1 (Figure 1C) or Akt (Figure 1E) was not affected by these treatments.
Figure 1.
ADN inhibits insulin (Ins) signaling and insulin-stimulated amino acid transport. PHT cells were incubated with Ins (5.8 ng/mL), ADN (5 μg/mL), or Ins (5.8 ng/mL) + ADN (5 μg/mL), and protein lysates were examined by immunoblotting or systems A and L amino acid transport activity was measured. A, Representative immunoblots of P-IRS-1 (Y612), IRS-1, P-Akt (T308), Akt, and β-actin. Histograms illustrate relative protein expression of (B) phosphorylated IRS-1 (Y612), (C) IRS-1, (D) phosphorylated Akt (T308), and (E) Akt. F, system A activity was determined as sodium-dependent methyl-aminoisobutyric acid uptake and (G) system L activity by BCH-inhibitable leucine uptake. Data represent fold change from vehicle control (PBS, 0.1% vol/vol). Mean + SEM, n = 6 (cell signaling), n = 4 (amino acid transport); one-way ANOVA; *, P < .05; **, P < .01; ****, P < .0001 vs Cnt; or # #, P < .01; # # #, P < .001; # # # #, P < .0001 vs Ins. Ins, insulin; Y612, tyrosine 612; T308, threonine 308.
Insulin stimulated system A (Figure 1F) and system L (Figure 1G) transport activity by 2- and 1.5-fold, respectively. Interestingly, ADN alone also stimulated systems A and L activity, whereas combining ADN with insulin markedly attenuated the increase in amino acid transport in response to insulin.
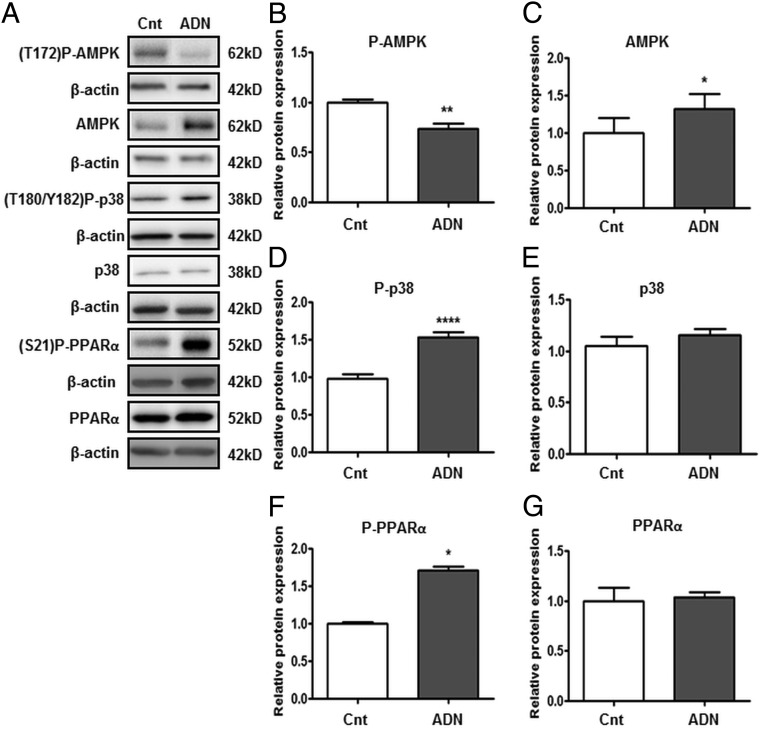
ADN inhibits AMPK phosphorylation but activates p38 MAPK and PPARα in PHT cells
ADN treatment decreased the phosphorylation of AMPK (T172; Figure 2B) but increased total AMPK expression (Figure 2C). Phosphorylation of p38 MAPK (T180/Y182; Figure 2D) and PPARα (S21; Figure 2F) was increased by ADN, whereas the total expression of p38 MAPK (Figure 2E) or PPARα (Figure 2G) was not affected by ADN treatment.
Figure 2.
Influence of ADN on AMPK, p38 MAPK, and PPARα signaling pathways. PHT cells were incubated with ADN (5 μg/mL) and protein lysates were examined by immunoblotting analyses. A, Representative immunoblots of P-AMPK (T172), AMPK, P-p38 (T180/Y182), p38, P-PPARα (Ser21), PPARα, and β-actin. Histograms illustrate relative protein expression of (B) phosphorylated AMPK (T172), (C) AMPK, (D) phosphorylated p38 (T180/Y182), (E) p38, (F) phosphorylated PPARα (Ser21), and (G) PPARα. Data represent fold change from vehicle control (dimethylsulfoxide [DMSO], 0.1% vol/vol). Mean + SEM, n = 6; Student's t test; *, P < .05; **, P < .01; ****, P < .0001 vs control (Cnt).
Activation of PPARα inhibits insulin signaling and insulin-mediated amino acid transport
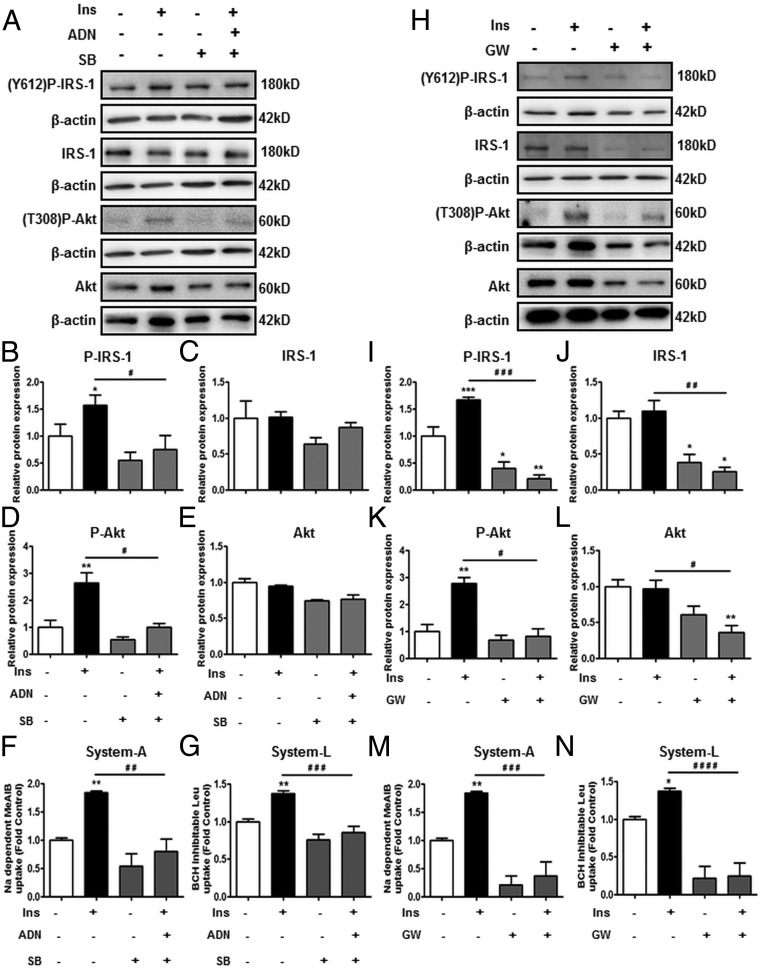
Based on the findings that ADN activated p38 MAPK and PPARα, we first targeted these proteins using pharmacologic agents. PHTs were treated with the p38 MAPK antagonist SB (20 μM), at concentrations previously shown to inhibit p38 MAPK activity in trophoblasts (37, 38). We hypothesized that inhibition of p38 MAPK would reverse ADN-mediated inhibition of insulin signaling and insulin-stimulated amino acid transport. SB treatment by itself did not influence P-IRS-1 (Figure 3B) or P-Akt (Figure 3D) expression, or system A (Figure 3F) and system L (Figure 3G) activity. In combination with insulin and ADN, SB did not reverse the effects of ADN, which was still able to prevent insulin stimulation of P-IRS-1 (Figure 3B), P-Akt (Figure 3D), system A (Figure 3F), and system L (Figure 3G) activity. Total IRS-1 and Akt expression were not affected by SB treatments (Figure 3, C and E). Collectively, these observations suggest that p38 MAPK is not involved in mediating the effects of ADN on trophoblast insulin signaling.
Figure 3.
The role of p38 MAPK and PPARα in mediating the effect of ADN on insulin (Ins) signaling and insulin-stimulated amino acid transport targeting by pharmacologic agents. In panels A–G, PHT cells were treated with Ins (5.8 ng/mL), p38 MAPK antagonist SB (20 μM) with/without Ins (5.8 ng/mL) + ADN (5 μg/mL). Panels H–N show PHT cells treated with PPARα agonist GW (0.1 μM) with/without Ins (5.8 ng/mL). A and H, Representative immunoblots of P-IRS-1 (Y612), IRS-1, P-Akt (T308), Akt, and β-actin. Histograms illustrate relative protein expression of (B and I) phosphorylated IRS-1 (Y612), (C and J) IRS-1, (D and K) phosphorylated Akt (T308), (E and L) Akt, and (F and M) system A and (G and N) system L amino acid transport activity. Data represent fold change from vehicle control (dimethylsulfoxide [DMSO], 0.1% vol/vol). Mean + SEM, n = 4 (cell signaling); n = 5 (amino acid transport); one-way ANOVA; *, P < .05; **, P < .01; ***, P < .001 vs Cnt; or #, P < .05; # #, P < .01; # # #, P < .001; # # # #, P < .0001 vs Ins. Ins, insulin; Y612, tyrosine 612; T308, threonine 308.
Because a selective PPARα inhibitor is not readily available, PPARα was pharmacologically targeted using the agonist GW (0.1 μM). GW stimulated PPARα (S21) phosphorylation by up to 5-fold in PHTs (Supplemental Figure F). GW treatment by itself, or with insulin, decreased P-IRS-1 (Figure 3I) and P-Akt (Figure 3K) expression. Total IRS-1 (Figure 3J) and Akt (Figure 3L) expression was also decreased with GW stimulation with or without insulin. Moreover, GW inhibited insulin-stimulated system A (Figure 3M) and system L (Figure 3N) activity. Taken together, GW activation of PPARα inhibits insulin signaling and insulin-stimulated amino acid transport.
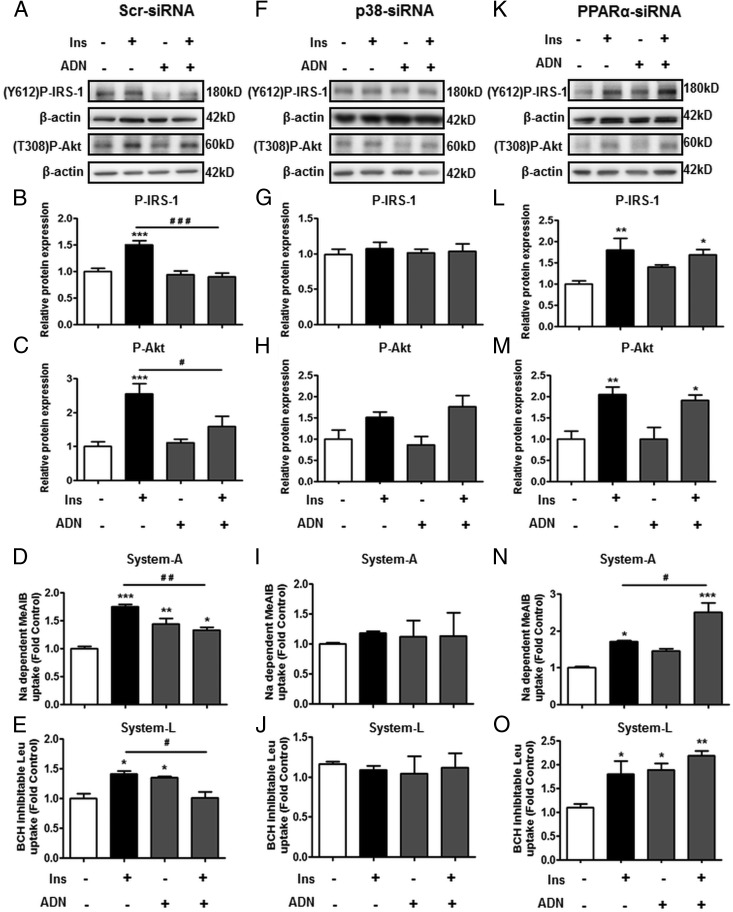
PPARα-siRNA knockdown prevents ADN-mediated inhibition of insulin signaling and amino acid transport
In addition to pharmacologic agents, both p38 MAPK and PPARα expression was inhibited using gene silencing approaches. siRNA knockdown of p38 MAPK and PPARα decreased their protein expression by 70–80% (Supplemental Figure A). In scramble (control)-siRNA-transfected PHTs, treatment with insulin and/or ADN had similar effects on insulin signaling and amino acid transport (Figure 4, A–E) as in nontransfected cells (Figure 1, A–G), ie, insulin increased the phosphorylation of IRS-1 (Figure 4B) and Akt (Figure 4C) and stimulated system A (Figure 4D) and system L (Figure 4E) activity. ADN alone did not influence insulin signaling (Figure 4, B and C) but it did increase amino acid transport (Figure 4, D and E). When combined with insulin, ADN prevented the insulin-stimulated increase in insulin signaling (Figure 4, B and C) and amino acid transport (Figure 4, D and E).
Figure 4.
The role of p38 MAPK and PPARα in mediating the effect of ADN on insulin signaling and insulin-stimulated amino acid transport targeting by siRNA. PHTs were transfected with (A–E) nontargeting scramble-siRNA (F–J) p38-siRNA or (K–O) PPARα-siRNA, and then stimulated with Ins (5.8 ng/mL), ADN (5 μg/mL), or Ins (5.8 ng/mL) + ADN (5 μg/mL) as described in Materials and Methods. A, F, and K, Representative immunoblots of P-IRS-1 (Y612), P-Akt (T308), and β-actin. Histograms illustrate relative protein expression of (B, G, and L) phosphorylated IRS-1 (Y612) and (C, H, and M) phosphorylated Akt (T308) and (D, I, and N) system A and (E, J, and O) system L amino acid transport activity. Data represent fold change from vehicle control (PBS, 0.1% vol/vol). Mean + SEM, n = 5 (cell signaling), n = 4 (amino acid transport); one-way ANOVA; *, P < .05; **, P < .01; ***, P < .001 vs Cnt or #, P < .05; # #, P < .01; # # #, P < .001 vs Ins. Ins, insulin; Y612, tyrosine 612; T308, threonine 308. Scr siRNA, scramble siRNA.
Silencing of p38 MAPK inhibited both the insulin and ADN responses (Figure 4, F–J). Specifically, in PHTs transfected with p38-siRNA, insulin did not stimulate IRS-1 (Figure 4G) or Akt (Figure 4H) phosphorylation, and there was no increase in system A (Figure 4I) or system L (Figure 4J) activity. Likewise, the stimulatory effects of ADN on system A (Figure 4I) and system L (Figure 4J) activity were also abrogated by p38 knockdown. Because p38 silencing prevented both the effects of insulin and ADN in isolation, combining insulin with ADN did not further alter P-IRS-1 (Figure 4G) and P-Akt (Figure 4H) expression, or amino acid transport (Figure 4, I and J) in these cells.
PPARα-knockdown, on the other hand, reversed the inhibitory effects of ADN on insulin signaling and amino acid transport (Figure 4, K–O). Similar to Scr-siRNA, insulin increased P-IRS-1 (Figure 4L) and P-Akt (Figure 4M) expression, as well as stimulated system A (Figure 4N) and system L (Figure 4O) uptake in PPARα-silenced cells. Treatment with ADN in PPARα-siRNA cells did not influence basal insulin signaling (Figure 4, L and M) but it did stimulate amino acid transport (Figure 4O), akin to findings in Scr-siRNA. In PPARα-silenced cells, ADN did not inhibit the insulin-stimulated increase in phosphorylation of IRS-1 (Figure 4L) and Akt (Figure 4M), or insulin-mediated system A (Figure 4N) and system L (Figure 4O) activity, indicating a reversal of the effects of ADN on insulin signaling and function.
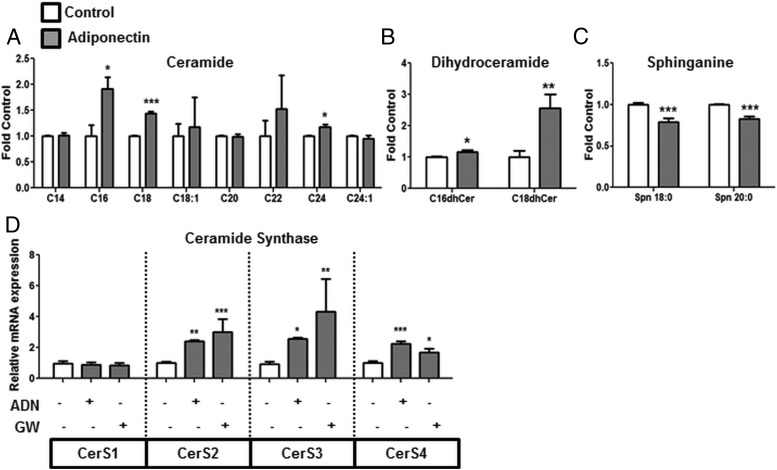
ADN increases cellular ceramide levels via the de novo pathway associated with increased CerS expression
Cellular levels of C16, C18, and C24 ceramide species were increased by ADN treatment (Figure 5A). In order to determine which pathway was involved in the accumulation of ceramide following ADN incubation, sphingolipid metabolites directly upstream or downstream of ceramide were measured. Sphingosine and sphingosine-1-phosphate were not altered (Supplemental Figure Gi) whereas inconsistent changes in sphingomyelin levels (decreased SM 18:0 and increased SM 24:0) were observed with ADN (Supplemental Figure Gii). However, C16 and C18 dihydroceramides were elevated by ADN (Figure 5B), and sphinganine 18:0 and 20:0 were decreased with ADN (Figure 5C). These findings suggest that sphinganine is being metabolized to dihydroceramide, which is then reduced to ceramide.
Figure 5.
ADN increases the expression of CerS and promotes ceramide biosynthesis. PHT cells were incubated with ADN (5 μg/mL), and lipids were extracted for HPLC-ESI-MS/MS analysis. ADN treatment is associated with alterations in cellular levels of long chain (A) ceramide, (B) dihydroceramide, and (C) sphinganine. Target lipid concentrations were normalized against total lipids and expressed as fold change from vehicle control (PBS, 0.1% vol/vol). Mean + SEM, n = 5. Student's t test; *, P < .05; **, P < .01; ***, P < .001 vs Cnt. D, mRNA expression of CerS1–4 was analyzed by Q-PCR following ADN and GW treatment. Mean + SEM, n = 4; one-way ANOVA; *, P < .05; **, P < .01; ***, P < .001 vs control (Cnt).
Because CerS is involved in the acylation of sphinganine to dihydroceramide (39), the mRNA expression of CerS1–4 was measured by Q-PCR, following ADN or GW (PPARα agonist) exposure. CerS2, -3, and -4 mRNA expression was increased with both ADN and GW treatment, but CerS1 expression did not change (Figure 5D). Collectively, these results suggest that ADN and PPARα activation is associated with increased CerS expression, which may promote the metabolism of sphinganine to dihydroceramide and ceramide.
CerS mediates the effects of ADN on insulin signaling and insulin-stimulated amino acid transport
To determine whether ADN, CerS, and ceramide are mechanistically linked, we tested the hypothesis that treatment of PHTs with ceramide inhibits insulin signaling and insulin-stimulated amino acid transport, whereas CerS antagonism reverses the inhibitory effects of ADN on insulin signaling and function.
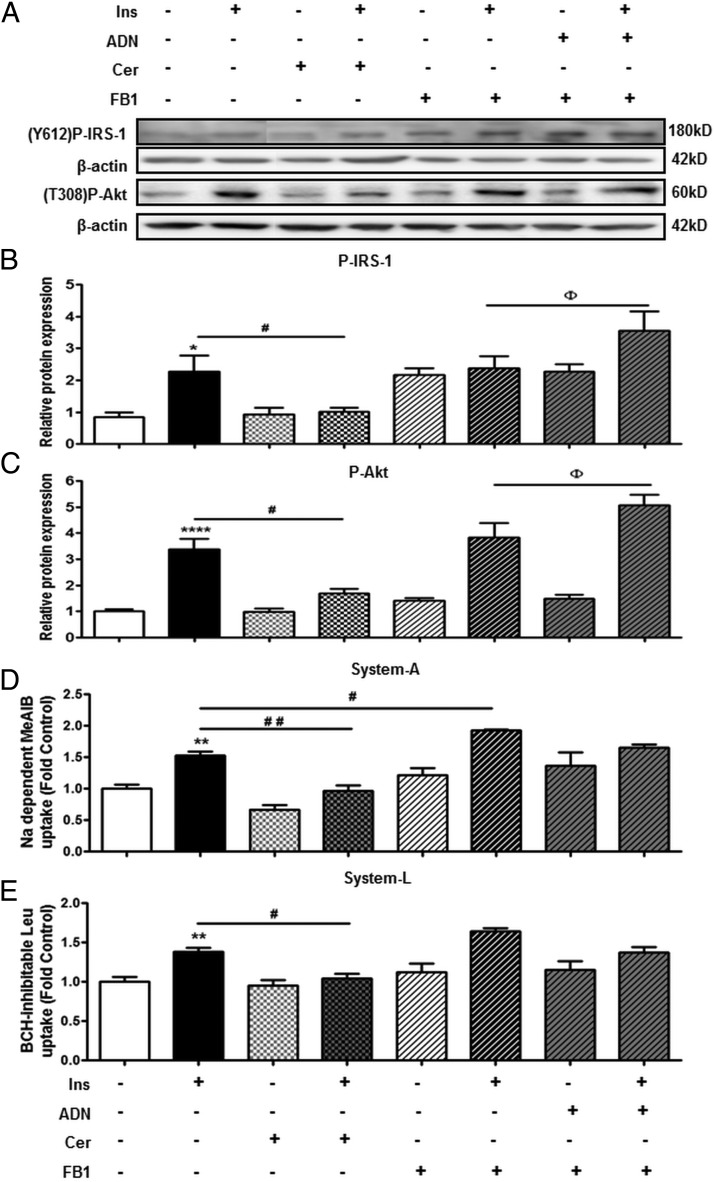
Having previously established that ADN increases cellular ceramide production (Figure 5A), we determined whether treatment with cell-permeable C2-Cer also inhibits insulin signaling and function. Although C2-Cer alone did not influence basal insulin signaling or amino acid transport (Figure 6B), C2-Cer inhibited the insulin-stimulated phosphorylation of IRS-1 (Figure 6B) and Akt (Figure 6C). Moreover, C2-Cer also inhibited the insulin-mediated increase in system A (Figure 6D) and system L (Figure 6E) activity.
Figure 6.
The role of ceramide in mediating the effect of ADN on insulin signaling and insulin-stimulated amino acid transport. Solid filled bars represent PHTs treated with Ins (5.8 ng/mL) or vehicle control. Checkered bars illustrate PHTs treated with cell- permeable C2-Cer (10 μM) with/without Ins (5.8 ng/mL). Striped bars indicate PHTs incubated with FB1 (50 μM) prior to stimulation with Ins (5.8 ng/mL), ADN (5 μg/mL), or Ins (5.8 ng/mL) + ADN (5 μg/mL). A, Representative immunoblots of P-IRS-1 (Y612), P-Akt (T308), and β-actin. Histograms illustrate relative protein expression of (B) phosphorylated IRS-1 (Y612) and (C) phosphorylated Akt (T308) and (D) system A and (E) system L amino acid transport activity. Data represent fold change from vehicle control (BSA, 0.1% vol/vol). Mean + SEM, n = 5 (cell signaling and amino acid transport); one-way ANOVA; *, P < .05; **, P < .01; ****, P < .0001 vs Cnt; #, P < .05; # #, P < .01; # # #, P < .0001 vs Ins; or Φ, P < .05 vs Ins + FB1. Ins, insulin; Cer, C2-ceramide; Y612, tyrosine 612; T308, threonine 308.
Because ADN increased CerS expression (Figure 5D), we investigated the effect of CerS inhibition on the effects of insulin and ADN on insulin signaling and insulin-stimulated amino acid transport. CerS activity was inhibited with FB1, which prevents CerS-mediated conversion of sphinganine to dihydroceramide (40). In FB1-treated cells, ADN did not inhibit insulin-stimulated phosphorylation of IRS-1 (Figure 6B) or Akt (Figure 6C). Rather, ADN combined with insulin further increased the phosphorylation of IRS-1 (Figure 6B) and Akt (Figure 6C) compared with treatment with insulin alone, in FB1-treated cells. Similarly, in FB1-treated cells, insulin-stimulated system A (Figure 6D) and system L (Figure 6E) activity was not inhibited by ADN. Collectively, these data suggest that ADN-mediated trophoblast insulin resistance is dependent on ceramide biosynthesis involving CerS based on the following: 1) ceramide inhibits insulin signaling and function, mirroring the effects of ADN; and 2) inhibition of CerS with FB1 reversed the inhibitory effects of ADN on insulin signaling and amino acid transport.
Discussion
We report, for the first time, that PPARα-stimulated ceramide synthesis constitutes a mechanistic link between ADN and inhibition of insulin signaling in cultured primary human trophoblast cells. Maternal ADN levels are inversely correlated with birth weight, and insulin signaling in the placenta plays a critical role in regulating placental function and fetal growth (41). Therefore, these novel findings will increase our understanding of the mechanisms linking maternal adiposity to fetal growth and long-term outcomes.
The discovery of ADN as an antidiabetic hormone has prompted numerous investigators to explore the cellular functions of ADN. Many of the metabolic effects of ADN are related to its insulin-sensitizing properties in tissues such as adipose, liver, and skeletal muscle. In sharp contrast, we have previously reported that ADN inhibits insulin signaling and function in cultured PHTs (10) and in the mouse placenta in vivo (31), and in this study we explored the underlying mechanisms.
We found that insulin stimulated the phosphorylation of IRS-1 and Akt, and increased systems A and L amino acid transport in cultured PHTs, in accordance with earlier studies (10, 33). ADN prevented the stimulatory effects of insulin on phosphorylation of IRS-1 and Akt, and systems A and L transport activity. In contrast to our previous report (10), ADN treatment alone stimulated both systems A and L transport in PHTs. One possible explanation for this discrepancy is differences in cell culture treatments. Whereas PHTs were maintained in 10% FCS-supplemented media during treatments in the previous study (10), we performed PHT treatments in 1% FCS-supplemented media to establish robust insulin signaling and activity.
Several studies have demonstrated that the biological effects of ADN are mediated via activation of AMPK, p38 MAPK, and PPARα (16, 42). Moreover, it has been shown that ADN sequentially activates AMPK/p38 MAPK followed by PPARα (16). Therefore, we investigated whether similar mechanisms are involved in ADN-mediated insulin resistance in PHTs. ADN activated both p38 MAPK and PPARα. In contrast, ADN decreased AMPK phosphorylation, which is contrary to the findings in muscle and liver (43). These results show that AMPK activation is not involved in mediating the effects of ADN on trophoblast insulin signaling. This difference may underlie the distinctly divergent responses of trophoblast cells to ADN compared with muscle and liver cells.
The role of p38 MAPK in the effect of ADN on insulin signaling and insulin-stimulated amino acid transport was determined by targeting p38 MAPK with pharmacologic agents and siRNA gene silencing. p38 MAPK is an essential kinase associated with inflammatory signaling, but it can also be activated by growth factors (44). Contrary to our hypothesis, pharmacologic antagonism of p38 MAPK with SB did not reverse the effect of ADN on insulin signaling or insulin-stimulated amino acid transport. Although originally recognized as a specific p38 MAPK inhibitor (45), SB also affects other kinases, including an inhibitory effect on Akt (46) and activation of Erk and JNK-MAPKs (47). Therefore, we determined the effect of ADN on insulin signaling and insulin-stimulated amino acid transport in PHT cells following siRNA-mediated silencing of p38 MAPK. Interestingly, p38 MAPK silencing blunted the effects of both insulin and ADN on insulin signaling and amino acid transport. This suggests that p38 MAPK is required for maintaining insulin sensitivity and function in primary trophoblasts. Furthermore, our data indicate that p38 MAPK mediates the effects of ADN on basal amino acid transport, which may be distinct from its effects on insulin signaling and function. Indeed, p38 MAPK has been associated with the regulation of glucose (48) and amino acid uptake (49) including system A transport activity (50) in other tissues. However, the mechanism(s) by which p38 MAPK silencing inhibits insulin signaling and basal amino acid uptake in PHTs are currently unknown and warrant further investigation.
Activation of PPARα with GW stimulated PPARα phosphorylation and inhibited insulin signaling and insulin-mediated amino acid transport, similar to the effects observed with ADN. Activation of PPARα was previously shown to inhibit insulin-signaling proteins IRS-1 and Akt in cancer cell lines (51). Moreover, in this study, PPARα-knockdown reversed the effects of ADN on insulin signaling and insulin-stimulated amino acid transport, thereby establishing a cause-and-effect relationship between ADN and PPARα activity in inhibiting the insulin response. This implies that activation of PPARα may reduce fetal growth by inhibiting placental insulin signaling and amino acid transport. In support of this hypothesis, administration of PPARα agonists to pregnant rats prevented fetal overgrowth in an animal model of type 1 diabetes (52). Furthermore, decreased PPARα expression was reported in the placentas of women entering pregnancy with type 2 diabetes (53). Because these women also delivered larger babies than their body mass index-matched controls, without any differences in maternal fasting glucose (53), it is possible that reduced PPARα activity in the placentas of these women contributed to fetal overgrowth.
PPARα regulates lipid metabolism in a number of tissues including the placenta (54), and treatment with PPARα agonists has been associated with the accumulation of ceramide in several tissues (23, 24). Therefore, we explored the possibility that the mechanisms linking ADN-mediated PPARα activation to inhibition of insulin signaling in PHT cells involves changes in sphingolipid metabolism promoting ceramide accumulation. Indeed, ADN increased levels of several long-chain ceramides, including C16, C18, and C24, which are the predominant ceramide species in syncytiotrophoblasts (55). At the same time, there was a decrease in sphinganine and elevated dihydroceramide levels with ADN treatment. Because CerS is required for the acylation of sphinganine to dihydroceramide, which is then further reduced to ceramide (39), these findings suggest that ADN-stimulated ceramide production is dependent on activation of CerS. ADN or PPARα agonist increased CerS2–4 expression, further implicating the involvement of increased ceramide synthesis in the effects of ADN and PPARα on insulin signaling. These observations of increased ceramide production in PHTs are in contrast to published reports of decreased ceramide accumulation in skeletal muscle and liver of rodents following ADN administration (56). Therefore, these findings may represent a unique mechanism of ADN signaling in the placenta.
In liver and muscle, ADN increases insulin sensitivity; however, the existence of adaptor proteins known as APPL1 and APPL2, which mediate distinct effects on insulin signaling, have been reported (57). Whereas APPL1 sensitizes the insulin-signaling pathway, APPL2 is associated with inhibition of APPL1 activity. In addition, APPL1 phosphorylation sites associated with insulin resistance were recently demonstrated (58). Although both AdipoR1 and AdipoR2 have been reported to be expressed in the placenta (26), it is currently unknown which of the adaptor proteins is expressed in the placenta. We speculate that the divergent effects of ADN on trophoblast amino acid transport mediated by the activation of p38 MAPK and PPARα represent distinct signaling pathways mediated by ADN receptors and their adaptor proteins. Hence, future studies exploring the upstream mechanisms of ADN signaling will play a critical role in our understanding of the interaction between ADN and insulin signaling in the regulation of placental amino acid transport.
Increased ceramide production in PHTs may explain ADN-mediated insulin resistance, because treating PHTs with a cell-permeable ceramide inhibited insulin signaling and insulin-mediated amino acid uptake. Generation of ceramide induces insulin resistance in a number of tissues through ceramide-mediated activation of protein kinase ζ and/or protein phosphatase 2A (20), which inhibit tyrosine phosphorylation of IRS-1 and phosphatidylinositol-3-kinase enzyme activity (59, 60). Ceramide inhibition of Akt phosphorylation was previously demonstrated in PHTs (19). Consistent with these reports, ceramide also inhibited phosphorylation of IRS-1 and Akt in this study. Insulin-stimulated systems A and L transport was also inhibited with ceramide, although basal amino acid uptake was not affected by ceramide treatment alone. Previous studies have reported similar effects of ceramide on inhibition of amino acid and glucose transport (21). Inhibition of CerS with FB1 has been shown in several cell types to reverse the insulin resistance caused by saturated fatty acids and TNF-α (61, 62). We propose that ADN-mediated insulin resistance in PHTs occurs through CerS-dependent ceramide production, because inhibition of CerS with FB1 reversed the inhibitory effects of ADN on the insulin response.
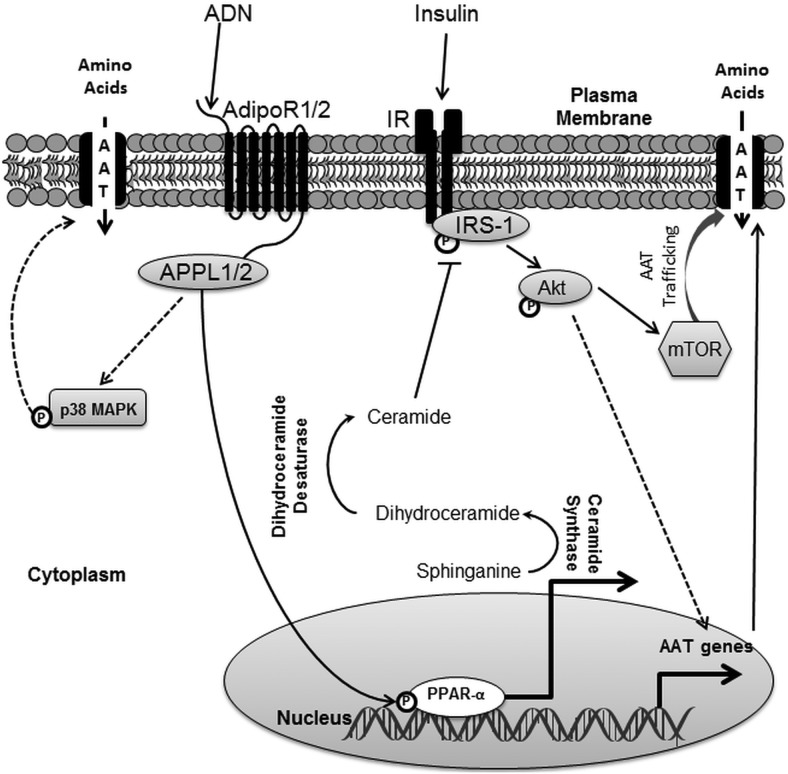
In conclusion, ADN activates PPARα and increases ceramide production, which inhibits insulin signaling and insulin-stimulated amino acid transport (Figure 7). Insulin regulates amino acid transport in PHTs by both transcriptional mechanisms (10) and posttranslational processes, which involve mechanistic target of rapamycin-dependent trafficking of amino acid transporters to the plasma membrane (63). Therefore, ADN inhibition of insulin signaling likely impacts upon insulin-dependent mechanistic target of rapamycin activation and regulation of amino acid transport. These findings indicate a novel role of PPARα in regulating trophoblast insulin sensitivity. Because placental amino acid transport is an important determinant of fetal growth, we propose that maternal hormones, such as ADN, which regulates placental insulin signaling, have profound effects on fetal development. Therefore ADN constitutes an important endocrine link between maternal adipose tissue and fetal growth, mediated by its effects on placental insulin sensitivity.
Figure 7.
Model of ADN-mediated inhibition of insulin signaling and function. ADN activates p38 MAPK and PPARa. ADN activation of p38 MAPK is associated with increased amino acid uptake. PPARa activation by ADN leads to increased conversion of sphinganine to dihydroceramide mediated by ceramide synthase. Dihydroceramide is then further reduced to ceramide, which inhibits IRS-1 and subsequently Akt. Inhibition of insulin signaling by ADN is associated with attenuation of insulin stimulated systems A and L amino acid transport, which is known to be mediated by increased expression of amino acid transporter genes and trafficking of amino acid transporters to the plasma membrane via mTOR. AAT, amino acid transporters; mTOR, mechanistic target of rapamycin.
Additional material
Supplementary data supplied by authors.
Acknowledgments
We thank Evelyn Miller and the staff at the Labor and Delivery Unit at the University Hospital for their assistance in collecting placentas.
This work was supported by National Institutes of Health grant HD065007 (to T.J. and T.L.P.). Mass spectrometry analyses were conducted in the University of Texas Health Science Center San Antonio Institutional Mass Spectrometry Laboratory, with instrumentation funded in part by National Institutes of Health grant 1S10RR031586–01 (to S.T.W.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ADN
- adiponectin
- AMPK
- AMP-activated protein kinase
- AdipoR
- ADN receptor
- C2-Cer
- C2-ceramide
- CerS
- ceramide synthase
- ESI-MS/MS
- electrospray ionization tandem mass spectrometry
- FB1
- Fumonisin B1
- FCS
- fetal calf serum
- GDM
- gestational diabetes mellitus
- GW
- GW7647
- hCG
- human chorionic gonadotropin
- IRS
- insulin receptor substrate
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- P-
- phosphorylation
- PHT
- primary human trophoblast
- PPARα
- peroxisome proliferator-activated receptor α
- Q-PCR
- quantitative PCR
- S1P
- sphingosine-1-phosphate
- SB
- SB203580, 20 μM
- siRNA
- small interfering RNA.
References
- 1. Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PloS One. 2013;8(4):e61627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marshall NE, Guild C, Cheng YW, Caughey AB, Halloran DR. The effect of maternal body mass index on perinatal outcomes in women with diabetes. Am J Perinatol. 2014;31(3)249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32(6):1076–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3):e290–296. [DOI] [PubMed] [Google Scholar]
- 5. Gaccioli F, Lager S, Powell TL, Jansson T. Placental transport in response to altered maternal nutrition. J Dev Origins HealthDis. 2013;4(2):101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jansson T, Aye IL, Goberdhan DC. The emerging role of mTORC1 signaling in placental nutrient-sensing. Placenta. 2012;33(Suppl 2):e23–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lager S, Powell TL. Regulation of nutrient transport across the placenta. J Pregnancy. 2012;2012:179827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sferruzzi-Perri AN, Vaughan OR, Forhead AJ, Fowden AL. Hormonal and nutritional drivers of intrauterine growth. Curr Opin Clin Nutr Metab Care. 2013;16(3):298–309. [DOI] [PubMed] [Google Scholar]
- 9. Jansson N, Nilsfelt A, Gellerstedt M, et al. Maternal hormones linking maternal body mass index and dietary intake to birth weight. Am J Clin Nutr. 2008;87(6):1743–1749. [DOI] [PubMed] [Google Scholar]
- 10. Jones HN, Jansson T, Powell TL. Full-length adiponectin attenuates insulin signaling and inhibits insulin-stimulated amino acid transport in human primary trophoblast cells. Diabetes. 2010;59(5):1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roos S, Lagerlöf O, Wennergren M, Powell TL, Jansson T. Regulation of amino acid transporters by glucose and growth factors in cultured primary human trophoblast cells is mediated by mTOR signaling. Am J Physiol Cell Physiol. 2009;297(3):C723–C731. [DOI] [PubMed] [Google Scholar]
- 12. Hiden U, Glitzner E, Hartmann M, Desoye G. Insulin and the IGF system in the human placenta of normal and diabetic pregnancies. J Anat. 2009;215(1):60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laviola L, Perrini S, Belsanti G, et al. Intrauterine growth restriction in humans is associated with abnormalities in placental insulin-like growth factor signaling. Endocrinology. 2005;146(3):1498–1505. [DOI] [PubMed] [Google Scholar]
- 14. Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270(45):26746–26749. [DOI] [PubMed] [Google Scholar]
- 15. Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423(6941):762–769. [DOI] [PubMed] [Google Scholar]
- 16. Yoon MJ, Lee GY, Chung JJ, Ahn YH, Hong SH, Kim JB. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes. 2006;55(9):2562–2570. [DOI] [PubMed] [Google Scholar]
- 17. Holland WL, Miller RA, Wang ZV, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17(1):55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh AT, Dharmarajan A, Aye IL, Keelan JA. Sphingosine-sphingosine-1-phosphate pathway regulates trophoblast differentiation and syncytialization. Reprod Biomed Online. 2012;24(2):224–234. [DOI] [PubMed] [Google Scholar]
- 19. Singh AT, Dharmarajan A, Aye IL, Keelan JA. Ceramide biosynthesis and metabolism in trophoblast syncytialization. Mol Cell Endocrinol. 2012;362(1–2):48–59. [DOI] [PubMed] [Google Scholar]
- 20. Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab. 2012;15(5):585–594. [DOI] [PubMed] [Google Scholar]
- 21. Guenther GG, Peralta ER, Rosales KR, Wong SY, Siskind LJ, Edinger AL. Ceramide starves cells to death by downregulating nutrient transporter proteins. Proc Natl Acad Sci USA. 2008;105(45):17402–17407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zabielski P, Blachnio-Zabielska A, Baranowski M, Zendzian-Piotrowska M, Gorski J. Activation of PPARα by bezafibrate negatively affects de novo synthesis of sphingolipids in regenerating rat liver. Prostaglandins Other Lipid Mediat. 2010;93(3–4):120–125. [DOI] [PubMed] [Google Scholar]
- 23. Baranowski M, Blachnio A, Zabielski P, Górski J. PPARα agonist induces the accumulation of ceramide in the heart of rats fed high-fat diet. J Physiol Pharmacol. 2007;58(1):57–72. [PubMed] [Google Scholar]
- 24. Yamane T, Kobayashi-Hattori K, Oishi Y. A high-fat diet reduces ceramide synthesis by decreasing adiponectin levels and decreases lipid content by modulating HMG-CoA reductase and CPT-1 mRNA expression in the skin. Mol Nutr Food Res. 2011;55(Suppl 2):S186–S192. [DOI] [PubMed] [Google Scholar]
- 25. Benaitreau D, Dos Santos E, Leneveu MC, De Mazancourt P, Pecquery R, Dieudonne MN. Adiponectin promotes syncytialisation of BeWo cell line and primary trophoblast cells. Reprod Biol Endocrinol. 2010;8:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McDonald EA, Wolfe MW. Adiponectin attenuation of endocrine function within human term trophoblast cells. Endocrinology. 2009;150(9):4358–4365. [DOI] [PubMed] [Google Scholar]
- 27. Lappas M, Permezel M, Rice GE. Leptin and adiponectin stimulate the release of proinflammatory cytokines and prostaglandins from human placenta and maternal adipose tissue via nuclear factor-κB, peroxisomal proliferator-activated receptor-γ and extracellularly regulated kinase 1/2. Endocrinology. 2005;146(8):3334–3342. [DOI] [PubMed] [Google Scholar]
- 28. Benaitreau D, Dieudonné MN, Dos Santos E, Leneveu MC, Mazancourt Pd, Pecquery R. Antiproliferative effects of adiponectin on human trophoblastic cell lines JEG-3 and BeWo. Biol Reprod. 2009;80(6):1107–1114. [DOI] [PubMed] [Google Scholar]
- 29. Lowe LP, Metzger BE, Lowe WL, Jr, Dyer AR, McDade TW, McIntyre HD. Inflammatory mediators and glucose in pregnancy: results from a subset of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. J Clin Endocrinol Metab. 2010;95(12):5427–5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mazaki-Tovi S, Kanety H, Pariente C, et al. Maternal serum adiponectin levels during human pregnancy. J Perinatol. 2007;27(2):77–81. [DOI] [PubMed] [Google Scholar]
- 31. Rosario FJ, Schumacher MA, Jiang J, Kanai Y, Powell TL, Jansson T. Chronic maternal infusion of full-length adiponectin in pregnant mice down-regulates placental amino acid transporter activity and expression and decreases fetal growth. J Physiol. 2012;590(Pt 6):1495–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF., 3rd Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118(4):1567–1582. [DOI] [PubMed] [Google Scholar]
- 33. Aye IL, Jansson T, Powell TL. Interleukin-1β inhibits insulin signaling and prevents insulin-stimulated system A amino acid transport in primary human trophoblasts. Mol Cell Endocrinol. 2013;381(1–2):46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 35. Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gao X, Zhang Q, Meng D, et al. A reversed-phase capillary ultra-performance liquid chromatography-mass spectrometry (UPLC-MS) method for comprehensive top-down/bottom-up lipid profiling. Anal Bioanal Chem. 2012;402(9):2923–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ge YC, Li JN, Ni XT, et al. Cross talk between cAMP and p38 MAPK pathways in the induction of leptin by hCG in human placental syncytiotrophoblasts. Reproduction. 2011;142(2):369–375. [DOI] [PubMed] [Google Scholar]
- 38. Daoud G, Amyot M, Rassart E, Masse A, Simoneau L, Lafond J. ERK1/2 and p38 regulate trophoblasts differentiation in human term placenta. J Physiol. 2005;566(Pt 2):409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Levy M, Futerman AH. Mammalian ceramide synthases. IUBMB Life. 2010;62(5):347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Merrill AH, Jr, Liotta DC, Riley RT. Fumonisins: fungal toxins that shed light on sphingolipid function. Trends Cell Biol. 1996;6(6):218–223. [DOI] [PubMed] [Google Scholar]
- 41. Aye IL, Powell TL, Jansson T. Review: adiponectin—the missing link between maternal adiposity, placental transport and fetal growth? Placenta. 2013;34(Suppl):S40–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xin X, Zhou L, Reyes CM, Liu F, Dong LQ. APPL1 mediates adiponectin-stimulated p38 MAPK activation by scaffolding the TAK1-MKK3–p38 MAPK pathway. Am J Physiol Endocrinol Metab. 2011;300(1):E103–E110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11):1288–1295. [DOI] [PubMed] [Google Scholar]
- 44. Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–1912. [DOI] [PubMed] [Google Scholar]
- 45. Cuenda A, Rouse J, Doza YN, et al. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364(2):229–233. [DOI] [PubMed] [Google Scholar]
- 46. Lali FV, Hunt AE, Turner SJ, Foxwell BM. The pyridinyl imidazole inhibitor SB203580 blocks phosphoinositide-dependent protein kinase activity, protein kinase B phosphorylation, and retinoblastoma hyperphosphorylation in interleukin-2-stimulated T cells independently of p38 mitogen-activated protein kinase. J Biol Chem. 2000;275(10):7395–7402. [DOI] [PubMed] [Google Scholar]
- 47. Henklova P, Vrzal R, Papouskova B, et al. SB203580, a pharmacological inhibitor of p38 MAP kinase transduction pathway activates ERK and JNK MAP kinases in primary cultures of human hepatocytes. Eur J Pharmacol. 2008;593(1–3):16–23. [DOI] [PubMed] [Google Scholar]
- 48. Ho RC, Alcazar O, Fujii N, Hirshman MF, Goodyear LJ. p38γ MAPK regulation of glucose transporter expression and glucose uptake in L6 myotubes and mouse skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2004;286(2):R342–R349. [DOI] [PubMed] [Google Scholar]
- 49. Tsai RY, Tai YH, Tzeng JI, et al. Ultra-low dose naloxone restores the antinociceptive effect of morphine in pertussis toxin-treated rats and prevents glutamate transporter downregulation by suppressing the p38 mitogen-activated protein kinase signaling pathway. Neuroscience. 2009;159(4):1244–1256. [DOI] [PubMed] [Google Scholar]
- 50. Bruscalupi G, Massimi M, Spagnuolo S, Fiore AM, Leoni S. Hypertonic stress regulates amino acid transport and cell cycle proteins in chick embryo hepatocytes. Cell Biol Int. 2012;36(2):203–213. [DOI] [PubMed] [Google Scholar]
- 51. Martínez de Ubago M, García-Oya I, Pérez-Pérez A, et al. Oleoylethanolamide, a natural ligand for PPAR-α, inhibits insulin receptor signalling in HTC rat hepatoma cells. Biochim Biophys Acta. 2009;1791(8):740–745. [DOI] [PubMed] [Google Scholar]
- 52. Martínez N, White V, Kurtz M, Higa R, Capobianco E, Jawerbaum A. Activation of the nuclear receptor PPARα regulates lipid metabolism in foetal liver from diabetic rats: implications in diabetes-induced foetal overgrowth. Diabetes Metab Res Rev. 2011;27(1):35–46. [DOI] [PubMed] [Google Scholar]
- 53. Capobianco E, Martinez N, Fornes D, et al. PPAR activation as a regulator of lipid metabolism, nitric oxide production and lipid peroxidation in the placenta from type 2 diabetic patients. Mol Cell Endocrinol. 2013;377(1–2):7–15. [DOI] [PubMed] [Google Scholar]
- 54. Jawerbaum A, Capobianco E. Review: Effects of PPAR activation in the placenta and the fetus: implications in maternal diabetes. Placenta. 2011;32 (Suppl 2):S212–S217. [DOI] [PubMed] [Google Scholar]
- 55. Baig S, Lim JY, Fernandis AZ, et al. Lipidomic analysis of human placental syncytiotrophoblast microvesicles in adverse pregnancy outcomes. Placenta. 2013;34(5):436–442. [DOI] [PubMed] [Google Scholar]
- 56. Patel SA, Hoehn KL, Lawrence RT, et al. Overexpression of the adiponectin receptor AdipoR1 in rat skeletal muscle amplifies local insulin sensitivity. Endocrinology. 2012;153(11):5231–5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang C, Xin X, Xiang R, et al. Yin-Yang regulation of adiponectin signaling by APPL isoforms in muscle cells. J Biol Chem. 2009;284(46):31608–31615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu M, Zhou L, Wei L, et al. Phosphorylation of adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain, and leucine zipper motif 1 (APPL1) at Ser430 mediates endoplasmic reticulum (ER) stress-induced insulin resistance in hepatocytes. J Biol Chem. 2012;287(31):26087–26093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Miura A, Kajita K, Ishizawa M, et al. Inhibitory effect of ceramide on insulin-induced protein kinase Czeta translocation in rat adipocytes. Metab Clin Exp. 2003;52(1):19–24. [DOI] [PubMed] [Google Scholar]
- 60. Mandavia C, Sowers JR. Phosphoprotein phosphatase PP2A regulation of insulin receptor substrate 1 and insulin metabolic signaling. Cardiorenal Med. 2012;2(4):308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lennon R, Pons D, Sabin MA, et al. Saturated fatty acids induce insulin resistance in human podocytes: implications for diabetic nephropathy. Nephrol Dial Transplant. 2009;24(11):3288–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fernández-Veledo S, Hernandez R, Teruel T, Mas JA, Ros M, Lorenzo M. Ceramide mediates TNF-α-induced insulin resistance on GLUT4 gene expression in brown adipocytes. Arch Physiol Biochem. 2006;112(1):13–22. [DOI] [PubMed] [Google Scholar]
- 63. Rosario FJ, Kanai Y, Powell TL, Jansson T. Mammalian target of rapamycin signalling modulates amino acid uptake by regulating transporter cell surface abundance in primary human trophoblast cells. J Physiol. 2013;591(Pt 3):609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.