Abstract
The regulation of behavior by the molecular components of the circadian clock is not well understood. Here we report that mice lacking the nuclear receptor Rev-erbα, a potent transcriptional repressor and core clock component, displayed marked hyperactivity and impaired response habituation in novel environments. In addition, Rev-erbα knockout (KO) mice were deficient in short-term, long-term, and contextual memories and also showed impairment in nest-building ability. Together, these results suggest that Rev-erbα KO mice manifest defective hippocampal function. Interestingly, the changes in novelty-induced locomotor activity of Rev-erbα KO mice were comparable at multiple times of day, potentially due to the muted amplitude of Rev-erbα oscillation in the hippocampus of wild-type mice. Hippocampal dopamine turnover was increased in Rev-erbα KO mice, due to up-regulation of tyrosine hydroxylase, the rate-limiting enzyme in dopamine production, and pharmacologic inhibition of tyrosine hydroxylase activity partially rescued locomotor hyperactivity. These findings reveal a novel, nonredundant function for Rev-erbα that links a core component of the circadian gene-regulatory network to the control of dopaminergic and hippocampus-dependent behaviors.
Rev-erbα is an unusual member of the nuclear receptor superfamily because it lacks a canonical activation domain and, therefore, functions primarily as a potent repressor of transcription (1). Rev-erbα is expressed in a circadian manner in many tissues (2–6), and its repression of the core clock gene Bmal1 (7, 8) has been implicated in a feedback loop that impacts the molecular clock. Mice lacking Rev-erbα display a measurable but modest reduction in locomotor activity period (7), and loss of Rev-erbα together with highly related Rev-erbβ leads to arrhythmia in cells (9) and mice (10).
Many physiological functions have circadian rhythms (11–14), and circadian disruption increases risk for metabolic disorders and cancer (6, 15–17). Impaired circadian rhythm and mutations or polymorphisms in clock genes have also been associated with behavioral and psychiatric disorders, including affective disorders (15, 18), attention deficit/hyperactivity disorder (19), and schizophrenia (20). Studies of mice with mutations in core clock genes including Clock (21–27), Per1 and Per2 (28, 29), Cry1 and Cry2 (30), and the CRY modifiying gene Fbxl3 (31) have consistently demonstrated abnormal behaviors including hyperactivity, lowered anxiety and depression-like behaviors, and increased exploratory and escape-seeking behaviors.
In addition to its role in the clock, Rev-erbα also has direct effects on many tissue-specific genes that have been uncovered in peripheral tissues including liver (32–34), muscle (35), macrophages (36–38), white adipose tissue (39–41) and brown fat (42). The effects of Rev-erbα on behavior have been less well characterized. Here we report that mice lacking Rev-erbα display novelty-induced hyperactivity, impaired habituation, and cognitive deficits. These abnormal behaviors are observed throughout the day, suggesting that Rev-erbα is functioning independently of its role in the clock and may be explained by altered expression of genes regulating hippocampal dopaminergic activity. Indeed, abnormal behaviors of mice lacking Rev-erbα may be rescued by modulation of the dopaminergic system. These findings reveal a novel, nonredundant function for Rev-erbα in the control of behavior.
Materials and Methods
Animal subjects
Rev-erbα knockout (KO) mice were obtained from B. Vennström, and backcrossed >7 generations with C57Bl/6 mice. Rev-erbα KO and wild-type (WT) littermates were housed 4–5 per cage under standard 12-hour light/12-hour dark (LD) cycles, lights on at 7:00 am (Zeitgerber time 0 [ZT0]) and lights off at 7:00 pm (ZT12). All behaviors were performed between 7:00 am (ZT0) and 11:00 am (ZT4), except where indicated to time of day effects. All animal care and procedures followed the guidelines of the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Open-field (OF) test
Locomotor activity was assessed with the automated Photobeam Activity System (San Diego Instruments). Mice were individually placed in a clear 16 × 16 inches arena fitted with photocells to detect horizontal and rearing activities for 10 minutes. The arena was cleaned with 70% ethanol between trials. For amphetamine-sensitivity test, mice were injected with 1 mg/Kg 30 minutes before the OF test. To determine the effect of tyrosine hydroxylase (TH) inhibition on OF, mice were injected with 200 mg/Kg of α-methyl-p-tyrosine (AMPT) 45 minutes before the test.
Home cage activity
Mice were individually housed in cages fitted with photocells to measure activity (Columbus Instruments). After 2 days of acclimation, locomotor activity was assessed by counting the number of beam breaks per hour.
Y-maze spontaneous alternations
Mice were individually placed into a Y-shaped maze with 3 white arms at 120° angles (San Diego Instruments). Mice were allowed to freely explore the maze for 5 minutes, and sequential entries into each of the arms were noted. Trials were videotaped and graded by an observer blind to group designation. An arm entry was scored when all 4 limbs were within an arm. Percentage alternation was calculated as the (number of alternations − 2)/total arm entries. The maze was cleaned with 70% ethanol between trials.
Spatial object recognition (SOR)
Mice were tested for SOR as described by Wimmer et al (43). Mice were allowed to habituate to a gray arena with one side decorated with black and white vertical stripes to serve as a spatial cue for 10 minutes. Mice were removed, the arena cleaned, and 2 unique objects (metal cube and plastic cylinder) were placed in the corners of the arena, opposite to the stripes about 1.5 inches from the walls. Mice were allowed to explore the arena and objects for 3 × 10-minute trials. The objects and arena were cleaned between trials. Twenty-four hours after the training, the mice were placed in the same arena with one object displaced to a new location (displaced object, DO) while the other object was not moved (nondisplaced object [NDO]). The DO (metal cube or plastic cylinder) was alternated within each group of mice. The 10-minute testing sessions were recorded and scored by an observer blind to group designation. Preference for the DO was calculated as the percentage of time spent exploring the DO vs NDO.
Contextual fear conditioning
Mice were placed in fear-conditioning chambers within sound-attenuating cabinets (MedAssociates). Mice were allowed to explore the unique context of the chambers for 3 minutes and then received a single 2-second 1 mA foot shock. Mice remained in the chambers for an additional 30 seconds. Twenty-four hours later mice were returned to the chamber, and the percentage of time spent in freezing posture was analyzed with software (FreezeScan). Freezing in response to reexposure to the context is a form of hippocampal-dependent learning. The chambers were cleaned with 70% ethanol between mice.
Nest-construction task
Mice were individually housed in standard cages 1 week before the test. A pressed white cotton Neslet was weighed and placed into the cage. Twenty-four hours later, the quality of nest construction was scored between 1 and 5 (where 1= no nest and 5 = perfect nest) as described by Deacon (44), and the remaining intact portion was weighed. Nests were scored by an individual blind to genotype.
HPLC analysis of catecholamines
Mice were euthanized, and the hippocampus were dissected and frozen at −80°C until processing. Biogenic amines and their metabolites were analyzed by using reverse-phase chromatography combined with electrochemical detection as previously described (45). Briefly, specimens were weighed and prepared in buffer containing the internal standard, 3,4-dihydroxybenzlamine. Standards containing between 0 and 10 pmol were injected every fifth specimen. Levels in brain tissue were determined from this standard curve and corrected for loss using the internal standard. A Dionex Coulochem III electrochemical detector equipped with a 5011A analytical cell was used for these studies (Thermo Scientific). The analyses were done by an individual blind to genotype.
Gene-expression analysis
Total RNA was extracted from tissue using the RNeasy Mini Kit (QIAGEN) and treated with DNase (QIAGEN). The RNA was reversed transcribed using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems) and analyzed by quantitative PCR. Quantitative PCR was performed with Power SYBR Green PCR Mastermix on the PRISM 7500 (Applied Biosystems). Gene expression was normalized to mRNA levels of housekeeping gene 36B4 and the level of the gene of interest in the control samples. 36B4 (forward), 5′-TCCAGGCTTTGGGCATCA-3′; 36B4 (reverse), 5′-CTTTATCAGCTGCACATCACTCAGA-3′; Rev-erbα (forward), 5′-GTCTCTCCGTTGGCATGTCT-3′; Rev-erbα (reverse), 5′-CCAAGTTCATGGCGCTCT-3′; Vmat2 (forward), 5′-TTCTTCGAAGTCCACCTGCT-3′; Vmat2 (reverse), 5′-ACATTGGGCAACGTTAGAGG-3′; Dat (forward), 5′-CCTGGTTCTACGGTGTCCAG-3′; Dat (reverse), 5′-GCTGACCACGACCACATACA-3′; Drd1a (forward), 5′-GAACCCAGAAGACAGGTGGA-3′; Drd1a (reverse), 5′-GCTTAGCCCTCACGTTCTTG-3′; Drd2 (forward), 5′-TATGGCTTGAAGAGCCGTGC-3′; Drd2 (reverse), 5′-CCCTTCGGACCCATTGAAGG-3′; Th (forward), 5′-TGCAGCCCTACCAAGATCAAAC-3′; Th (reverse), 5′-CGCTGGATACGAGAGGCATAGTT-3′; Nurr1 (forward), 5′-GCCTAGCTGTTGGGATGG-3′; Nurr1 (reverse), 5′-TTGCCTGGAACCTGGAAT-3′; Nur77 (forward), 5′-GCACAGCTTGGGTGTTGATG-3′; Nur77 (reverse), 5′-CAGACGTGACAGGCAGCTG-3′; Nor1 (forward), 5′-AGACGCCGAAACCGATGT-3′; Nor1 (reverse), 5′-CTCGGACAAGGGCATTCA-3′.
Reagents
Antibodies to Rev-erbα (catalog no. 2124) and ERK (catalog no. 4695) were purchased from Cell Signaling Technology. TH antibody was purchased from Millipore Corp (MAB318). The TH inhibitor AMPT and amphetamine were purchased from Sigma.
Western blot
Hippocampus samples were dounced in cold extraction buffer (25 mM Tris-HCl, 2 mM EDTA, 0.1% sodium dodecyl sulfate, 150 mM NaCl, 50 mM KCl, 1% Triton X-100, 0.08% deoxycholate, pH 8) supplemented with Complete protease inhibitors (Roche). SDS-PAGE was performed using 30 μg protein loaded onto a 10% Tris-glycine gel (Invitrogen), followed by transfer to a polyvinylidene difluoride membrane (Invitrogen). After incubation with antibodies, blots were developed using the enhanced chemiluminescence kit from PerkinElmer.
Statistical analysis
Comparisons between 2 groups were performed using Student's t test. Multiple group comparisons were performed by a two-way ANOVA. Statistical significance was defined as P < .05. All data are presented as mean ± SEM.
Results
Rev-erbα KO mice display novelty-induced hyperactivity and impaired habituation
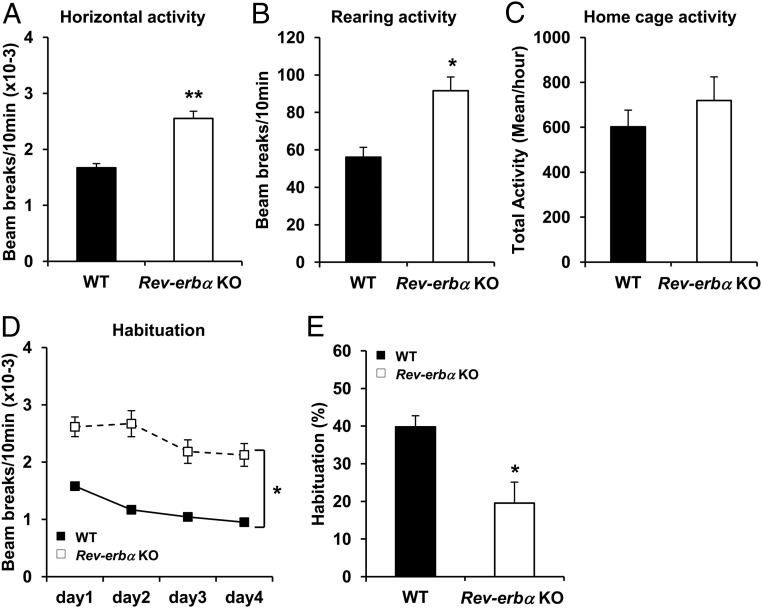
During daily observation and husbandry, Rev-erbα KO mice were noticeably more reactive to routine perturbations than their WT littermates. We investigated the response of Rev-erbα KO mice to a novel environment, because increased spontaneous locomotor activity in the OF is considered a salient feature of models of manic behavior (46). Rev-erbα KO mice showed higher horizontal (Figure 1A) and rearing (Figure 1B) activities compared with WT mice, revealing a marked locomotor hyperactivity phenotype. To test further whether basal locomotor activity was affected in Rev-erbα KO mice, we recorded the home cage activity at the same period of the day as the OF test. Importantly, Rev-erbα KO mice were not significantly more active in their home cage compared with WT mice (Figure 1C), indicating that the locomotor hyperactivity in the OF was induced by exposure to a novel environment. Hyperactivity in response to a novel environment could be explained by a defect of habituation; therefore mice were reexposed to the same OF for 4 consecutive days. Although both WT and Rev-erbα KO mice showed a habituation response (Figure 1D), the Rev-erbα KO mice showed a lower percentage of habituation between day 1 and day 4 compared with WT mice (19.5 ± 5.6 vs 39.8 ± 2.9, Figure 1E), revealing a deficit in habituation.
Figure 1.
Novelty-induced hyperactivity and impaired habituation in Rev-erbα KO mice. A and B, WT and Rev-erbα KO mice were tested in OF during a 10-minute session. Horizontal (A) and rearing (B) locomotor activities were measured. Data are expressed as the number of beam breaks for 10 minutes ± SEM (Student's t test: *, P < .01 and **, P < .001 vs WT; n = 9–10). C, Total locomotor activity in home cage of WT and Rev-erbα KO mice. Data are expressed as the mean of beam breaks per hour ± SEM (n = 10). D and E, Habituation to a new environment was assessed by measuring the total locomotor activity during a 10-minute session in the OF for 4 consecutive days. D, Data are expressed as the number of beam breaks for 10 minutes ± SEM (two-way repeated measures ANOVA: *, P < .001 vs genotype and vs time; n = 15–16). E, Data are expressed as percentage of habituation between day 1 and day 4. (Student's t test: *, P < .01 vs WT; n = 15–16).
Rev-erbα KO mice exhibit cognitive deficits and impaired hippocampus-dependent behavior
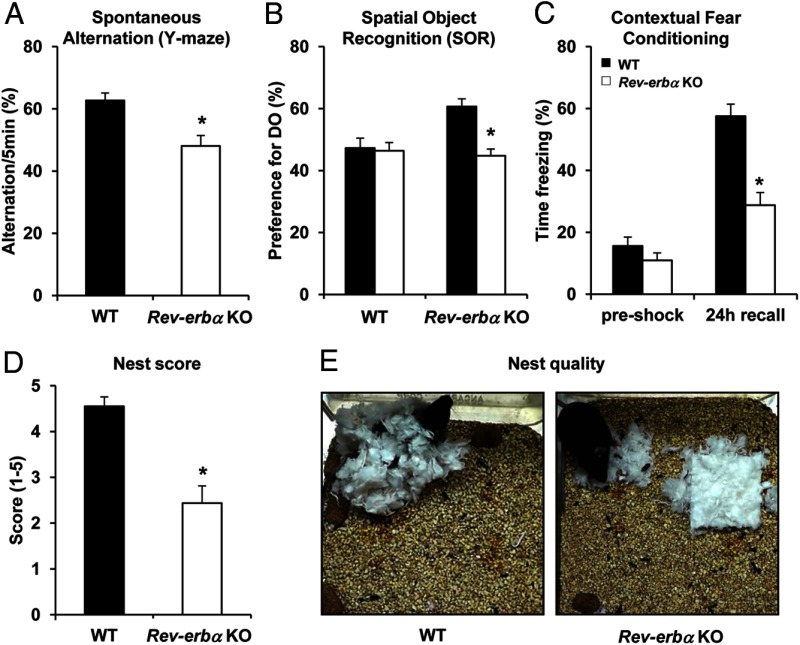
Cognitive disorders are often associated with affective diseases and are seen in animal models of mania (47). In order to explore the role of Rev-erbα in learning and memory, we assessed the performance of Rev-erbα KO mice in a variety of tests. Spontaneous alternation in a Y-maze is considered a test of short-term or working memory. Mice have a tendency to avoid an arm they have just visited and alternate their entries among the arms; therefore, reentry into an arm just visited suggests memory impairment (48). In the Y-maze task, Rev-erbα KO mice showed reduced spontaneous alternations between the arms compared with WT mice (Figure 2A), suggesting a deficit in working and short-term memory.
Figure 2.
Cognitive deficit and impaired hippocampus-dependent behavior in Rev-erbα KO mice. A, Short-term spatial memory was assessed in WT and Rev-erbα KO mice using the Y-maze test. Data are expressed as the percentage of spontaneous alternation between the arms ± SEM (Student's t test: *, P < .01 vs WT; n = 17–19). B, Long-term spatial memory was assessed in WT and Rev-erbα KO mice using the SOR test. Data are expressed as the percentage of preference for the DO ± SEM (two-way ANOVA: *, P < .05 genotype × condition; n = 15–17). C, Associative memory was assessed in WT and Rev-erbα KO mice using the contextual fear-conditioning test. Data are expressed as the percentage of time freezing ± SEM during the training (preshock) and the next day during the test (24-hour recall) (two-way ANOVA: *, P < .001 genotype × condition; n = 18–22). D, The quality of the nest construction was assessed on a rating scale of 1–5. The data are expressed as the mean score ± SEM (Student's t test: *, P < .001 vs WT; n = 10–16). E, Representative pictures of the nest construction.
We also assessed long-term memory using the SOR test, which relies on the innate propensity of mice to explore their environment and later recall where objects are located (43). Mice were trained to learn the position of objects and then, 24 hours later, one object was moved, with the type of object displaced being alternated within each group. “Intact” mice will recall the position of the NDO and exploration of the DO is favored. Neither WT nor Rev-erbα KO mice showed a preference for either object during training sessions, but Rev-erbα KO mice showed less preference for the DO than WT mice when tested 24 hours later (Figure 2B), suggesting impaired long-term spatial memory. We then performed a second test of long-term memory, contextual fear conditioning, in which mice are trained to associate an environment (or context) with an aversive unconditional stimulus (foot shock). Upon reexposure to the context, a mouse will freeze if an association has been made between the context and shock, and reduced freezing indicates a memory deficit (49). Rev-erbα KO mice demonstrated reduced time spent freezing compared with WT mice 24 hours after the training (Figure 2C), supporting a long-term memory deficit associated with Rev-erbα KO.
These learning and memory tests require an intact hippocampus (50), suggesting hippocampal dysfunction in the Rev-erbα KO mice. Thus, we investigated nest building, a hippocampus-dependent behavior (51). Rev-erbα KO mice were less capable at nest construction, using less material (Figure 2D) and building nests of poorer quality than WT mice (Figure 2E). Taken together, these results indicate impaired hippocampal functions in Rev-erbα KO mice.
Novelty-induced locomotor activity is not circadian
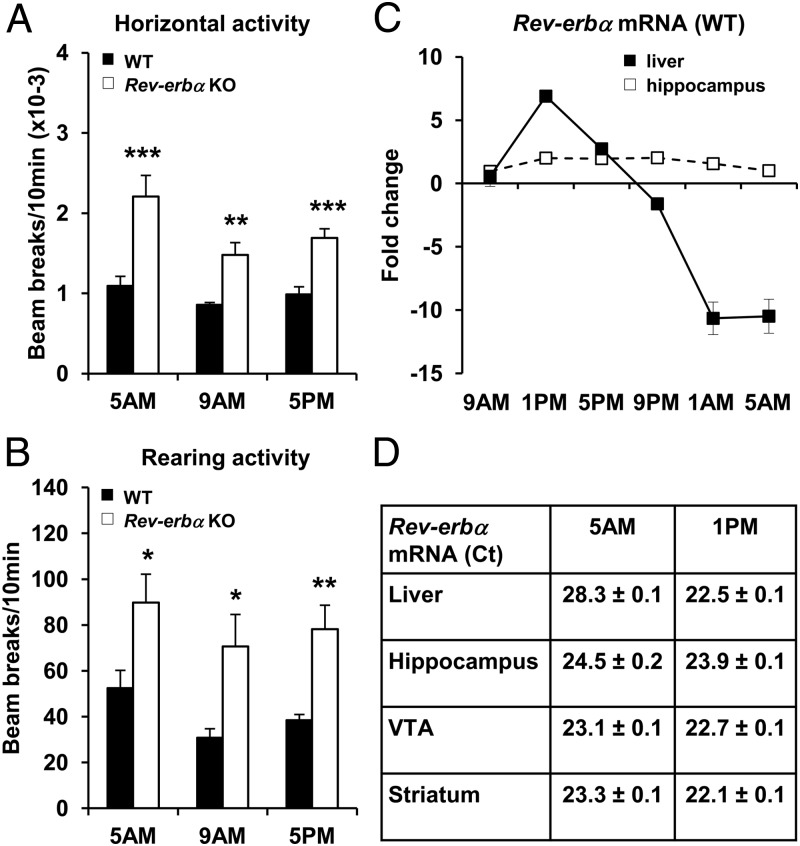
Because Rev-erbα is a core clock component that is expressed with a significant circadian rhythm in liver, skeletal muscle, and adipose tissue, as well as in the brain (www.nursa.org/10.1621/datasets.02001), we hypothesized that the change in novelty-induced locomotor activity might be a circadian phenomenon. To test this, we performed the OF test at either 4:00 to 6:00 pm, when Rev-erbα expression is highest in WT mice, or at 4:00 to 6:00 am, when Rev-erbα expression is lowest in WT mice. Of note, Rev-erbα KO mice displayed increased horizontal and rearing activities at both times of day, comparable to the magnitude of effect observed at the time of day that the earlier experiments were performed, 8:00 to 10:00 am (Figure 3, A and B). Thus the effects of Rev-erbα on novelty-induced locomotor activity did not appear to be circadian.
Figure 3.
Novelty-induced locomotor activity is not circadian. A and B, WT and Rev-erbα KO mice were tested in OF during a 10-minute session at either 5:00 am, 9:00 am or 5:00 pm. Horizontal (A) and rearing (B) locomotor activities were measured. Data are expressed as the number of beam breaks for 10 minutes ± SEM (Student's t test: *P < .05, **,P < .01, and ***, P < .001 vs WT same time; n = 5–15). C, Fold change of Rev-erbα mRNA level in the liver and the hippocampus of WT mice harvested every 4 hours of 24 hours (9AM, 1PM, 5PM, 9PM, 1AM, 5AM). Data are the mean of fold change ± SEM (Nonparametric algorithm JTK_CYCLE (66) for significance of oscillation: P < .05 for the liver; P = .61 for the hippocampus; n = 4–6). D, Ct values for Rev-erbα mRNA in the liver, the hippocampus, the VTA (ventral tegmental area), and the striatum of WT mice at 5:00 am and 1:00 pm. Data are the mean of the Ct ± SEM (n = 4–6).
Interestingly, whereas Rev-erbα mRNA expression was highly circadian in liver as expected, this was not the case in hippocampus, which is a major regulator of novelty detection and habituation (Figure 3C). Rev-erbα mRNA levels peaked at 1:00 pm (Ct 22.5 ± 0.1, Figure 3D) and were almost undetectable at 5:00 am (Ct 28.3 ± 0.2, Figure 3D) in liver, whereas in the hippocampus, Rev-erbα mRNA was detectable throughout the day (Ct 23.9 ± 0.1 at 1:00 pm and Ct 24.5 ± 0.2 at 5:00 am, Figure 3D). Furthermore, Rev-erbα mRNA level was also detectable throughout the day in other parts of the brain related to the control of locomotor activity, including the ventral tegmental area (VTA) and the striatum (Figure 3D and Supplemental Figure 1 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). These results likely explain the absence of variation in novelty-induced locomotor activity throughout the day.
Rev-erbα KO mice exhibit up-regulation of dopaminergic gene expression and higher dopamine turnover compared with WT mice
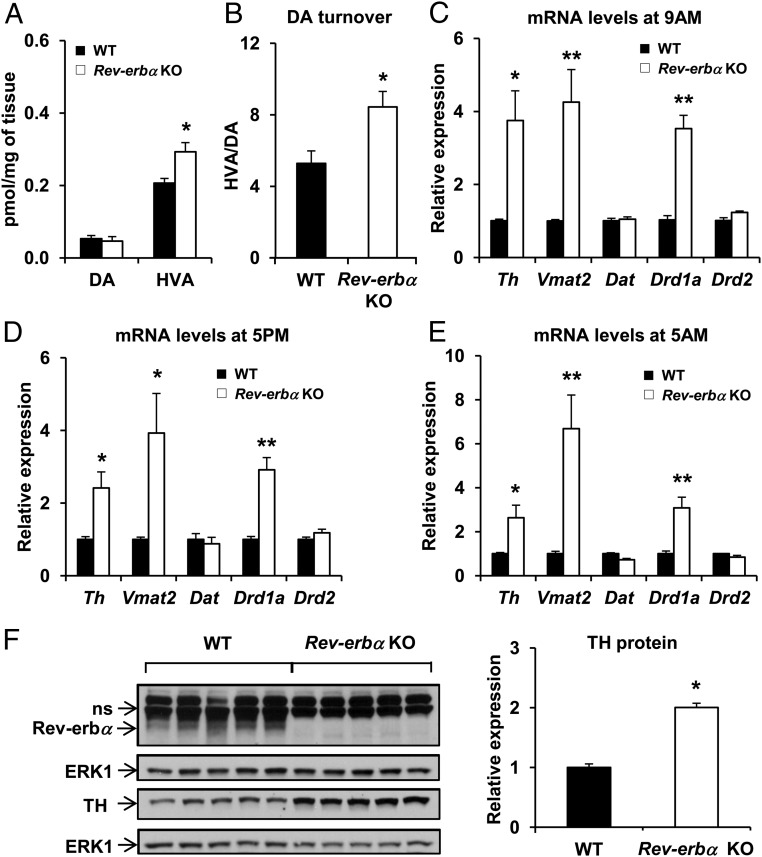
Mice lacking a functional dopamine transporter display hyperactivity and impaired response habituation in novel environments (52, 53), and increasing evidence suggests that dopamine plays an important role in the detection of novel information (54). This is especially relevant in the hippocampus in which extracellular dopamine concentrations can be elevated by exposure of animals to novel environments (55) and activation of hippocampal dopamine receptors can induce locomotor hyperactivity (56). Although hippocampal dopamine levels were not higher, levels of the extraneuronal dopamine metabolite, homovanillic acid, as well as the ratio of homovanillic acid to dopamine were increased in Rev-erbα KO mice (Figure 4, A and B), indicating greater presynaptic activity and dopamine turnover.
Figure 4.
TH expression shows a circadian pattern of expression and is up-regulated in Rev-erbα KO mice. A, Concentration of dopamine (DA) and its metabolite homovanillic acid (HVA) and dopamine turnover (B) in the hippocampus of WT and Rev-erbα KO mice. Data are expressed as picomoles/mg of tissue and are the mean ± SEM (Student's t test; HVA: *, P < .01 and HVA/DA: *, P < .01 vs WT; n = 13–14). C–E, Relative mRNA expression of Th, Vmat2, Dat, Drd1a, Drd2 at 9:00 am (C), 5:00 pm (D), and 5:00 am (E) in the hippocampus of WT and Rev-erbα KO mice. Data are the mean ± SEM (Student's t test: *, P < .01 and **, P < .001 vs WT; n = 4–6). F, Western blot analysis of Rev-erbα (ns, nonspecific band) TH, and ERK1 (loading control) in the hippocampus of WT and Rev-erbα KO mice. Representative immunoblots and quantification of TH protein level are presented (Student's t test: *, P < .001 vs WT; n = 5).
Consistent with the function of Rev-erbα as a transcriptional repressor (1), several genes involved in dopamine transmission were up-regulated in the hippocampus of Rev-erbα KO mice at different times of day (Figure 4, C–E). These included TH, the rate limiting-enzyme in dopamine biosynthesis (57), the vesicular monoamine transporter 2 (Vmat2), which limits the rate of neurotransmitter accumulation, and the dopamine receptor d1a (Drd1a). The level of TH protein was correspondingly increased (Figure 4F).
Because Th mRNA is compartmentalized in axons and terminals of catecholaminergic neurons (58), we measured the expression of Th in different regions of the brain that receive dopaminergic projections originating from the VTA and other brainstem nuclei. Although Rev-erbα mRNA was detected in all of the brain regions we analyzed, up-regulation of Th mRNA was not detected in the striatum, frontal cortex, or hypothalamus of Rev-erbα KO mice (Supplemental Figure 2). We also measured the expression of nuclear receptors involved in the regulation of dopaminergic signaling, but we did not detect significant changes in the mRNA levels of Nurr1, Nur77, and Nor1 in the VTA of the Rev-erbα KO (Supplemental Figure 3). Nevertheless, together these results suggest that deletion of Rev-erbα induced the up-regulation of dopaminergic genes throughout the day, leading to increased dopamine turnover in the hippocampus.
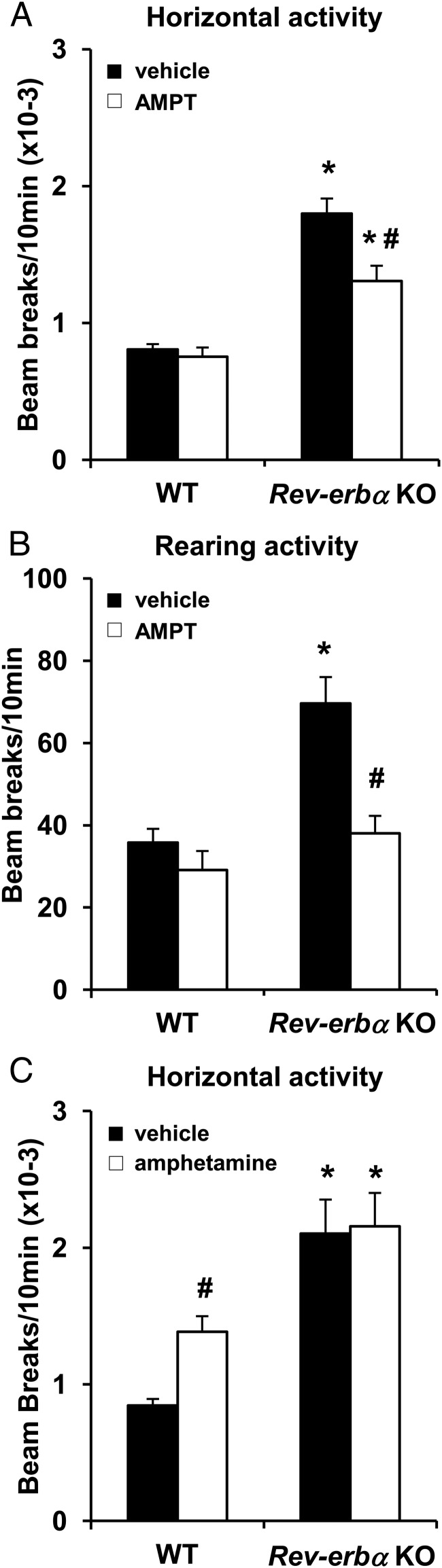
Inhibition of TH rescues the hyperactivity of Rev-erbα KO mice
To test whether the increases in dopaminergic gene expression drive the hyperactive phenotype, mice were treated with a pharmacologic inhibitor of TH, AMPT, and then subjected to OF testing. TH inhibition partially rescued the horizontal locomotor hyperactivity phenotype (Figure 5A) and completely rescued the rearing activity (Figure 5B) of Rev-erbα KO mice whereas the inhibitor had no effect on WT mice (Figure 5, A and B). We then tested the effect of amphetamine, which releases dopamine into the extracellular space (59). WT mice treated with amphetamine showed an increase in locomotor activity, whereas amphetamine had no significant effect on the Rev-erbα KO mice (Figure 5C), suggesting that synaptic activity and dopamine turnover were already maximal in Rev-erbα KO mice. These results suggest that increased dopaminergic activity mediated the observed novelty-induced hyperactivity in Rev-erbα KO mice.
Figure 5.
Inhibition of TH partially rescues the hyperactivity of Rev-erbα KO. A and B, WT and Rev-erbα KO mice were administrated with vehicle or AMPT (TH inhibitor) at dose of 200 mg/Kg and 45 minutes before the OF test. Horizontal (A) and rearing (B) locomotor activities were measured. Data are expressed as the number of beam breaks for 10 minutes ± SEM (two-way ANOVA and Turkey's post hoc: *, P < .01 vs WT same treatment and #, P < .01 vs vehicle same genotype; n = 11–26). C, WT and Rev-erbα KO mice were administrated with vehicle or amphetamine at dose of 1 mg/Kg and 30 minutes before the OF test. Horizontal locomotor activity was measured. Data are expressed as the number of beam breaks for 10 minutes ± SEM (two-way ANOVA and Turkey's post hoc: *, P < .01 vs WT same treatment and #, P < .01 vs vehicle same genotype; n = 5–9).
Discussion
We have demonstrated, for the first time, that loss of the nuclear receptor Rev-erbα leads to novelty-induced hyperactivity and impaired learning and memory. Moreover, Rev-erbα is an important regulator of dopaminergic genes in neurons projecting to the hippocampus, which may explain these behavioral changes. These findings reveal an important and previously unknown role for Rev-erbα in behavior.
Previous studies of Clock mutant mice have demonstrated that Clock regulates behavior through up-regulation of Th and other genes involved in control of dopaminergic signaling (21–24). Because Clock functions primarily as a heterodimer with Bmal1 to activate transcription (60), it is plausible that the up-regulation of genes in the Clock mutant mice could be mediated by reduced expression of Rev-erbα, which is normally induced by Clock/Bmal1 (7), leading to derepression of these genes.
Our finding of hippocampal-specific up-regulation of dopaminergic tone is curious and suggests that Rev-erbα may regulate projection-specific changes in gene expression in the VTA. This would be consistent with recent findings of projection-specific changes in Th expression in dopaminergic neurons originating in the VTA (61). Although we did not detect any change in the mRNA level of Nurr1, Nur77, and Nor1 in the VTA of the Rev-erbα KO mice, it is possible that Rev-erbα does regulate the expression of these nuclear receptors in a minority population of the cells in the VTA, such that the gene regulation is drowned out by the lack of regulation in most cells. Thus, further investigation is needed to delineate Rev-erbα and dopaminergic pathway interactions. Alternatively, Rev-erbα may regulate proteins associated with axonal transport or stability of mRNA.
Dopaminergic tone can greatly influence behavior. The detection of novelty triggers the firing of dopaminergic neurons in the VTA (62), leading to the release of dopamine in the hippocampus, which is crucial for consolidation processes of long-term memory (47, 63). We found that Rev-erbα KO mice were hyperactive in a novel environment and that subsequent reexposure to the OF demonstrated a deficit in habituation of Rev-erbα KO mice. Furthermore we found deficits in 2 forms of long-term hippocampal-dependent learning, spatial object recognition and contextual fear learning. Rev-erbα KO mice also demonstrated a working-memory deficit in spontaneous alternations in the Y-maze, another cognitive task influenced by hippocampal dopaminergic tone (48). Of note, mutations in other clock genes, including Npas2 or Per2, also alter learning in mice (64, 65). Remarkably, the novelty-induced hyperactivity was reversed by treatment with the TH inhibitor (AMPT). Thus, loss of Rev-erbα up-regulates Th (and other dopamine-associated genes) to cause hyperactivity and directly blocking TH can reverse the hyperactivity. This mechanism makes Rev-erbα KO mouse a useful tool for investigating models of affective disorders associated with increased dopaminergic tone. It should be noted that Rev-erbα is widely expressed during development as well as in adult life, and therefore the behavioral effect observed in the Rev-erbα KO mice could also be due to defects during brain formation.
Although in many tissues, including liver (34) and brown fat (42), circadian expression of Rev-erbα plays an important physiological role, we found that Rev-erbα mRNA oscillations are not nearly as great in brain regions involved in the control of locomotor activity including the hippocampus, the VTA, and the striatum. These findings likely explain the absence of variation in novelty-induced locomotor activity throughout the day and suggest that clock genes like Rev-erbα might also regulate noncircadian functions in brain regions outside the suprachiasmatic nucleus. Interestingly, the circadian phenotypes of Rev-erbα KO mice are relatively modest due to redundancy with Rev-erbβ (9, 10). Thus, the robust hyperactive phenotype of the Rev-erbα KO mice, despite minimally disrupted circadian behavior, suggests a nonredundant role for Rev-erbα independent of its function in the core clock mechanism. The critical role of Rev-erbα in dopamine-regulated behaviors suggests that it could be a pharmacologic target for the treatment of dopamine-related behavioral disorders without major disruption of the molecular circadian clock.
Additional material
Supplementary data supplied by authors.
Acknowledgments
We thank Dr Olivier Berton for helpful discussions and technical advice. We thank the Neurobehavior Testing Core of the Penn Medicine Neuroscience Center. We also thank Ravi Dhir and Rex Ahima of the Mouse Metabolic Phenotyping Core of the Penn Diabetes Research Center for home cage activity measurements.
This work was supported by National Institutes of Health (NIH) R01 DK45586 (to M.A.L.), the Cox Institute for Medical Research, and a grant from the Institute for Regenerative Medicine at the University of Pennsylvania, School of Medicine (to P.S.K.). The Analytical Neurochemistry and Spectroscopy Core is supported as part of the institutional Intellectual and Developmental Disabilities Research Center (P30 HD26979) and was responsible for analyses of dopamine and metabolites. The Mouse Metabolic Phenotyping Core of the Penn Diabetes Research Center is supported by NIH P30 DK19525.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AMPT
- α-methyl-p-tyrosine
- DO
- displaced object
- KO
- knockout
- NDO
- nondisplaced object
- OF
- open field
- SOR
- spatial object recognition
- TH
- tyrosine hydroxylase
- VTA
- ventral tegmental area
- WT
- wild type
- ZT
- Zeitgerber time.
References
- 1. Harding HP, Lazar MA. The monomer-binding orphan receptor Rev-Erb represses transcription as a dimer on a novel direct repeat. Mol Cell Biol. 1995;15:4791–4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–676. [DOI] [PubMed] [Google Scholar]
- 3. Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–661. [DOI] [PubMed] [Google Scholar]
- 4. Yamamoto T, Nakahata Y, Soma H, Akashi M, Mamine T, Takumi T. Transcriptional oscillation of canonical clock genes in mouse peripheral tissues. BMC Mol Biol. 2004;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wijnen H, Young MW. Interplay of circadian clocks and metabolic rhythms. Annu Rev Genet. 2006;40:409–448. [DOI] [PubMed] [Google Scholar]
- 6. Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Preitner N, Damiola F, Lopez-Molina L, et al. , The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. [DOI] [PubMed] [Google Scholar]
- 8. Yin L, Lazar MA. The orphan nuclear receptor Rev-erbα recruits the N-CoR/histone deacetylase 3 corepressor to regulate the circadian Bmal1 gene. Mol Endocrinol. 2005;19:1452–1459. [DOI] [PubMed] [Google Scholar]
- 9. Bugge A, Feng D, Everett LJ, et al. Rev-erbα and Rev-erbβ coordinately protect the circadian clock and normal metabolic function. Genes Dev. 2012;26:657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cho H, Zhao X, Hatori M, et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature. 2012;485:123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gachon F, Nagoshi E, Brown SA, Ripperger J, Schibler U. The mammalian circadian timing system: from gene expression to physiology. Chromosoma. 2004;113:103–112. [DOI] [PubMed] [Google Scholar]
- 12. Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet. 2004;5:407–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stratmann M, Schibler U. Properties, entrainment, and physiological functions of mammalian peripheral oscillators. J Biol Rhythms. 2006;21:494–506. [DOI] [PubMed] [Google Scholar]
- 14. Kohsaka A, Bass J. A sense of time: how molecular clocks organize metabolism. Trends Endocrinol Metab. 2007;18:4–11. [DOI] [PubMed] [Google Scholar]
- 15. Mansour HA, Monk TH, Nimgaonkar VL. Circadian genes and bipolar disorder. Ann Med. 2005;37:196–205. [DOI] [PubMed] [Google Scholar]
- 16. Sahar S, Sassone-Corsi P. Metabolism and cancer: the circadian clock connection. Nat Rev Cancer. 2009;9:886–896. [DOI] [PubMed] [Google Scholar]
- 17. Huang W, Ramsey KM, Marcheva B, Bass J. Circadian rhythms, sleep, and metabolism. J Clin Invest. 2011;121:2133–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McClung CA. Circadian rhythms and mood regulation: insights from pre-clinical models. Eur Neuropsychopharmacol. 2011;21(Suppl 4):S683–S693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Imeraj L, Sonuga-Barke E, Antrop I, et al. Altered circadian profiles in attention-deficit/hyperactivity disorder: an integrative review and theoretical framework for future studies. Neurosci Biobehav Rev. 2012;36:1897–1919. [DOI] [PubMed] [Google Scholar]
- 20. Pritchett D, Wulff K, Oliver PL, et al. Evaluating the links between schizophrenia and sleep and circadian rhythm disruption. J Neural Transm. 2012;119:1061–1075. [DOI] [PubMed] [Google Scholar]
- 21. Easton A, Arbuzova J, Turek FW. The circadian Clock mutation increases exploratory activity and escape-seeking behavior. Genes Brain Behav. 2003;2:11–19. [DOI] [PubMed] [Google Scholar]
- 22. McClung CA, Sidiropoulou K, Vitaterna M, et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci USA. 2005;102:9377–9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roybal K, Theobold D, Graham A, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci USA. 2007;104:6406–6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mukherjee S, Coque L, Cao JL, et al. Knockdown of Clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biol Psychiatry. 2010;68:503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coque L, Mukherjee S, Cao JL, et al. Specific role of VTA dopamine neuronal firing rates and morphology in the reversal of anxiety-related, but not depression-related behavior in the ClockΔ19 mouse model of mania. Neuropsychopharmacology. 2011;36:1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jiang WG, Li SX, Liu JF, et al. Hippocampal CLOCK protein participates in the persistence of depressive-like behavior induced by chronic unpredictable stress. Psychopharmacology (Berl). 2013;227:79–92. [DOI] [PubMed] [Google Scholar]
- 27. Ozburn AR, Larson EB, Self DW, McClung CA. Cocaine self-administration behaviors in ClockΔ19 mice. Psychopharmacology (Berl). 2012;223:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hampp G, Ripperger JA, Houben T, et al. Regulation of monoamine oxidase A by circadian-clock components implies clock influence on mood. Curr Biol. 2008;18:678–683. [DOI] [PubMed] [Google Scholar]
- 29. Spencer S, Falcon E, Kumar J, et al. Circadian genes Period 1 and Period 2 in the nucleus accumbens regulate anxiety-related behavior. Eur J Neurosci. 2013;37:242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kondratova AA, Dubrovsky YV, Antoch MP, Kondratov RV. Circadian clock proteins control adaptation to novel environment and memory formation. Aging (Albany NY). 2010;2:285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Keers R, Pedroso I, Breen G, et al. Reduced anxiety and depression-like behaviours in the circadian period mutant mouse afterhours. PLoS One. 2012;7:e38263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Duez H, Staels B. Nuclear receptors linking circadian rhythms and cardiometabolic control. Arterioscler Thromb Vasc Biol. 2010;30:1529–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Le Martelot G, Claudel T, Gatfield D, et al. REV-ERBα participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 2009;7:e1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Feng D, Liu T, Sun Z, et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331:1315–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Woldt E, Sebti Y, Solt LA, et al. Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat Med. 2013;19:1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fontaine C, Rigamonti E, Pourcet B, et al. The nuclear receptor Rev-erbα is a liver X receptor (LXR) target gene driving a negative feedback loop on select LXR-induced pathways in human macrophages. Mol Endocrinol. 2008;22:1797–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gibbs JE, Blaikley J, Beesley S, et al. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci USA. 2012;109:582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lam MT, Cho H, Lesch HP, et al. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature. 2013;498:511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fontaine C, Dubois G, Duguay Y, et al. The orphan nuclear receptor Rev-Erbα is a peroxisome proliferator-activated receptor (PPAR) γ target gene and promotes PPARγ-induced adipocyte differentiation. J Biol Chem. 2003;278:37672–37680. [DOI] [PubMed] [Google Scholar]
- 40. Laitinen S, Fontaine C, Fruchart JC, Staels B. The role of the orphan nuclear receptor Rev-Erb α in adipocyte differentiation and function. Biochimie. 2005;87:21–25. [DOI] [PubMed] [Google Scholar]
- 41. Wang J, Lazar MA. Bifunctional role of Rev-erbα in adipocyte differentiation. Mol Cell Biol. 2008;28:2213–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gerhart-Hines Z, Feng D, Emmett MJ, et al. The nuclear receptor Rev-erbα controls circadian thermogenic plasticity. Nature. 2013;503:410–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wimmer ME, Hernandez PJ, Blackwell J, Abel T. Aging impairs hippocampus-dependent long-term memory for object location in mice. Neurobiol Aging. 2012;33:2220–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Deacon RM. Assessing nest building in mice. Nat Protoc. 2006;1:1117–1119. [DOI] [PubMed] [Google Scholar]
- 45. Stein JM, Bergman W, Fang Y, et al. Behavioral and neurochemical alterations in mice lacking the RNA-binding protein translin. J Neurosci. 2006;26:2184–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Young JW, Henry BL, Geyer MA. Predictive animal models of mania: hits, misses and future directions. Br J Pharmacol. 2011;164:1263–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zierhut K, Bogerts B, Schott B, et al. The role of hippocampus dysfunction in deficient memory encoding and positive symptoms in schizophrenia. Psychiatry Res. 2010;183:187–194. [DOI] [PubMed] [Google Scholar]
- 48. Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev. 2002;26:91–104. [DOI] [PubMed] [Google Scholar]
- 49. Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Deacon RM, Bannerman DM, Kirby BP, Croucher A, Rawlins JN. Effects of cytotoxic hippocampal lesions in mice on a cognitive test battery. Behav Brain Res. 2002;133:57–68. [DOI] [PubMed] [Google Scholar]
- 51. Deacon RM, Croucher A, Rawlins JN. Hippocampal cytotoxic lesion effects on species-typical behaviours in mice. Behav Brain Res. 2002;132:203–213. [DOI] [PubMed] [Google Scholar]
- 52. Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. [DOI] [PubMed] [Google Scholar]
- 53. Ralph-Williams RJ, Paulus MP, Zhuang X, Hen R, Geyer MA. Valproate attenuates hyperactive and perseverative behaviors in mutant mice with a dysregulated dopamine system. Biol Psychiatry. 2003;53:352–359. [DOI] [PubMed] [Google Scholar]
- 54. Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res. 1996;77:23–43. [DOI] [PubMed] [Google Scholar]
- 55. Ihalainen JA, Riekkinen P, Jr, Feenstra MG. Comparison of dopamine and noradrenaline release in mouse prefrontal cortex, striatum and hippocampus using microdialysis. Neurosci Lett. 1999;277:71–74. [DOI] [PubMed] [Google Scholar]
- 56. Smialowski A, Maj J. Repeated treatment with imipramine potentiates the locomotor effect of apomorphine administered into the hippocampus in rats. Psychopharmacology (Berl). 1985;86:468–471. [DOI] [PubMed] [Google Scholar]
- 57. Daubner SC, Le T, Wang S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys. 2011;508:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Melia KR, Trembleau A, Oddi R, Sanna PP, Bloom FE. Detection and regulation of tyrosine hydroxylase mRNA in catecholaminergic terminal fields: possible axonal compartmentalization. Exp Neurol. 1994;130:394–406. [DOI] [PubMed] [Google Scholar]
- 59. Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998;18:1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gekakis N, Staknis D, Nguyen HB, et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. [DOI] [PubMed] [Google Scholar]
- 61. Niwa M, Jaaro-Peled H, Tankou S, et al. Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science. 2013;339:335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. [DOI] [PubMed] [Google Scholar]
- 63. Lisman JE, Coyle JT, Green RW, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Garcia JA, Zhang D, Estill SJ, et al. Impaired cued and contextual memory in NPAS2-deficient mice. Science. 2000;288:2226–2230. [DOI] [PubMed] [Google Scholar]
- 65. Wang LM, Dragich JM, Kudo T, et al. Expression of the circadian clock gene Period2 in the hippocampus: possible implications for synaptic plasticity and learned behaviour. ASN Neuro. 2009;1:e00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hughes ME, Hogenesch JB, Kornacker K. JTK_CYCLE: an efficient nonparametric algorithm for detecting rhythmic components in genome-scale data sets. J Biol Rhythms. 2010;25:372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.