Nonalcoholic fatty liver disease (NAFLD) occurs in 20–30% of Americans and is the most prevalent form of liver disease (1,2). NAFLD is commonly linked to hepatic and whole-body insulin resistance and, in some individuals NAFLD can lead to steatohepatitis, cirrhosis, and cancer (3). The selective hepatic insulin resistance that typically accompanies NAFLD results in increased hepatic glucose production, hepatic lipogenesis, and very low-density lipoprotein (VLDL) secretion, thereby contributing to the hyperglycemia and triglyceridemia observed in insulin-resistant and type 2 diabetic subjects. Despite their close association, NAFLD and insulin resistance can be uncoupled, suggesting that not all NAFLD is the same (4).
Although dietary and environmental factors have a large influence, genetics alone or in combination with external factors also influence NAFLD etiology. A point mutation in PNPLA3 (also known as adiponutrin) is prevalent in ∼20–50% of people depending upon ethnicity and is the single best genetic predictor of NAFLD (5). A recent meta-analysis of nearly 3,000 subjects showed that carriers of the I148M mutant have 73% higher liver triacylglycerol (TAG) content compared with those with the normal I148I allele (6). In addition, the I148M variant has been associated with complications or progression of NAFLD, including development of nonalcoholic steatohepatitis, alcoholic liver disease and its progression to cirrhosis, and the severity of hepatitis C–induced steatosis (7). Despite its important role in NAFLD etiology, the physiological function of wild-type and variant PNPLA3 remains under intense debate. PNPLA3 was first characterized as a lipase with preference toward TAG, and the I148M loss-of-function mutation causes steatosis by reducing TAG breakdown (8). The confusion perhaps started when PNPLA3 knockout mice were observed to have no overt phenotype (9,10). In contrast to a catabolic role of PNPLA3, a study by Zechner and colleagues (11) showed that PNPLA3 is a lysophosphatidic acid acyltransferase and the I148M mutation causes a gain in function to promote TAG synthesis. There are additional studies supporting each of these lines of thought; thus, researchers are largely divided on the exact role of PNPLA3.
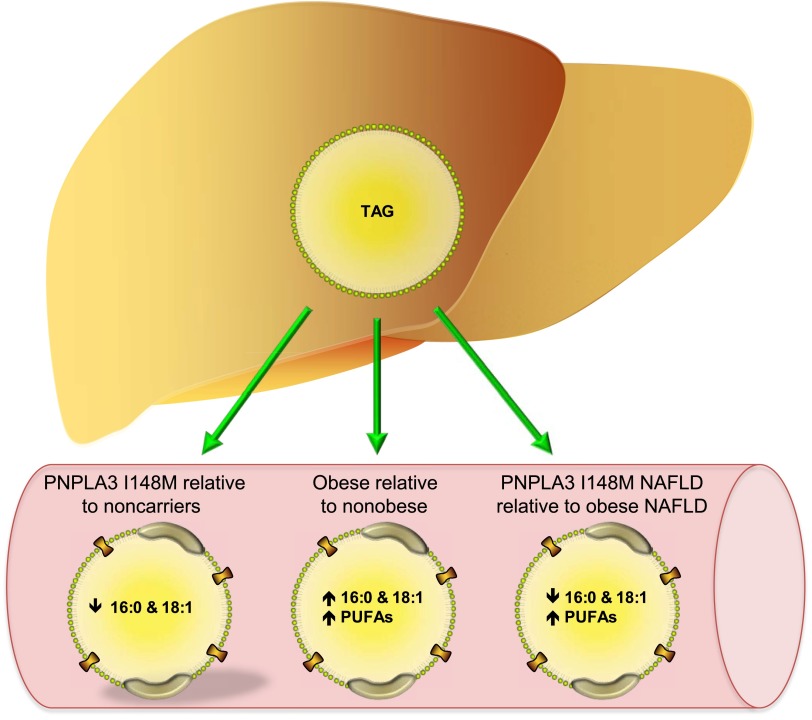
In this issue, Hyysalo et al. (12) investigated how obesity, PNPLA3 variant expression, and NAFLD influence circulating TAG content and composition. The authors found that subjects expressing the variant PNPLA3 tended to have lower circulating TAG concentrations, which is in line with previous work demonstrating that PNPLA3 I148M decreases VLDL secretion (13). However, expression of the variant was associated with decreased saturated and monounsaturated fatty acids in circulating TAG (Fig. 1). Previous studies show that mice overexpressing the PNPLA3 variant have elevated 16:1 and 18:1 fatty acids in liver TAG, consistent with PNPLA3's preference to hydrolize monounsaturated fatty acids (14). Approximately 70% of fatty acids are esterified and stored in cytosolic lipid droplets prior to their hydrolysis and re-esterification into TAG destined for VLDL secretion (15). Thus, the composition of VLDL-TAG should largely mirror that of liver TAG. When taken together, the authors suggest that the data support a lipolytic role for PNPLA3, whereby reduced activity due to the mutation results in monounsaturated fatty acids accumulating in hepatic TAG and a subsequent reduction in their secretion. Indeed, this is a logical conclusion and the lipolytic role of PNPLA3 may have contributed to these findings.
Figure 1.
Absolute changes in serum TAG composition in response to expression of the PNPLA3 variant, obesity, and NAFLD sub-types. PUFAs, polyunsaturated fatty acids.
However, one must also consider that the anabolic function of PNPLA3 could also contribute to the observed changes in serum TAG composition. PNPLA3 is highly regulated by factors that stimulate lipogenesis, such as refeeding, high-carbohydrate diet, carbohydrate-responsive element–binding protein, and sterol regulatory element–binding protein-1c, and in vitro and in vivo studies provide evidence for a role of PNPLA3 I148M in de novo lipogenesis (11,14). In support of this concept, dietary carbohydrate content is correlated with liver fat content in humans with the PNPLA3 I148M variant, but not in subjects heterozygous for the variant or expressing the normal allele (16). It is known that fatty acids generated from de novo lipogenesis are not secreted at the same rate as fatty acids derived exogenously, suggesting that different pools of fatty acids may exist (17). For example, 16:1 or 18:1 derived from de novo lipogenesis may be esterified into a specific position of TAG or a different pool of lipid droplets that make them less likely to be secreted. Thus, the contribution of the lysophosphatidic acid acyltransferase activity of PNPLA3 cannot be ruled out as a contributor to altered serum TAG composition. It is also quite possible that PNPLA3 has more than one physiological function—surely, this would not be the first bifunctional enzyme.
Despite its association with NAFLD and the tight association between NAFLD and hepatic insulin resistance, most studies do not demonstrate any relationship between the PNPLA3 variant and insulin resistance (18,19), suggesting that the PNPLA3 variant could, at least in part, explain why some fatty livers are not associated with impaired insulin signaling. Hyysalo et al. (12) demonstrates that serum TAG composition differs greatly between subjects with NAFLD that are obese and lean individuals with liver steatosis that express the PNPLA3 variant. Serum TAG in the NAFLD subjects with the PNPLA3 variant had less saturated and monounsaturated fatty acids and more longer-chain polyunsaturated fatty acids compared with obese NAFLD subjects. The data, consistent with prior studies, point toward significant differences in hepatic fatty acid metabolism and secretion between these two NAFLD subtypes. The authors attribute these differences to the fact that the PNPLA3 variant is not associated with insulin resistance, whereas the NAFLD associated with obesity is typically linked to insulin resistance. Previous studies have shown a positive association between saturated and monounsaturated fatty acid content of serum TAG and insulin resistance, whereas longer-chain polyunsaturated fatty acids are negatively associated with insulin resistance (19,20). Thus, expression of the PNPLA3 variant results in a lipid profile that would be expected to promote insulin sensitivity. The data clearly point to the complexity of linking NAFLD to insulin resistance, but also highlights the utility of the PNPLA3 variant as a model to help us further understand the etiology of hepatic insulin resistance.
In summary, Hyysalo et al. (12) highlight the importance of genetic (PNPLA3) and environmental (obesity) factors that more adequately define NAFLD. The data strengthen the concept that not all NAFLD is created equal, which is an important factor to consider as we strive to design and tailor therapies to treat NAFLD and its complications.
Article Information
Funding. D.G.M. receives support from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (DK-0903634) and the Minnesota Obesity Center (DK050456). A.S.G. receives support from the National Institute of Environmental Health Sciences (UO1-ES-020958, RO3-ES-0227), NIDDK-Boston Nutrition Obesity Research Center (P30-DK-46200), and the U.S. Department of Agriculture, Agricultural Research Service, under agreement no. 58-1950-7-70.
Duality of Interest. D.G.M. receives support from Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 312.
References
- 1.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387–1395 [DOI] [PubMed] [Google Scholar]
- 2.Wattacheril J, Chalasani N. Nonalcoholic fatty liver disease (NAFLD): is it really a serious condition? Hepatology 2012;56:1580–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science 2011;332:1519–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farese RV, Jr, Zechner R, Newgard CB, Walther TC. The problem of establishing relationships between hepatic steatosis and hepatic insulin resistance. Cell Metab 2012;15:570–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008;40:1461–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology 2011;53:1883–1894 [DOI] [PubMed] [Google Scholar]
- 7.Zimmer V, Lammert F. Genetics and epigenetics in the fibrogenic evolution of chronic liver diseases. Best Pract Res Clin Gastroenterol 2011;25:269–280 [DOI] [PubMed] [Google Scholar]
- 8.He S, McPhaul C, Li JZ, et al. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J Biol Chem 2010;285:6706–6715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basantani MK, Sitnick MT, Cai L, et al. PNPLA3/adiponutrin deficiency in mice does not contribute to fatty liver disease or metabolic syndrome. J Lipid Res 2011;52:318–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W, Chang B, Li L, Chan L. Patatin-like phospholipase domain-containing 3/adiponutrin deficiency in mice is not associated with fatty liver disease. Hepatology 2010;52:1134–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumari M, Schoiswohl G, Chitraju C, et al. Adiponutrin functions as a nutritionally regulated lysophosphatidic acid acyltransferase. Cell Metab 2012;15:691–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyysalo J, Gopalacharyulu P, Bian H, et al. Circulating triacylglycerol signatures in nonalcoholic fatty liver disease associated with the I148M variant in PNPLA3 and with obesity. Diabetes 2014;63:312–322 [DOI] [PubMed] [Google Scholar]
- 13.Pirazzi C, Adiels M, Burza MA, et al. Patatin-like phospholipase domain-containing 3 (PNPLA3) I148M (rs738409) affects hepatic VLDL secretion in humans and in vitro. J Hepatol 2012;57:1276–1282 [DOI] [PubMed] [Google Scholar]
- 14.Li JZ, Huang Y, Karaman R, et al. Chronic overexpression of PNPLA3I148M in mouse liver causes hepatic steatosis. J Clin Invest 2012;122:4130–4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibbons GF, Wiggins D. Intracellular triacylglycerol lipase: its role in the assembly of hepatic very-low-density lipoprotein (VLDL). Adv Enzyme Regul 1995;35:179–198 [DOI] [PubMed] [Google Scholar]
- 16.Davis JN, Lê K-A, Walker RW, et al. Increased hepatic fat in overweight Hispanic youth influenced by interaction between genetic variation in PNPLA3 and high dietary carbohydrate and sugar consumption. Am J Clin Nutr 2010;92:1522–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vedala A, Wang W, Neese RA, Christiansen MP, Hellerstein MK. Delayed secretory pathway contributions to VLDL-triglycerides from plasma NEFA, diet, and de novo lipogenesis in humans. J Lipid Res 2006;47:2562–2574 [DOI] [PubMed] [Google Scholar]
- 18.Kantartzis K, Peter A, Machicao F, et al. Dissociation between fatty liver and insulin resistance in humans carrying a variant of the patatin-like phospholipase 3 gene. Diabetes 2009;58:2616–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotronen A, Johansson LE, Johansson LM, et al. A common variant in PNPLA3, which encodes adiponutrin, is associated with liver fat content in humans. Diabetologia 2009;52:1056–1060 [DOI] [PubMed] [Google Scholar]
- 20.Rhee EP, Cheng S, Larson MG, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest 2011;121:1402–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]