Abstract
Nephrotic syndrome was reported in a highly-sensitized patient receiving enzyme replacement therapy (ERT) for Pompe disease, but the prevalence of ERT-induced renal complications and mechanisms to facilitate readministration of ERT in these patients remain unexplored. This work identifies a new antigen responsible for secondary membranous nephropathy (MN) in a patient with mucopolysaccharidosis type VI caused by aryl sulfatase B (ASB) deficiency. ERT (recombinant human ASB [rhASB]; 1 mg/kg per week) started at the age of 4 years led to a high anti-rhASB titer and dramatically improved clinical manifestations. However, 16 months later, the patient suddenly developed nephrotic syndrome resistant to steroid therapy 1 week after orthopedic surgery. Examination of the kidney biopsy specimen revealed glomerular deposition of IgG (mostly IgG4, C3, and C5b-9) in a granular pattern typical of MN. Double immunofluorescence staining showed that subepithelial granular deposits contained rhASB colocalized with IgG. Ig eluted from the patient’s biopsy specimen reacted specifically with rhASB. On discontinuation of ERT, proteinuria progressively decreased, but the patient's clinical condition markedly deteriorated. Induction of tolerance to rhASB was initiated by coadministration of low-dose corticosteroids, rituximab, intravenous Igs, and oral methotrexate. ERT was resumed 8 weeks after starting immunosuppressive therapy without inducing a rebound of antibody titer or an increase in proteinuria. We conclude that the allo-immune response to the recombinant rhASB caused the nephropathy. Considering the critical requirement for ERT in patients with such enzyme deficiencies, immune tolerance induction should be advocated in the patients with allo-immune MN.
In 2002, we described allo-immune membranous nephropathy (MN) in a neonate born to a mother genetically deficient in neutral endopeptidase.1.2 Allo-immunization may also occur when neoantigens are presented by the grafted kidney. Next to histocompatibility antigens, potential targets include antigens genetically absent in the native kidney, such as the α3/4/5 trimer of collagen type IV in Alport syndrome3 and nephrin in severe congenital Finnish syndrome.4 A third category of allo-immune reactions occurs during enzyme replacement therapy (ERT).5–7 Because of the absence or very low levels of enzyme in many patients, therapeutic proteins are potential allo-antigens that commonly trigger immunization. Allo-antibodies may be without clinical significance or lead to hypersensitivity reactions, decreased bioavailability, and reduced efficacy of the therapeutic proteins. A single case of nephrotic syndrome with mesangial and subepithelial Ig deposits was reported in a highly-sensitized patient with Pompe disease,8 but the prevalence of ERT-induced renal complications is probably underestimated.
Here, we report the case of a boy age 5.5 years born to consanguineous parents who was diagnosed at birth with mucopolysaccharidosis type VI (MPS VI), or Maroteaux–Lamy syndrome, and developed an allo-immune MN in the setting of ERT. MPS VI is an autosomal recessive lysosomal storage disorder caused by mutations in the ARSB gene encoding the aryl sulfatase B (ASB) enzyme. It leads to cellular and tissular accumulation of undegraded glycosaminoglycans. If untreated, patients experience progressive physical multiorgan deterioration and premature death without renal involvement.9.10 Our patient had an homozygous ARSB missense mutation c.176A>T (p.Asp59Val) responsible for the absence of ASB protein (not shown). Weekly infusions of 1 mg/kg body wt recombinant human ASB (rhASB), galsulfase (Naglazyme; Biomarin, Novato), was started at the age of 4 years. During the first 1 year of ERT, the child’s general condition and growth markedly improved, upper airway infections decreased, and liver volume normalized. Regular dipstick urine controls did not detect proteinuria.
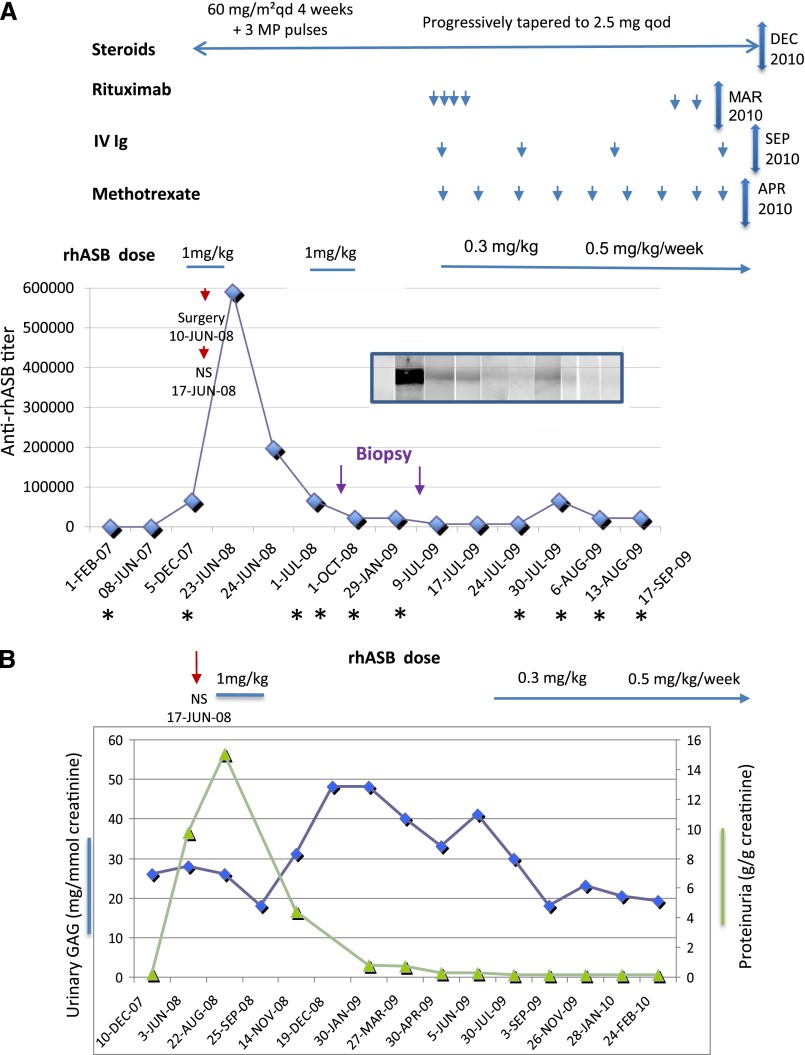
After 18 months of ERT, the child underwent orthopedic surgery for hip dysplasia. Drugs used in the perianesthetic period are shown in Supplemental Table 1. One week later, the patient developed peripheral edema and arterial hypertension (145/95 mmHg). Laboratory investigations showed nephrotic range proteinuria (38.4 g/L; 9.7 g/g creatinine) with hypoalbuminemia (10.8 g/L), microscopic hematuria, normal serum creatinine (0.2 mg/dl; 21 µmol/L), and normal levels of complement component (C3, 1.0 g/L; C4, 0.35 g/L). Anti-nuclear antibodies were absent, and screening for hepatitis B and C and HIV infection was negative. Ultrasound examination of kidney was normal. Anti-rhASB antibodies in patient’s sera were markedly elevated at time of surgery (Figure 1A).
Figure 1.
Beneficial effects of immune tolerance induction therapy. Time course of administration of rhASB, combined therapy, and anti-rhASB IgG antibody titer measured by ELISA and Western blot. (A) Dates (abscissa) when the sera were taken for Western blot analysis are marked with an asterisk. The Western blot is shown on the graph. IV, intravenous; NS, nephrotic syndrome; qd, every day; qod, every other day. (B) Time course of proteinuria and urinary glucosaminoglycan (GAG) excretion.
ERT was suspended for 2 weeks and then resumed at the same dose. Despite prednisone therapy given for 4 weeks at a dose of 60 mg/m2 per day followed by three methylprednisolone pulses (1 g/1.73 m2), proteinuria persisted (3.7 g/L; 15 g/g creatinine) and a kidney biopsy was performed.
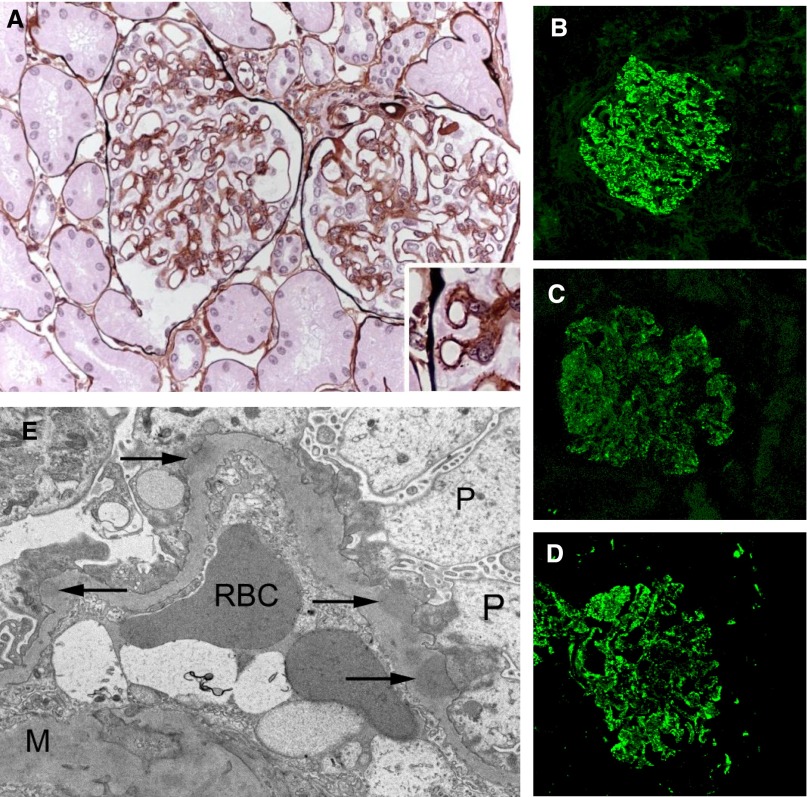
Light microscopy showed thickened glomerular basement membranes without cell proliferation, interstitium infiltration, and vascular lesion (Figure 2A, Supplemental Figure 1). Immunofluorescence examination revealed granular subepithelial deposits of IgG, C3, and C5b-9 (Figure 2, B–D), but not C1q (not shown). Electron microscopy confirmed the presence of subepithelial electron-dense deposits associated with foot process effacement without mesangial deposits (Figure 2E).
Figure 2.
Characteristics of membranous nephropathy. Light microscopy and immunofluorescence study of specimens of the first kidney biopsy (August 2008). (A) Glomerular basement membranes have diffuse spikes and clubber aspects (Jone’s staining, ×400). (B–D) Glomerulus with diffuse, finely granular deposition of (B) IgG, (C) C3, and (D) C5b-9 along the outer surface of all capillary walls. (E) Electron microscopy showing subepithelial electron dense deposits (×10,000). M, mesangium; P, podocyte; RBC, red blood cell. Original magnification, ×400 in A–D; ×10,000 in E.
One week after the kidney biopsy, prednisone was progressively tapered to 2.5 mg four times per day. Given the possible implication of rhASB, ERT was discontinued 1 month later. Anti-rhASB antibody titer dramatically decreased after stopping galsulfase (Figure 1A). During the next 6 months, proteinuria progressively decreased to 0.57 g/L (0.8 g/g creatinine) (Figure 1B).
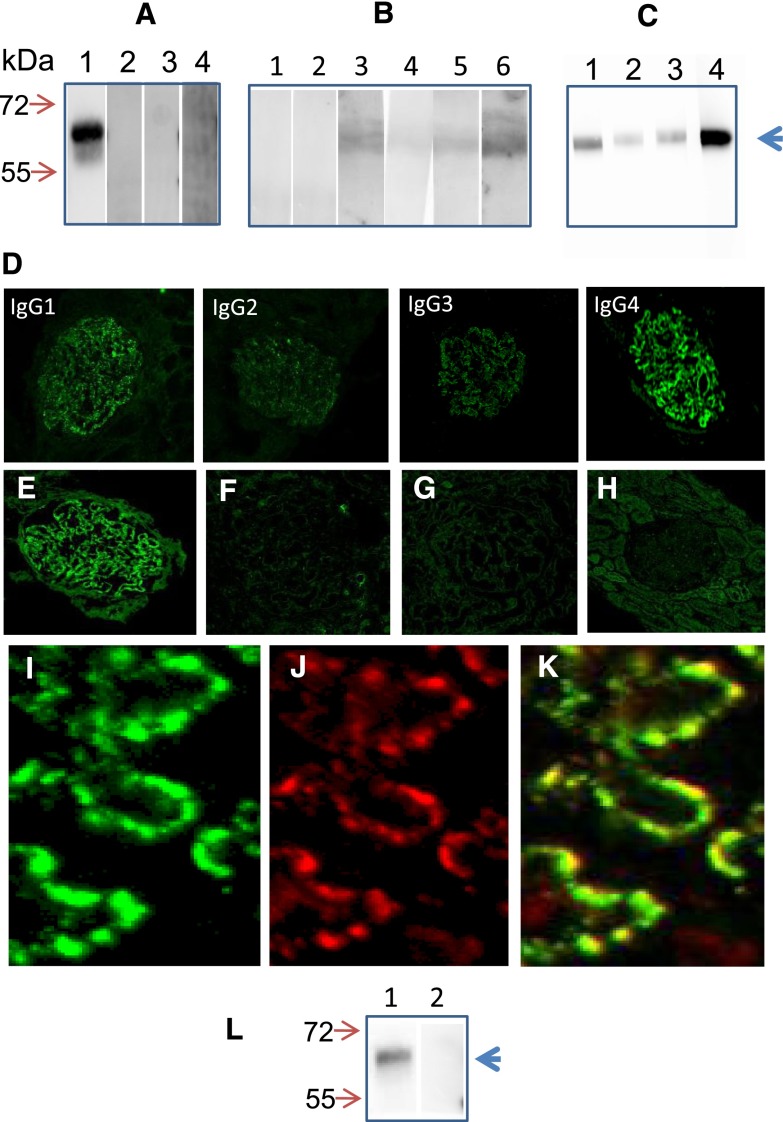
In the meantime, we showed a strong reactivity of the patient’s serum by Western blot with rhASB at the expected molecular mass of 60 kDa (Figure 3A). No reactive band appeared when rhASB was incubated with control sera, including sera from patients with idiopathic MN and IgA nephropathy as well as healthy controls (Figure 3A). Other patients receiving galsulfase produced anti-rhASB antibodies, albeit at a much lower titer (Figure 3B). Anti-rhASB antibodies belonged to all Ig subclasses, although the highest reactivity was observed with IgG4 (Figure 3C).The reactivity profile observed in the patient’s serum matched the distribution of IgG subclasses in the subepithelial deposits in the patient’s biopsy specimen (Figure 3D). The patient’s serum did not cross-react with glomerular proteins lysates (Supplemental Figure 3) and PLA2R as tested by immunofluorescence test (data not shown). We could not detect circulating immune complexes by C1q and Raji enzymatic immunoassay (Supplemental Table 2).
Figure 3.
Patient has anti-rhASB antibodies in serum and rhASB and anti-rhASB in glomerular immune deposits. (A) Equal amounts of rhASB were resolved by SDS-PAGE and incubated with (lane 1) patient’s serum, (lane 2) normal serum, and sera from patients with (lane 3) idiopathic MN and (lane 4) IgA nephropathy. (B) Other patients on rhASB enzymotherapy had low titer of anti-rhASB antibodies or were negative. (C) Distribution of rhASB-specific IgG subclasses in the patient’s serum. (D) Staining of the second kidney biopsy (March 2009) with anti-IgG subclass antibodies. (E–K) Confocal images of the second kidney biopsy specimen. (E) Anti-rhASB antibody revealed granular staining along capillary loops only in our patient. F shows an adjacent section, in which the anti-rhASB antiserum was preincubated with 20 µg rhASB protein. (G) Patient with idiopathic MN. (H) PLA2R is not present in subepithelial deposits in our patient. I, J, and K show confocal images of cryosections of the patient’s second kidney biopsy specimen, which have been double-labeled with (I) rabbit polyclonal anti-rhASB antibody (green) and (J) anti-human IgG antibodies (red). L and K show the merged image. Original magnification, ×400 in D–H; ×1200 in I, J, and K. (L) IgG was eluted from (lane 1) the patient’s second kidney biopsy and (lane 2) a patient with MN. Only IgG eluted from the patient’s biopsy identified rhASB.
We then searched for the presence of rhASB in glomeruli. By using a specific rabbit anti-rhASB antibody, we found a granular pattern of fluorescence along the capillary wall in the patient’s biopsy specimen (Figure 3E). Specificity of the binding of rhASB antibody was confirmed by extinction of fluorescence when the antibody was preincubated with an excess of rhASB (Figure 3F). Normal human kidney sections and biopsies from patients with other types of glomerulopathies, including idiopathic MN, showed a negative staining (Figure 3G, Supplemental Figure 2). No PLA2R antigen could be detected in deposits (Figure 3H). Colocalization of rhASB and IgG in the immune deposits was established by confocal microscopy. Many areas of colocalization were seen as the yellow staining in the merge image, whereas other areas of the glomerular capillary wall mostly featured the green staining of rhASB (Figure 3, I–K). Finally, to confirm that the IgG deposited in the glomeruli was reactive with rhASB, we eluted IgG from the biopsy specimens of our patient and a patient with idiopathic MN. Reactivity of IgG was analyzed by Western blotting with rhASB. Only IgG eluted from the biopsy specimen of our patient reacted with rhASB (Figure 3L).
As the patient’s clinical condition deteriorated, with a marked increase in urinary glucosaminoglycans (Figure 1B), we started a treatment with low-dose prednisone, four weekly rituximab infusions (375 mg/m2), intravenous Igs (0.5 g/kg per month), and oral methotrexate (0.5 mg/kg per week) to induce tolerance to rhASB (Figure 1A). Galsulfase was restarted at a lower dose (0.3 mg/kg per day) after a washout period of 9.5 months (8 weeks after starting high-dose immunosuppressive therapy). When ERT was resumed, CD19 and CD20 were undetectable, proteinuria and microalbuminuria were in the normal range, and anti-rhASB antibody was below detection threshold. Galsulfase dose was increased to 0.5 mg/kg 3 months later. The patient’s clinical condition markedly improved, with rapid decrease in liver and spleen volume and less frequent upper airway infectious episodes. He remained free from proteinuria 14 months after discontinuation of the immunosuppressive treatment and only developed a transient small rise of anti-rhASB titer together with reappearance of CD19-positive cells, which were controlled with rituximab.
He died at age 9 years and 2 months after severe anoxia secondary to an acute laryngospasm during anesthesia induction for cervical spine decompression, a common neurosurgical complication of MPS VI.
This report is the first report of MN in a patient with MPS VI treated with human rhASB. The finding of high titers of circulating anti-rhASB antibodies, which peaked at the onset of the nephrotic syndrome, indicates a mechanism of allo-immunization against ASB in a patient who had no measurable enzyme activity and no detectable anti-PLA2R or antiglomerular antibody. The clinical circumstances in this case (particularly, the resolution of proteinuria when ERT was suspended), the colocalization of rhASB antigen and Ig within immune deposits, and the finding that IgG eluted from the biopsy specimen reacted specifically with rhASB strongly suggest that the allo-immune response to the recombinant enzyme is the cause of the disease. This case, thus, adds a new cause to the list of MN etiologies. Considering the critical requirement for ERT in patients with such enzyme deficiencies, it also shows that intensive immunosuppressive therapy can allow reintroduction of ERT, resulting in a dramatic improvement of the patient’s condition.
Another case of ERT-induced nephrotic syndrome was previously reported in a patient with Pompe disease treated with recombinant human α-glucosidase (rhGAA).8 However, both the setting and glomerular lesions were different. First, the nephrotic syndrome occurred during an experimental immune tolerance regimen based on escalating doses of rhGAA, whereas our patient was receiving recommended stable doses of rhARB. Second, subepithelial immune deposits were associated with mesangium expansion, numerous mesangial deposits by immunofluorescence and electron microscopy, and presence of rhGAA antigen in the mesangium. These findings recapitulate the immune complex GN observed in early chronic serum sickness induced by repeated injections of exogenous protein.11 The nephrotic syndrome resolved after enzymotherapy was decreased. Our patient showed a typical MN without mesangium involvement.
Our case leads to discussion of the mechanisms of subepithelial immune deposit formation and the reason why the nephrotic syndrome suddenly appeared 1 week after surgery. There are three possible, nonmutually exclusive mechanisms of the formation of subepithelial deposits in experimental models of and patients with MN.12.13 The first mechanism is the deposition of immune complexes from the circulation. We could not find such complexes using two different methods, although we cannot exclude the presence of low levels of small-size IgG4–containing immune complexes, which are not detected by usual methods. The second mechanism involves in situ formation of immune complexes through the reaction of circulating autoantibody to a native podocyte antigen, such as PLA2R.14 We could not detect a specific reactivity with membrane glomerular antigens or PLA2R antigen in subepithelial immune deposits. The third mechanism also involves the in situ formation of immune complexes but with a nonnative (extrinsic) antigen bound to the capillary wall.15
Why did our patient develop a nephrotic syndrome, whereas other patients receiving galsulfatase did not, although anti-rhASB antibodies that do not seem to affect urinary glucosaminoglycan levels, efficacy, or safety are detected in most of them?16.17 First, our patient produced a relatively high level of anti-rhASB antibodies. Second, he underwent surgery requiring a cocktail of anesthetic drugs, which were shown to increase glomerular permeability to proteins and alter podocyte function.18 Third, sevoflurane, which was used in our patient, can affect the conductance of Ca2+-activated K+ channel expressed on podocytes.19.20 Because of the rapid onset of nephrotic syndrome only 1 week after surgery in a patient with regular negative controls of proteinuria, we suggest that anesthetics might have been a triggering factor.
A limitation to ERT is the production of antienzyme allo-antibodies that are reported in all lysosomal storage disorders,16.17.21.22 which may compromise the efficacy of treatment. We identified a mutation in ARSB responsible for the absence of protein and enzymatic activity, a situation where a high rate of allo-immune response is expected.23 For patients with antibody-mediated severe adverse effects, it is of paramount importance to develop tolerance-inducing protocols aimed to reintroduce ERT. We used a combination of high-dose corticosteroids, rituximab, intravenous Ig, and methotrexate, which induced operational tolerance to rhASB and enabled us to resume rhASB treatment with dramatic improvement of the patient’s condition and without rebound of antibody response and relapse of renal manifestations.
Concise Methods
Analysis of Kidney Biopsy Specimen
The patient’s biopsy specimen was prepared for light, immunofluorescence, and electron microscopy using standard techniques.24 We analyzed cryosections from the patient’s biopsy specimen as well as from patients with MN (10 cases), lupus MN (3 cases), membranoproliferative GN (1 case), IgA nephropathy (2 cases), and normal kidney. For detection of IgG subclasses and complement components, cryosections of the biopsy specimen were incubated with the following antibodies: mouse monoclonal anti-human IgG1, IgG2, IgG3, and IgG4 antibodies (commercially provided by Margaret Goodall, University of Birmingham, Birmingham, UK), anti-human C3 complement (Dako), and monoclonal anti-human C5b-9 (Dako).
Analysis of the Composition of Glomerular Immune Deposits by Confocal Microscopy
Cryosections of the patient’s biopsy specimen were first incubated with rabbit polyclonal anti-rhASB antibodies (Biomarin) and then goat Alexa488-conjugated anti-rabbit Fab IgG antibodies and goat Alexa 568–conjugated anti-human IgG (Molecular Probes). After being washed, sections were examined under a confocal microscope (TCS-SP2; Leica) and analyzed with Leica Confocal Software, version 2.61.
Western Blots and Detection of Circulating Immune Complexes
rhASB and glomerular extracts were electrophoresed under nonreducing conditions and transferred to poly(vinylidene difluoride) membranes according to standard protocols. Detection antibodies were peroxidase-conjugated goat anti-human antibodies (Chemicon). Immunoreactive proteins were visualized with SuperSignal West Pico Chemiluminescent substrate (Pierce). IgG subclasses were identified with mouse monoclonal anti-human IgG1, IgG2, IgG3, and IgG4 antibodies (commercially provided by Margaret Goodall, University of Birmingham, Birmingham, UK) followed by peroxidase-conjugated sheep anti-mouse IgG (GE Healthcare). Glomeruli were isolated from kidneys that were unsuitable for transplantation with the use of graded sieving, and glomerular proteins were extracted with RIPA buffer (Pierce). Contaminating IgG was removed through incubation with Immobilized Protein G Plus (Fisher Scientific). Circulating immune complexes containing C1q or C3d were detected with the use of ELISA kits (Quidel).
Elution of IgG
Igs were acid-eluted from the cores of kidney biopsy specimens obtained from our patient and a patient with idiopathic MN. The eluted IgG was used to immunoblot the rhASB directly.
Assessment of Anti-rhASB Antibody
Anti-rhASB antibodies in patient’s sera were tested in the Biomarin Laboratory using an in-house routine test of ELISA (noncommercial assay).
Disclosures
None.
Supplementary Material
Acknowledgments
We are grateful to Adrian Quartel (Biomarin, Novato) for helpful discussion and financial support.
Research is supported by European Research Council Grant ERC-2012-ADG_20120314 (Grant Agreement 322947), Agence Nationale pour la Recherche Programme Blanc SVSE1 (2012) Decision ANR-12-BSE1-0002-01, Fondation pour la Recherche Médicale Equipe FRM 2012 grant, and 7th Framework Programme of the European Community Contract 2012-305608 (European Consortium for High-Throughput Research in Rare Kidney Diseases).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013030290/-/DCSupplemental.
References
- 1.Debiec H, Guigonis V, Mougenot B, Decobert F, Haymann JP, Bensman A, Deschênes G, Ronco PM: Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. N Engl J Med 346: 2053–2060, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Debiec H, Nauta J, Coulet F, van der Burg M, Guigonis V, Schurmans T, de Heer E, Soubrier F, Janssen F, Ronco P: Role of truncating mutations in MME gene in fetomaternal alloimmunisation and antenatal glomerulopathies. Lancet 364: 1252–1259, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Hudson BG: The molecular basis of Goodpasture and Alport syndromes: Beacons for the discovery of the collagen IV family. J Am Soc Nephrol 15: 2514–2527, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Patrakka J, Ruotsalainen V, Reponen P, Qvist E, Laine J, Holmberg C, Tryggvason K, Jalanko H: Recurrence of nephrotic syndrome in kidney grafts of patients with congenital nephrotic syndrome of the Finnish type: Role of nephrin. Transplantation 73: 394–403, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Richards SM: Immunologic considerations for enzyme replacement therapy in the treatment of lysosomal storage disorders. Clin Appl Immunol Rev 2: 241–253, 2002 [Google Scholar]
- 6.Brooks DA: Immune response to enzyme replacement therapy in lysosomal storage disorder patients and animal models. Mol Genet Metab 68: 268–275, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Koren E, Zuckerman LA, Mire-Sluis AR: Immune responses to therapeutic proteins in humans—clinical significance, assessment and prediction. Curr Pharm Biotechnol 3: 349–360, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Hunley TE, Corzo D, Dudek M, Kishnani P, Amalfitano A, Chen YT, Richards SM, Phillips JA, 3rd, Fogo AB, Tiller GE: Nephrotic syndrome complicating alpha-glucosidase replacement therapy for Pompe disease. Pediatrics 114: e532–e535, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Winchester B, Vellodi A, Young E: The molecular basis of lysosomal storage diseases and their treatment. Biochem Soc Trans 28: 150–154, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Valayannopoulos V, Nicely H, Harmatz P, Turbeville S: Mucopolysaccharidosis VI. Orphanet J Rare Dis 5: 5, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon FJ, Feldman JD, Vazquez JJ: Experimental glomerulonephritis. The pathogenesis of a laboratory model resembling the spectrum of human glomerulonephritis. J Exp Med 113: 899–920, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glassock RJ: Human idiopathic membranous nephropathy—a mystery solved? N Engl J Med 361: 81–83, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Ronco P, Debiec H: Pathogenesis of membranous nephropathy: Recent advances and future challenges. Nat Rev Nephrol 8: 203–213, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Beck LH, Jr., Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debiec H, Lefeu F, Kemper MJ, Niaudet P, Deschênes G, Remuzzi G, Ulinski T, Ronco P: Early-childhood membranous nephropathy due to cationic bovine serum albumin. N Engl J Med 364: 2101–2110, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Harmatz P, Giugliani R, Schwartz I, Guffon N, Teles EL, Miranda MC, Wraith JE, Beck M, Arash L, Scarpa M, Yu ZF, Wittes J, Berger KI, Newman MS, Lowe AM, Kakkis E, Swiedler SJ, MPS VI Phase 3 Study Group : Enzyme replacement therapy for mucopolysaccharidosis VI: A phase 3, randomized, double-blind, placebo-controlled, multinational study of recombinant human N-acetylgalactosamine 4-sulfatase (recombinant human arylsulfatase B or rhASB) and follow-on, open-label extension study. J Pediatr 148: 533–539, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Hendriksz CJ, Giugliani R, Harmatz P, Lampe C, Martins AM, Pastores GM, Steiner RD, Leão Teles E, Valayannopoulos V, CSP Study Group : Design, baseline characteristics, and early findings of the MPS VI (mucopolysaccharidosis VI) Clinical Surveillance Program (CSP). J Inherit Metab Dis 36: 373–384, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Giardino L, Armelloni S, Corbelli A, Mattinzoli D, Zennaro C, Guerrot D, Tourrel F, Ikehata M, Li M, Berra S, Carraro M, Messa P, Rastaldi MP: Podocyte glutamatergic signaling contributes to the function of the glomerular filtration barrier. J Am Soc Nephrol 20: 1929–1940, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Namba T, Ishii TM, Ikeda M, Hisano T, Itoh T, Hirota K, Adelman JP, Fukuda K: Inhibition of the human intermediate conductance Ca(2+)-activated K(+) channel, hIK1, by volatile anesthetics. Eur J Pharmacol 395: 95–101, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Morton MJ, Hutchinson K, Mathieson PW, Witherden IR, Saleem MA, Hunter M: Human podocytes possess a stretch-sensitive, Ca2+-activated K+ channel: Potential implications for the control of glomerular filtration. J Am Soc Nephrol 15: 2981–2987, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Kishnani PS, Nicolino M, Voit T, Rogers RC, Tsai AC, Waterson J, Herman GE, Amalfitano A, Thurberg BL, Richards S, Davison M, Corzo D, Chen YT: Chinese hamster ovary cell-derived recombinant human acid alpha-glucosidase in infantile-onset Pompe disease. J Pediatr 149: 89–97, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberg M, Kingma W, Fitzpatrick MA, Richards SM: Immunosurveillance of alglucerase enzyme therapy for Gaucher patients: Induction of humoral tolerance in seroconverted patients after repeat administration. Blood 93: 2081–2088, 1999 [PubMed] [Google Scholar]
- 23.Wang J, Lozier J, Johnson G, Kirshner S, Verthelyi D, Pariser A, Shores E, Rosenberg A: Neutralizing antibodies to therapeutic enzymes: Considerations for testing, prevention and treatment. Nat Biotechnol 26: 901–908, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Debiec H, Hanoy M, Francois A, Guerrot D, Ferlicot S, Johanet C, Aucouturier P, Godin M, Ronco P: Recurrent membranous nephropathy in an allograft caused by IgG3κ targeting the PLA2 receptor. J Am Soc Nephrol 23: 1949–1954, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.