Abstract
We previously reported life-supporting α1,3-galactosyltransferase knockout (GalTKO) thymokidney xenograft survival of >2 months in baboons. However, despite otherwise normal renal function, recipients developed proteinuria with morphologic changes (podocyte effacement), a condition that presents a major obstacle to long-term studies in this model. A recent clinical study showed that rituximab therapy after allogeneic transplant prevented proteinuria possibly associated with loss of sphingomyelin phosphodiesterase acid–like 3b (SMPDL-3b). Here, we demonstrate that rituximab prevents the disruption of pig podocytes in an SMPDL-3b–dependent manner in vitro and the early development of proteinuria after xenogeneic kidney transplantation in baboons. Immunofluorescence showed SMPDL-3b expression in pig glomerular epithelium; immunoprecipitation demonstrated rituximab binding to SMPDL-3b in glomeruli. Culture of isolated pig podocytes with naive baboon sera, which has preformed antipig natural antibodies, reduced SMPDL-3b expression, disrupted podocyte morphology, and decreased podocyte proliferation, whereas pretreatment with rituximab prevented these effects. Six baboons received rituximab before transplantation to deplete B cells and again in the peri-transplant period; 18 baboons treated only before transplantation served as historical controls. The onset of post-transplant proteinuria was significantly delayed in a B cell–independent manner in the animals that received peri-transplant rituximab treatment. Although further optimization of this protocol is required, these data provide intriguing clues to the mechanisms of post-transplant proteinuria in xenogeneic kidney transplantation and a potential strategy for its prevention.
With the advent of α1,3-galactosyltransferase (GalT) knockout donor animals (GalTKO),1–5 xenograft survival in our pig-to-baboon kidney transplant model was extended to an average of >50 days.6 Unfortunately, despite the achievement of immunologic hyporesponsiveness to pig antigens through a tolerance-inducing strategy using “thymokidney” grafts,6 most of the recipients developed severe post-transplant proteinuria. Histologic studies showed slight glomerulopathy in the kidney grafts; electron microscopic examination indicated effacement of the foot processes of the podocytes even though the creatinine level was stable.7 Similar to clinical situations in which proteinuria leads to a higher risk of death and cardiovascular disease events and is considered to be a predictor of graft loss,8–11 in the xenogeneic model it is associated with post-transplant hypoproteinemia, resulting in severe ascites, pleural effusion, and increased susceptibility to infection. Thus, studies to determine the mechanism responsible for this phenomenon and the development of preventive strategies are crucial to the success of future xeno-transplantation studies.
Recent reports have indicated that the loss of sphingomyelin phosphodiesterase acid–like 3b (SMPDL-3b) in allogeneic human kidney grafts was related to the development of post-transplant proteinuria in FSGS.12 Rituximab, a chimeric antihuman CD20 monoclonal antibody, unexpectedly cross-reacted with this protein, and rituximab treatment prevented the degradation of SMPDL-3b and thereby averted the development of post-transplant proteinuria in patients with FSGS.12,13 In this study, we examined whether rituximab inhibited the in vitro disruption of pig podocytes in an SMPDL-3b–dependent manner and evaluated its in vivo effect on proteinuria following preclinical pig-to-baboon xenogeneic GalTKO kindey transplant. We confirmed that (1) SMPDL-3b was expressed on pig kidneys; (2) rituximab bound to pig SMPDL-3b; (3) in vitro treatment of GalTKO pig podocytes with rituximab mitigated the damage caused by baboon anti-pig serum antibodies; and (4) rituximab administration in the peri-transplant period in vivo resulted in a marked delay in the development of proteinuria. These data indicate that rituximab impeded pig podocyte disruption in an SMPDL-3b–dependent manner and consequently delayed the development of proteinuria following xenogeneic GalTKO kidney transplant in nonhuman primates.
Results
Rituximab Bound to SMPDL-3b, Which Was Expressed on Epithelial Cells in the Pig Kidney
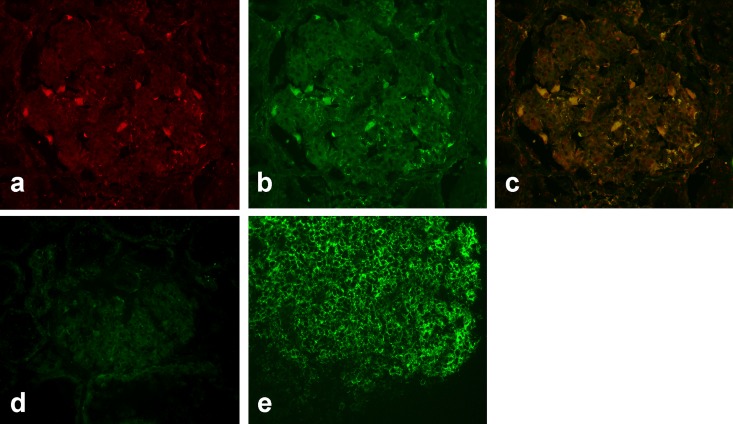
The presence of SMPDL-3b was determined by immunofluorescence microscopy with polyclonal anti–SMPDL-3b antibody. As shown in Figure 1A, SMPDL-3b was expressed on the epithelial cells in the naive pig kidney. Interestingly, rituximab staining of the same section (Figure 1B) clearly indicated that the binding site of this drug colocalized with SMPDL-3b (Figure 1C). The observation that a different anti-human CD20 monoclonal antibody (clone 2H7) failed to stain pig kidney epithelium (Figure 1, D and E) suggested that rituximab binds to a unique epitope on pig epithelial cells that is unrelated to CD20.
Figure 1.
Rituximab binds to the naive pig kidney. Binding of (A) anti–SMPDL-3b antibody and (B) rituximab to epithelial cells in a naive pig kidney. (C) Merged image of A and B. (D) Staining of a naive pig kidney and (E) naive baboon spleen with anti-human CD20 antibody.
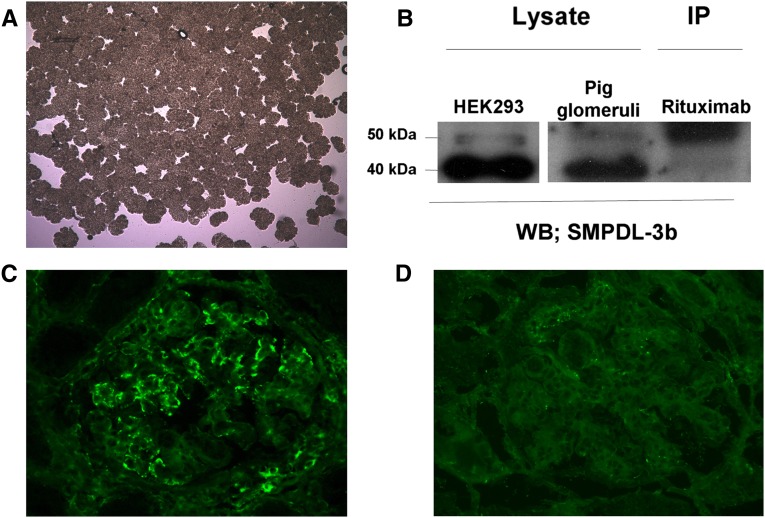
Glomeruli were isolated from naive pig kidneys by a sieving method with >95% purity (Figure 2A). Western blots of solubilized glomeruli were probed with polyclonal anti–SMPDL-3b antibody and proteins of 40 and 50 kD were detected; the same proteins were also detected in human embryonic kidney (HEK) 293 cells (Figure 2B). Lysed pig glomeruli were immunoprecipitated with rituximab, transferred to a Western blot, and probed with polyclonal anti–SMPDL-3b antibody (Figure 2B). Similar to results that were previously reported,12 immunoprecipitation with rituximab resulted in a major band of 50 kD, suggesting that it binds to SMPDL-3b in naive pig glomeruli. A blocking assay was performed in which kidney tissue sections were preincubated with anti–SMPDL-3b antibody (Figure 2D) and subsequent binding of rituximab was inhibited compared with a section that was not pretreated with anti–SMPDL-3b antibody (Figure 2C).
Figure 2.
Rituximab binds to SMPDL-3b in the naive pig kidney. (A) Isolation of pig glomeruli by conventional sieving method. (B) Western blot (WB) with anti–SMPDL-3b antibody of HEK293 cells (left lane) and naive pig glomeruli (middle lane). Western blot with anti–SMPDL-3b antibody of immunoprecipitation (IP) with rituximab (right lane). (C) Binding of rituximab to sections of a naive pig kidney and (D) following preincubation of anti–SMPDL-3b antibody.
Rituximab Prevented Podocyte Damage Induced by Baboon Serum Antibodies
Development and Characterization of the Pig Podocyte Culture
A technique for the culture of pig podocytes was developed in our laboratory. Before exposure to trypsin, the cells were confirmed to be podocytes on the basis of staining with anti-nephrin and anti-vimentin antibodies (Supplemental Figure 1C, left).14 After passaging in culture, the cells retained the characteristics of podocytes as demonstrated by staining with anti-nephrin and anti-podocin antibodies (Supplemental Figure 1D). The specificity of both antibodies was confirmed by immunofluorescence microscopy (Supplemental Figure 1A) and Western blotting (Supplemental Figure 1B). SMPDL-3b was expressed on the cultured podocytes (Supplemental Figure 1C, right).
Rituximab Protected Pig Podocytes from Damage Caused by Baboon Serum
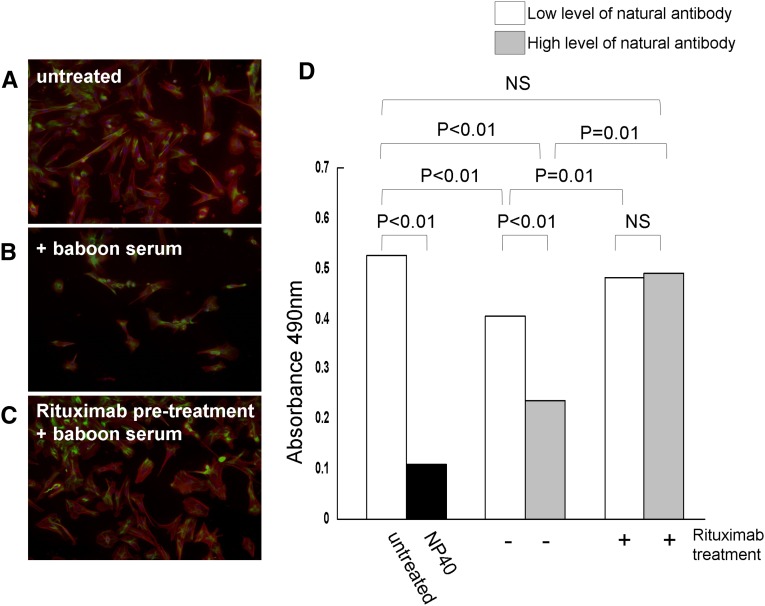
When pig podocytes were cultured overnight in the presence of 4% naive baboon serum (n=4), a marked morphologic disruption was detected by immunofluorescence microscopy with anti-nephrin and anti-vimentin antibodies (Figure 3, A and B). Pretreatment with rituximab prevented the disruption induced by the naive baboon sera (Figure 3C). We also tested the effect of naive baboon sera on the viability of podocytes using a cell proliferation assay. Sera from baboons with both low and high levels of preformed natural antibodies were used in this assay (Figure 3D). Podocytes incubated with the baboon sera had significantly lower rates of proliferation compared with untreated cells, and the extent of inhibition was proportional to level of natural antibody. Pretreatment of the podocytes with rituximab significantly increased the proliferation of the cells in the presence of both baboon sera, indicating that the drug had a protective effect.
Figure 3.
Rituximab therapy prevents podocyte injuries caused by the naive baboon sera. Immunofluorescence microscopy of antinephrin (red) and antivimentin (green) staining in (A) untreated podocyte cells, (B) cells incubated with 4% baboon serum, and (C) cells pretreated with rituximab and then incubated with 4% baboon serum. (D) Viability of the podocytes was assessed by a cell proliferation assay. Before the proliferation assay, cells were incubated with baboon serum containing low (white bars) or high (gray bars) levels of natural antibodies with (+) or without (−) rituximab pretreatment. The untreated cells were included, along with Nonidet P-40 (NP40)–treated cells.
Rituximab Prevented the Loss of SMPDL-3b by Pig Podocytes following Incubation with Baboon Serum
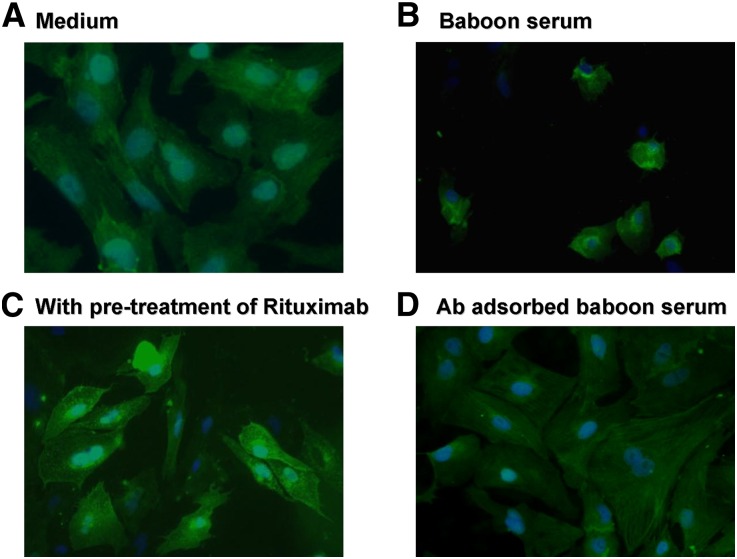
The amount of SMPDL-3b present was assessed by immunofluorescence staining of podocytes that were exposed to naive baboon serum. Following incubation with baboon serum (Figure 4B), the level of SMPDL-3b detected was substantially reduced relative to the normal control (Figure 4A). Preincubation of the podocytes with rituximab inhibited the loss of SMPDL-3b subsequent to baboon serum exposure (Figure 4C).
Figure 4.
Expression of SMPDL-3b was reduced by the naive baboon sera. Immunofluorescence with anti–SMPDL-3b antibody of (A) untreated podocytes, (B) cells treated with baboon serum, (C) cells pretreated with rituximab before incubation with baboon serum, and (D) cells treated with baboon serum after adsorption of preformed natural antibodies (Ab).
Preformed Natural Antibody Damaged the Pig Podocytes
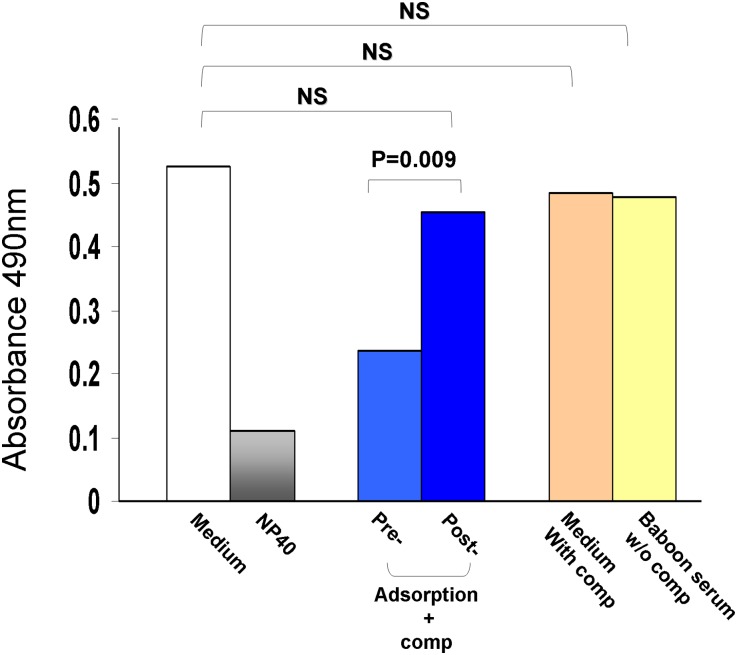
To test whether preformed natural antibodies were responsible for the podocyte injuries, naive baboon serum was incubated with GalTKO pig peripheral blood mononuclear cells (PBMCs) to adsorb pig reactive antibodies. FACS was performed with GalTKO PBMCs to confirm that the preformed natural antibodies were completely depleted from the naive baboon serum. Pig podocytes were incubated with normal or adsorbed baboon serum, and the viability was evaluated by proliferation assay. The viability of pig podocytes incubated with adsorbed serum was significantly higher than with untreated serum (Figure 5). To determine whether other soluble factors were involved in the damage, we performed the same assays using podocytes and serum in the absence of complement and podocytes with complement only. As shown in Figure 5, pig podocytes were killed only in the presence of the preformed natural antibodies with complement. Podocytes with baboon serum alone or complement alone had no killing, similar to the medium alone control. When the adsorbed serum was added in the cultured podocytes, SMPDL-3b expression was similar to that observed in control cells exposed to medium only (Figure 4D). These data provided further evidence that preformed natural antibodies were responsible for the loss of SMPDL-3b on the pig podocytes and subsequent cell death.
Figure 5.
Preformed natural antibody damaged the podocytes. Proliferation of podocytes that were incubated with baboon serum before (pre) or after (post) adsorption on GalTKO PBMCs. Controls included medium with rabbit complement (comp) only (orange bar), baboon serum (preabsorption) without rabbit complement (yellow bar), untreated cells (medium only, white bar), and Nonidet P-40 (NP40)–treated podocytes (gray bar).
Effects of Rituximab Therapy in Peri-Transplant Periods on Development of Proteinuria after GalT-KO Thymokidney Transplant in Baboons
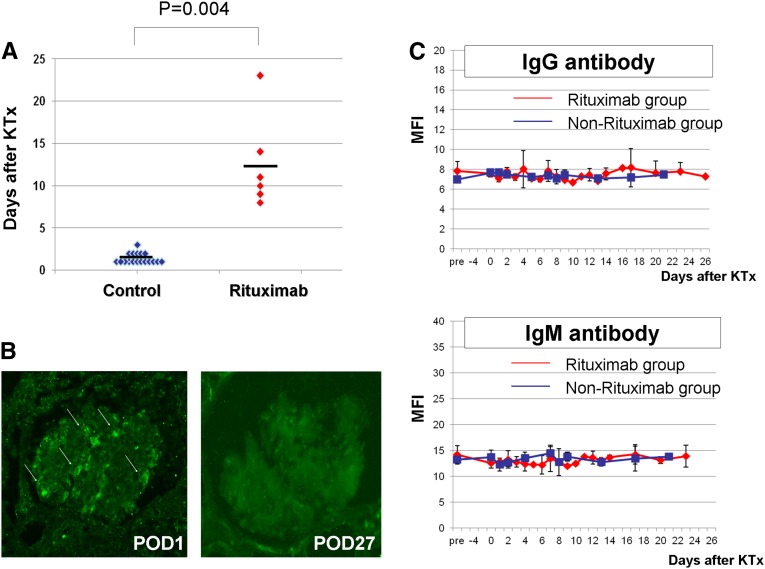
Rituximab was administered to six baboons in the peri-transplant period (treated group). Eighteen baboons that underwent GalTKO thymokidney transplant but did not receive rituximab in the peri-transplant period were retrospectively analyzed (control group). Table 1 summarizes the characteristics of both groups, as well as the effect of rituximab on post-transplant proteinuria in these recipients. All animals in both groups received rituximab on postoperative day (POD)-14 to deplete CD20+ B cells. Peripheral B cell counts during the pre- and peri-transplant periods, as well as after kidney transplant, did not significantly differ between the two groups. The anti–non-Gal antibody levels at the time of transplantation were similar in both groups (Table 1). The levels of antipig antibodies showed a similar time course in both groups (Figure 6C). However, the onset of 2+ post-transplant proteinuria was markedly delayed in the treated group compared with the control group (P=0.004) (Table 1, Figure 6A). Most of the baboons in the control group developed >2+ proteinuria within 2 days after transplant (1.39±0.61 days), while the average time of onset of 2+ proteinuria was >12.50±5.54 days in the treated group. One of the baboons in the treated group maintained trace or 1+ proteinuria until POD-22. We found that 3+ proteinuria was also delayed in the treated group (P=0.12). In addition, we assessed the urine protein-to-creatinine ratios in these two groups; in the untreated group (red lines in Supplemental Figure 2) the ratios were >5 within 7 days and then later exceeded 10, while in the treated group (black lines), all of the animals had ratios <1 in the first week (Supplemental Figure 2).
Table 1.
Clinical summary
| Variable | Control (n=18) | Treated (n=6) | P Value |
|---|---|---|---|
| Pretreatment B cell counts (cells/μl) | 1066.4±637.8 | 1353.0±1057.9 | 0.55 |
| B cell counts in peri-transplant period (cells/μl) | 0.67±1.30 | 0.03±0.08 | 0.06 |
| Anti–non-Gal antibody levels before transplant (MFI) | |||
| IgG | 7.38±0.65 | 7.97±1.02 | 0.32 |
| IgM | 13.32±1.16 | 14.17±2.06 | 0.50 |
| Onset of proteinuria after transplant (d) | |||
| 2+ proteinuriaa | 1.39±0.61 | >12.50±5.54 | 0.004 |
| 3+ proteinuriab | 8.25±5.69 | 19.75±10.87 | 0.12 |
Control animals did not receive rituximab in peri-transplant period. Treated animals received rituximab in peri-transplant period to prevent post-transplant proteinuria. The data are shown as mean±SD. MFI, mean fluorescence intensity by FACS
Estimated protein concentration is 100 mg/dl.
Estimated protein concentration is 500 mg/dl.
Figure 6.
Onset of post-transplant proteinuria, expression of SMPDL-3b in the xeno kidney grafts, and levels of anti-pig IgG and IgM. (A) Onset of 2+ proteinuria in animals treated with rituximab after xeno-kidney transplantation (KTx; red dots). (B) SMPDL-3b expression in kidney graft biopsies on POD-1 (left) and POD-27 (right). (C) Post-transplant time course of anti-pig IgG and IgM as detected by flow cytometry (upper, IgG; lower, IgM) in the treated (red line) and untreated (blue line) groups. MFI, mean fluorescence intensity.
We assessed the expression of SMPDL-3b in the pig kidney grafts by immunofluorescence microscopy. Recipient B333 died of complications on POD-1 at a time that the animal had trace proteinuria. At this time, the expression of SMPLD-3b was easily detected (Figure 6B, left). SMPDL-3b was not detected in kidney grafts from recipients with >3+ proteinuria (Figure 6B, right).
Discussion
Although one of the major obstacles in xenogeneic kidney transplant is post-transplant proteinuria, the underlying mechanisms of this complication have not been defined. To elucidate this mechanism in xeno-transplantation, we examined the role of SMPDL-3b on post-transplant proteinuria. In our GalTKO kidney transplant model, we found that rituximab treatment prevented in vitro damage to pig podocytes as well as the early development of proteinuria following xenogeneic GalTKO kidney transplant in baboons. Notably, treatment with rituximab correlated inversely with the level of SMPDL-3b expression, suggesting that the drug has a protective effect. To our knowledge, this is the first mechanistic study to examine the effects of rituximab treatment on proteinuria in xeno-transplantation.
Rituximab, which is a chimeric antibody against human CD20, has recently been used to treat lupus nephritis,15 type I membranoproliferative GN,16 idiopathic membranous nephropathy,17 and transplant glomerulopathy.18 In addition, rituximab has been used to prevent the recurrence of FSGS,19 membranous nephropathy,20 and membranoproliferative GN.21 Because antibodies play a key role in the pathogenesis of these diseases, rituximab was used to inhibit humoral immunity by modulating B cell function and suppressing antibody production. Proteinuria improved with rituximab administration,15–18,20,21 but there was no evidence that the drug reduced antibody production. Furthermore, no change was reported in the level of donor-specific antibody and the number of CD20-positive cells in the kidney graft was not related to the improvement of proteinuria. In our model, all of the animals treated with rituximab, both control and experimental animals, showed complete B-cell depletion (<1 B cell/μl) at the time of transplantation. Thus, the B cell–depleting properties of rituximab do not play a role in its ability to prevent proteinuria.
Although the exact mechanism of this fortuitous effect of rituximab has yet to be determined, there are some intriguing clues. The downregulation of SMPDL-3b renders podocytes more susceptible to actin remodeling, which leads to proteinuria,12 while the induction of urokinase plasminogen activator receptor signaling in podocytes results in foot process effacement and urinary protein loss via lipid-dependent activation of avβ3 integrin.22,23 Unfortunately, because of a lack of pig-specific reagents, we have not yet been able to determine whether avβ3 integrin was activated in our model.
The antiproteinuric effects of rituximab were not long-lasting in the current protocol. One dose of rituximab on POD-1–5 was effective for three weeks. Most of the baboons eventually developed 3+ proteinuria, and SMPDL-3b expression was not detectable in those kidney grafts. There are several explanations for this. First, the preformed natural antibodies that are responsible for the proteinuria were still present in the baboons. Circulating preformed natural antibodies could have damaged the podocytes once the rituximab levels fell, such that it was no longer protecting the podocytes. Second, CD80, which is the cause of a nephritic syndrome in minimal-change disease,24 may be involved in development of proteinuria in our model. Our most recent data in collaboration with Dr. Johnson’s and Dr. Rivart’s laboratories suggested that upregulation of CD80 by inflammatory responses after transplantation may cause the development of proteinuria in this model. Further studies are in progress.
Administration of rituximab after the development of 3+ proteinuria was not effective, possibly because of the loss of SMPDL-3b subsequent to podocyte injury. Therefore, we concluded that rituximab should be given in the peri-transplant period before any damage to the pig glomeruli occurs. A study to determine an optimal schedule of repeated doses of rituximab for durable protective effects is currently in progress.
In summary, we demonstrated that rituximab delays the onset of proteinuria in our pig-to-baboon xeno-transplantation model. To our knowledge, this is the first evidence that the prevention of pig podocyte disruption in an SMPDL-3b–dependent manner inhibits the early development of proteinuria following xenogeneic GalTKO kidney transplant in nonhuman primates.
Concise Methods
Animals
Massachusetts General Hospital (MGH) inbred GalTKO miniature swine that were produced as previously described were used for in vitro assay as well as in vivo experiments as donors.5,6 Baboons (Papio hamadryas) were purchased from Mannheimer Foundation (Homestead, FL). MGH Institutional Animal Care and Use Committee guidelines were followed. All animal care was performed in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (publication no. 86–23, revised 1996). The protocol was approved by the MGH subcommittee of research animal care.
Immunofluorescence Microscopy
Frozen tissue sections from GalTKO miniature swine and the baboons were stained with FITC-conjugated anti-human CD20 antibody (BD Pharmingen; 2H7), polyclonal rabbit anti–SMPDL-3b antibody (GeneWay), and rituximab. Tetramethylrhodamine isomer R conjugated anti-rabbit IgG antibody (Dako) and FITC conjugated anti-rabbit antibody (Southern Biotech) were used to detect the anti–SMPDL-3b. Rituximab was visualized with FITC conjugated anti-human IgG antibody (Dako). Goat anti-human nephrin and podocin (Santa Cruz Biotechnology, Inc.) antibodies were used to characterize the podocyte cultures. Both of these antibodies were detected with FITC-conjugated rabbit anti-goat IgG (Vector Laboratories) or Texas Red conjugated donkey anti-goat IgG (Thermo). FITC-conjugated anti-vimentin antibody (Sigma-Aldrich) was used to identify podocytes.
Podocyte Culture
The porcine podocyte culture was developed as follows. Briefly, the kidney harvest was performed from MGH inbred GalTKO miniature swine under general anesthesia. The kidneys were perfused with 50 ml of PBS containing 50 mg of iron powder (Wako). The kidney cortices were dissected and cut into small pieces with a surgical blade in Hanks’ balanced salt solution (Invitrogen). The tissues were digested in collagenase solution containing 1 mg/ml collagenase A (Roche Diagnostics) and 0.2 mg/ml deoxyribonuclease I (Roche Diagnostics) in Hanks’ balanced salt solution at 37°C for 60 minutes with gentle agitation. The collagenase-digested tissues were gently pressed through a 110-μm metal mesh using a flattened pestle. Glomeruli containing iron powder in the cell suspension were gathered by a magnetic particle concentrator (Invitrogen) and washed at least four times with PBS. Finally, collected glomeruli were suspended in suitable amount of PBS and the suspension was poured onto a 70-μm cell strainer (BD Biosciences). The cell strainer was washed with PBS and glomeruli on the cell strainer were carefully collected. During the procedure, kidney tissues were kept on ice except for the collagenase digestion at 37°C. Isolated glomeruli were cultured on tissue culture dishes in DMEM/F-12 (1:1) containing 5% FBS, supplemented with 0.5% insulin-transferrin-selenium-A liquid media supplement (Sigma-Aldrich), 0.5 mg/ml Primocin (InvivoGen). Cultures were incubated in a 37°C humidified incubator with 5% CO2.
To remove phagocytic cells from the culture, immune complex–coated magnetic beads were prepared by sequential binding of Dynabeads M-280 Sheep antirabbit IgG (Invitrogen) to rabbit anti-ovalbumin antibody (1 mg/ml) (Chemicon), ovalbumin (1 mg/ml) (Sigma-Aldrich), and fresh rabbit complement (Pel-Freez Biologicals). The opsonized beads were mixed with culture media and added to the primary culture at day 3. Subculture of primary cultured podocytes was performed after 4 days of culture of isolated glomeruli. Cellular outgrowths were detached with 0.25% trypsin-EDTA (Cellgro) and passed through a 25-μm sieve to remove the remaining glomerular cores. The cells that passed through the 25-μm sieve were put on a magnetic particle concentrator to remove the phagocytized beads. The remaining cells were cultured for further analysis. The filtered cells were cultured on collagen I–coated 96-well plates or collagen I–coated polystyrene cover slips (celltight C-I cell disk; Sumitomo Bakelite) for 2 more days. To test the effect of baboon sera on pig podocytes, media containing 4% naive baboon sera were added to the cells on day 5. Cells were pretreated with 100 μg/ml rituximab for 30 minutes before the addition of baboon sera, as previously described.12,25 The cultured podocytes were processed for the cell proliferation assay or immunohistochemical analysis at day 6.
Western Blotting and Immunoprecipitation
Pig glomeruli were partially purified from naive pig kidney cortex by a standard sieving method using stainless sieves with several different mesh sizes. Glomeruli trapped on a sieve of 106-μm mesh were collected and homogenized in lysis solution (20 mM Tris, pH 7.4, 150 mM NaCl, 1% TX-100, 0.1% SDS, 10% glycerol, 1 mM EDTA, 0.5% deoxycholate, protease inhibitor cocktail [Sigma-Aldrich]). HEK293 cells were used as a positive control. The protein concentration was determined by the bicinchoninic acid assay method (BCA protein assay kit; Thermo). Twenty micrograms of protein was loaded onto 4%–20% SDS-PAGE gels (Invitrogen), transferred to polyvinylidene difluoride membrane (Milipore) and probed with polyclonal rabbit anti–SMPDL-3b antibody (GeneWay) and horseradish peroxidase–conjugated anti-rabbit IgG antibody (Southern Biotech). The blot was developed by enhanced chemiluminescence (GE Healthcare). Two milligrams of protein lysate from naive pig glomeruli was immunoprecipitated with 4 μg of rituximab adsorbed on a mixture of protein A-Sepharose and protein G-agarose beads (Classic Immunoprecipitation Kit; Thermo). Immunoprecipitates were subjected to Western blotting as described previously and probed with anti–SMPDL-3b antibody.
Cell Proliferation Assay
To evaluate the viability of the cultured podocytes, a cell proliferation assay (Cell Titer 96 Aqueous One Solution Cell Proliferation Assay; Promega) was performed according to manufacturer’s directions. The untreated control was medium only, and Nonidet P-40 was the positive control. This technique was determined to produce a linear relationship between the number of viable podocytes and the absorbance at 490 nm.
Pig-to-Baboon Xeno-Kidney Transplant
Six experimental baboons received xeno-kidney transplant from MGH GalKO swine as previously reported.5 Briefly, the recipient baboons underwent splenectomy and thymectomy before transplant. B cell and T cells were depleted by 20 mg/kg rituximab on POD-14 and 10 mg/kg rabbit ATG on POD-3 and -2, respectively. Mycophenolate mofetil, 110 mg/kg, was given from POD-6 and tapered after transplant. Cobra venom factor was given from POD-1 to POD-14.
To assess the effect of rituximab on post-transplant proteinuria, 10 mg/kg rituximab was given during the peri-transplant period. Eighteen baboons that were not given rituximab in the peri-transplant period were used as controls. All historical control baboons received 20 mg/kg rituximab on POD-14 in the same manner as the experimental baboons.
After kidney transplant, creatinine was assessed as an indicator of kidney graft function. Proteinuria was measured by dipstick (Multistix; Siemens). The results from the dipstick are correlated with the mainly albumin concentration in the urine (trace, 15 mg/dl; 1+, 30 mg/dl; 2+, 100 mg/dl; 3+, 500 mg/dl; 4+, >500 mg/dl).
Assessments of Anti–Non-Gal IgM and IgG Antibodies
Anti–non-Gal IgM and IgG serum antibody was evaluated using GalTKO PBMCs and detected by indirect FACS using Becton-Dickinson FACScan (Sunnyvale, CA) and FITC-conjugated goat anti-human IgG or IgM (Invitrogen). Data were expressed as median fluorescence intensity.6
Statistical Analyses
Statistical assessment of the differences between control and experimental animals was done by t test. Differences were considered statistically significant at P<0.05.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dr. Eishin Yaoita for providing the method of podocyte culture, and Dr. David H. Sachs and Dr. Laurence A. Turka for critical review of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Rituximab: A Boot to Protect the Foot,” on pages 647–648.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013040363/-/DCSupplemental.
References
- 1.Cozzi E, White DJ: The generation of transgenic pigs as potential organ donors for humans. Nat Med 1: 964–966, 1995 [DOI] [PubMed] [Google Scholar]
- 2.McGregor CG, Davies WR, Oi K, Teotia SS, Schirmer JM, Risdahl JM, Tazelaar HD, Kremers WK, Walker RC, Byrne GW, Logan JS: Cardiac xenotransplantation: Recent preclinical progress with 3-month median survival. J Thorac Cardiovasc Surg 130: 844–851, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Kolber-Simonds D, Lai L, Watt SR, Denaro M, Arn S, Augenstein ML, Betthauser J, Carter DB, Greenstein JL, Hao Y, Im GS, Liu Z, Mell GD, Murphy CN, Park KW, Rieke A, Ryan DJ, Sachs DH, Forsberg EJ, Prather RS, Hawley RJ: Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci U S A 101: 7335–7340, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN, Murphy CN, Carter DB, Hawley RJ, Prather RS: Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 295: 1089–1092, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, O’Malley P, Nobori S, Vagefi PA, Patience C, Fishman J, Cooper DK, Hawley RJ, Greenstein J, Schuurman HJ, Awwad M, Sykes M, Sachs DH: Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med 11: 32–34, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Griesemer AD, Hirakata A, Shimizu A, Moran S, Tena A, Iwaki H, Ishikawa Y, Schule P, Arn JS, Robson SC, Fishman JA, Sykes M, Sachs DH, Yamada K: Results of gal-knockout porcine thymokidney xenografts. Am J Transplant 9: 2669–2678, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimizu A, Yamada K, Robson SC, Sachs DH, Colvin RB: Pathologic characteristics of transplanted kidney xenografts. J Am Soc Nephrol 23: 225–223, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.First MR, Vaidya PN, Maryniak RK, Weiss MA, Munda R, Fidler JP, Penn I, Alexander JW: Proteinuria following transplantation. Correlation with histopathology and outcome. Transplantation 38: 607–612, 1984 [PubMed] [Google Scholar]
- 9.Reichel H, Zeier M, Ritz E: Proteinuria after renal transplantation: Pathogenesis and management. Nephrol Dial Transplant 19: 301–305, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Halimi JM, Matthias B, Al-Najjar A, Laouad I, Chatelet V, Marlière JF, Nivet H, Lebranchu Y: Respective predictive role of urinary albumin excretion and nonalbumin proteinuria on graft loss and death in renal transplant recipients. Am J Transplant 7: 2775–2781, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Fernández-Fresnedo G, Escallada R, Rodrigo E, De Francisco AL, Cotorruelo JG, Sanz De Castro S, Zubimendi JA, Ruiz JC, Arias M: The risk of cardiovascular disease associated with proteinuria in renal transplant patients. Transplantation 73: 1345–1348, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, Jauregui AN, Li J, Mattiazzi A, Ciancio G, Chen L, Zilleruelo G, Abitbol C, Chandar J, Seeherunvong W, Ricordi C, Ikehata M, Rastaldi MP, Reiser J, Burke GW, 3rd: Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med 3: 85ra46, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perosa F, Favoino E, Caragnano MA, Dammacco F: Generation of biologically active linear and cyclic peptides has revealed a unique fine specificity of rituximab and its possible cross-reactivity with acid sphingomyelinase-like phosphodiesterase 3b precursor. Blood 107: 1070–1077, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Yaoita E, Franke WW, Yamamoto T, Kawasaki K, Kihara I: Identification of renal podocytes in multiple species: Higher vertebrates are vimentin positive/lower vertebrates are desmin positive. Histochem Cell Biol 111: 107–115, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Roccatello D, Sciascia S, Rossi D, Alpa M, Naretto C, Baldovino S, Menegatti E, La Grotta R, Modena V: Intensive short-term treatment with rituximab, cyclophosphamide and methylprednisolone pulses induces remission in severe cases of SLE with nephritis and avoids further immunosuppressive maintenance therapy. Nephrol Dial Transplant 26: 3987–3992, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Dillon JJ, Hladunewich M, Haley WE, Reich HN, Cattran DC, Fervenza FC: Rituximab therapy for type I membranoproliferative glomerulonephritis. Clin Nephrol 77: 290–295, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Fervenza FC, Cosio FG, Erickson SB, Specks U, Herzenberg AM, Dillon JJ, Leung N, Cohen IM, Wochos DN, Bergstralh E, Hladunewich M, Cattran DC: Rituximab treatment of idiopathic membranous nephropathy. Kidney Int 73: 117–125, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Rostaing L, Guilbeau-Frugier C, Fort M, Mekhlati L, Kamar N: Treatment of symptomatic transplant glomerulopathy with rituximab. Transpl Int 22: 906–913, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Pescovitz MD, Book BK, Sidner RA: Resolution of recurrent focal segmental glomerulosclerosis proteinuria after rituximab treatment. N Engl J Med 354: 1961–1963, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Gallon L, Chhabra D: Anti-CD20 monoclonal antibody (rituximab) for the treatment of recurrent idiopathic membranous nephropathy in a renal transplant patient. Am J Transplant 6: 3017–3021, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Damodar A, Mustafa R, Bhatnagar J, Panesar M, Gundroo A, Zachariah M, Blessios G, Tornatore K, Weber-Shrikant E, Venuto R: Use of anti-CD20 antibody in the treatment of post-transplant glomerulonephritis. Clin Transplant 25: 375–379, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Wei C, Möller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP, Cowan P, Kretzler M, Parrilla R, Bendayan M, Gupta V, Nikolic B, Kalluri R, Carmeliet P, Mundel P, Reiser J: Modification of kidney barrier function by the urokinase receptor. Nat Med 14: 55–63, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, Maiguel D, Karumanchi SA, Yap HK, Saleem M, Zhang Q, Nikolic B, Chaudhuri A, Daftarian P, Salido E, Torres A, Salifu M, Sarwal MM, Schaefer F, Morath C, Schwenger V, Zeier M, Gupta V, Roth D, Rastaldi MP, Burke G, Ruiz P, Reiser J: Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med 17: 952–960, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garin EH, Diaz LN, Mu W, Wasserfall C, Araya C, Segal M, Johnson RJ: Urinary CD80 excretion increases in idiopathic minimal-change disease. J Am Soc Nephrol 20: 260–266, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bezombes C, Grazide S, Garret C, Fabre C, Quillet-Mary A, Müller S, Jaffrézou JP, Laurent G: Rituximab antiproliferative effect in B-lymphoma cells is associated with acid-sphingomyelinase activation in raft microdomains. Blood 104: 1166–1173, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.