Abstract
Turnip seedlings (Brassica rapa L.) irradiated for 24 hours with radiation at 720 nanometers synthesize chlorophyll a and anthocyanin. Antimycin A and 2,4-dinitrophenol, which are known to reduce cyclic photophosphorylation, also reduce anthocyanin synthesis. Noncyclic photophosphorylation is inhibited by 3-(3,4-dichlorophenyl)-1,1-dimethylurea and o-phenanthroline. These compounds promote cyclic photophosphorylation and anthocyanin synthesis. On the basis of these findings it is suggested that the photomorphogenic response of anthocyanin synthesis in turnip seedlings arises in part through photosynthetic activity.
Phytochrome involvement in turnip seedling photomorphogenesis is evidenced by the photoreversibility of anthocyanin synthesis in response to 5-minute irradiations with red or far red light. The inhibition of anthocyanin synthesis by 2,4-dinitrophenol does not arise from a destruction of phytochrome photoreversibility.
It is suggested that plant photomorphogenic responses to prolonged far red irradiations arise through the photochemical activation of at least two pigment systems; namely, the photosynthetic pigments and phytochrome.
Full text
PDF
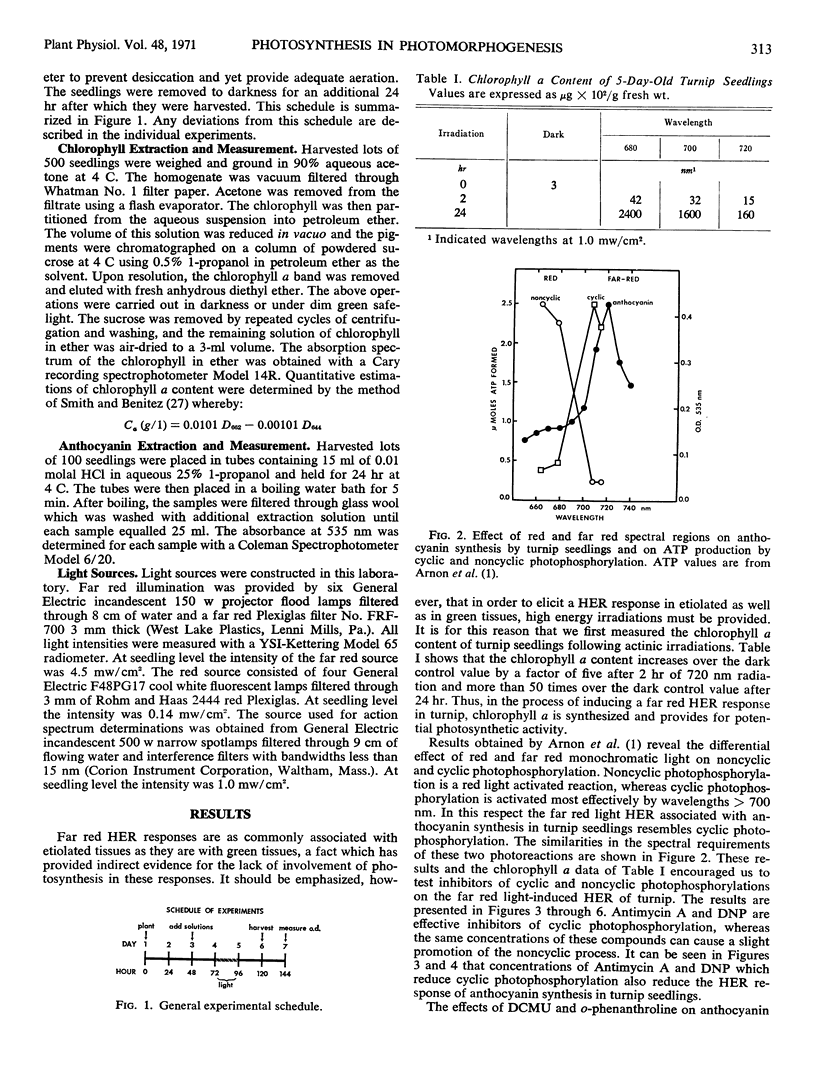
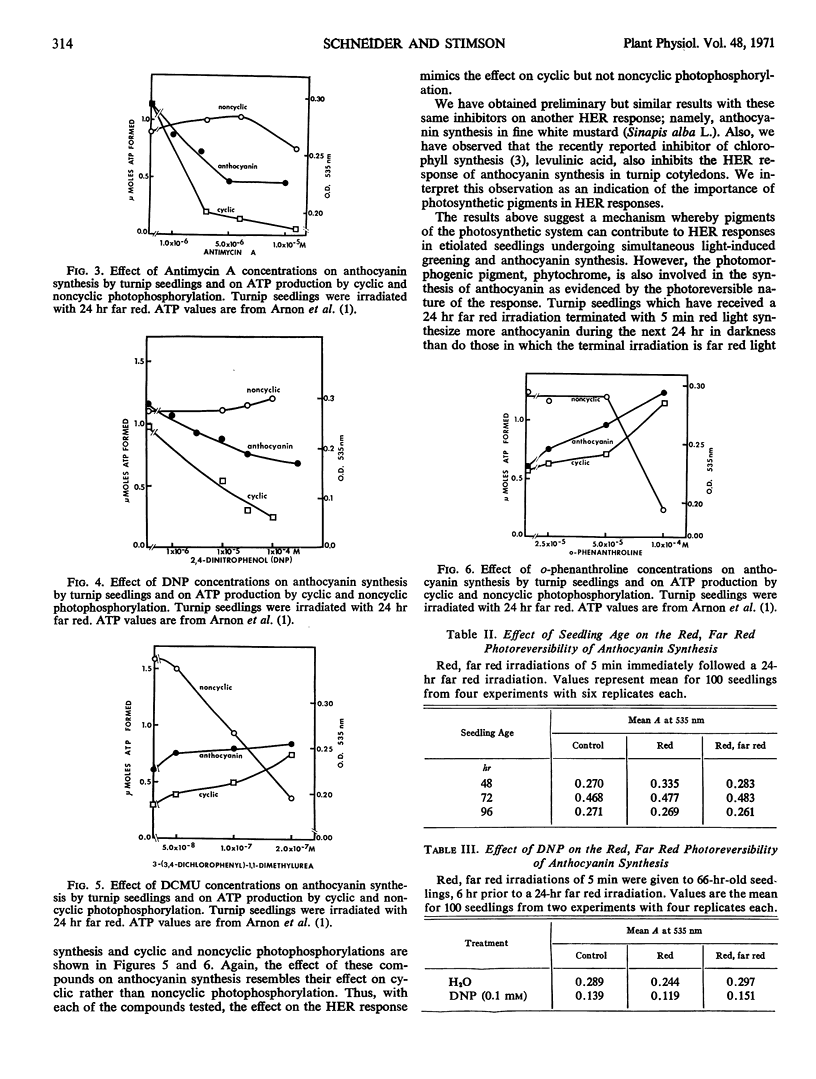

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I., Tsujimoto H. Y., McSwain B. D. Ferredoxin and photosynthetic phosphorylation. Nature. 1967 May 6;214(5088):562–566. doi: 10.1038/214562a0. [DOI] [PubMed] [Google Scholar]
- Beale S. I. The biosynthesis of delta-aminolevulinic acid in Chlorella. Plant Physiol. 1970 Apr;45(4):504–506. doi: 10.1104/pp.45.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black C. C., Mayne B. C. P(700) activity and chlorophyll content of plants with different photosynthetic carbon dioxide fixation cycles. Plant Physiol. 1970 Jun;45(6):738–741. doi: 10.1104/pp.45.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthwick H. A., Hendricks S. B., Schneider M. J., Taylorson R. B., Toole V. K. The high-energy light action controlling plant responses and development. Proc Natl Acad Sci U S A. 1969 Oct;64(2):479–486. doi: 10.1073/pnas.64.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satter R. L., Wetherell D. F. Photomorphogenesis in Sinningia speciosa, cv. Queen Victoria II. Stem Elongation: Interaction of a Phytochrome Controlled Process and a Red-requiring, Energy Dependent Reaction. Plant Physiol. 1968 Jun;43(6):961–967. doi: 10.1104/pp.43.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelman H. W., Hendricks S. B. Photocontrol of Alcohol, Aldehyde, and Anthocyanin Production in Apple Skin. Plant Physiol. 1958 Nov;33(6):409–413. doi: 10.1104/pp.33.6.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelman H. W., Hendricks S. B. Photocontrol of Anthocyanin Formation in Turnip and Red Cabbage Seedlings. Plant Physiol. 1957 Sep;32(5):393–398. doi: 10.1104/pp.32.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]


