Abstract
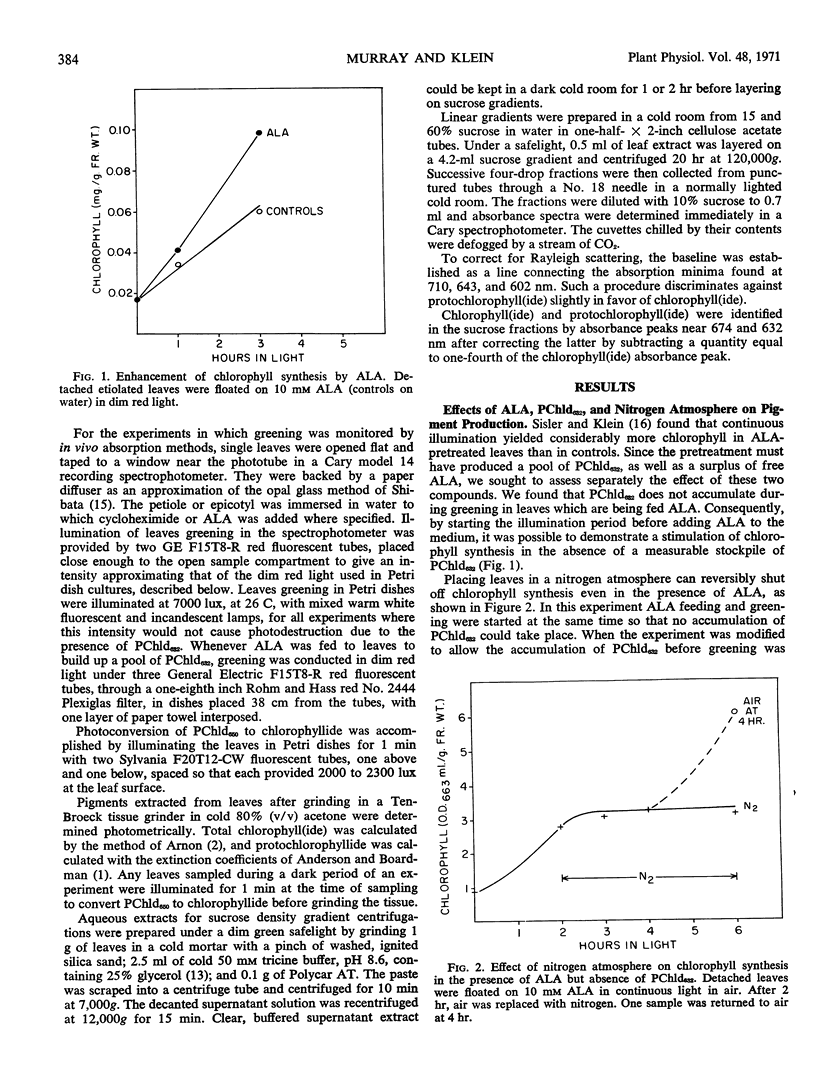
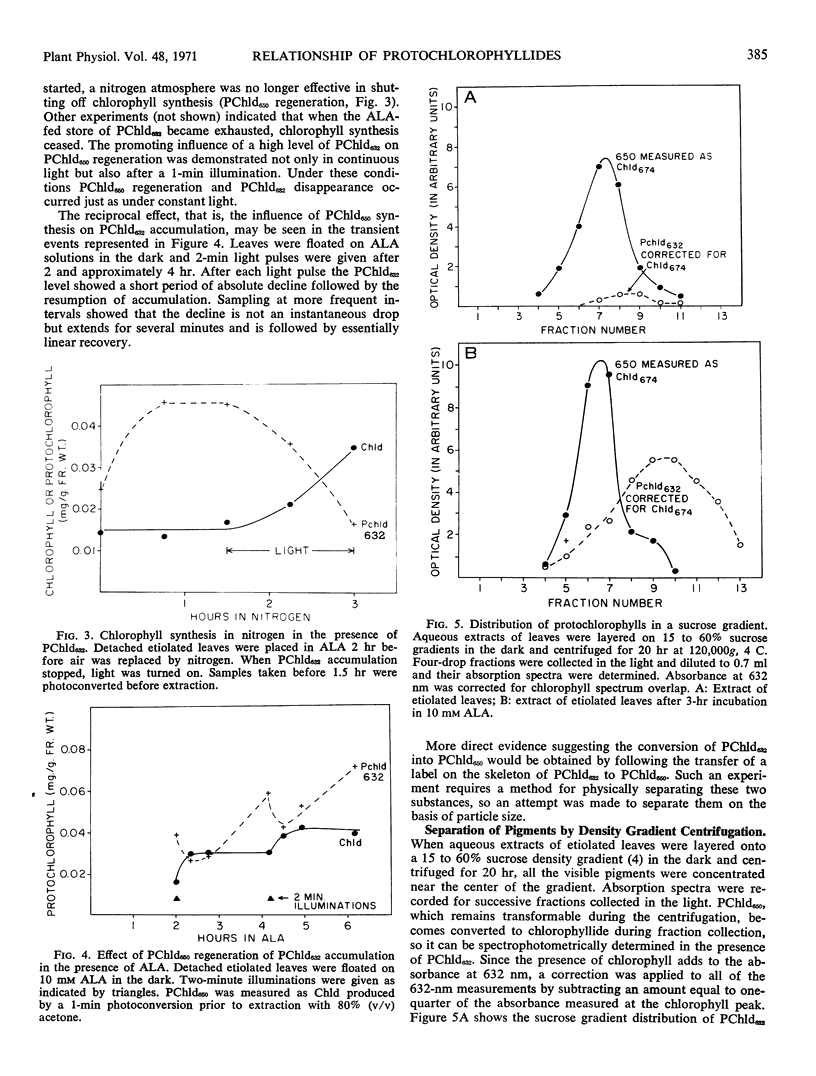
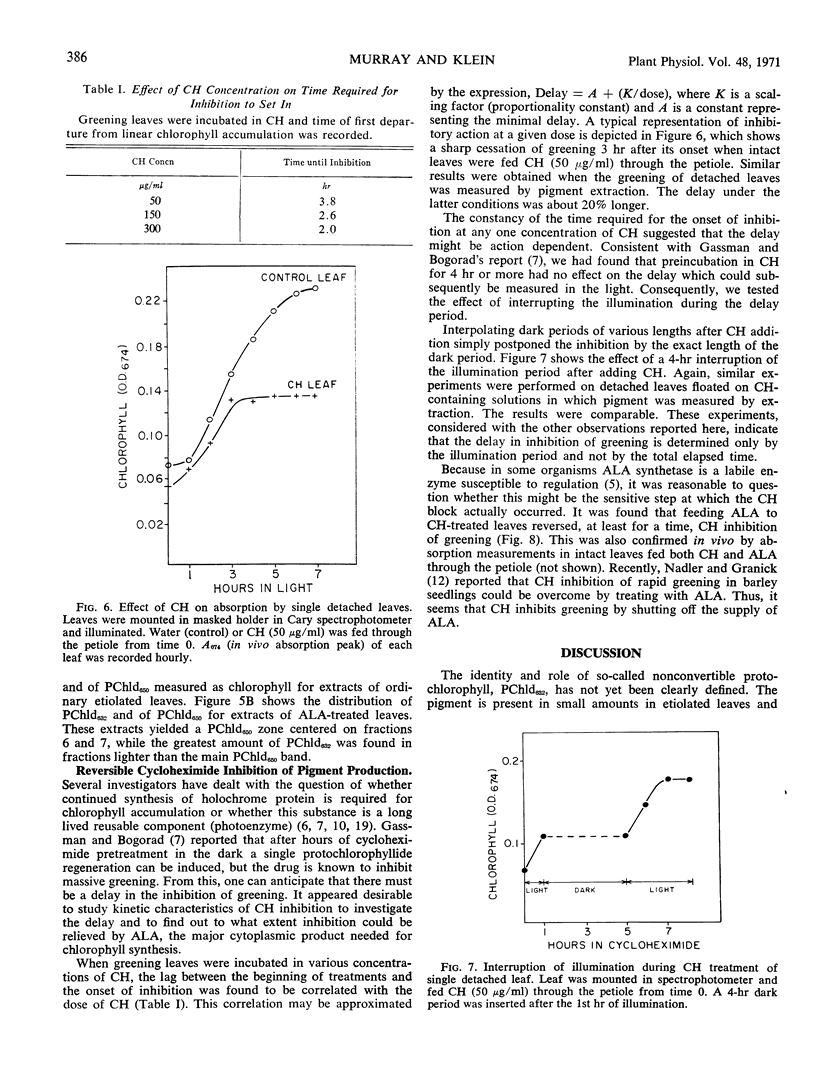
Two forms of protochlorophyllide are found in dark-grown bean (Phaseolus vulgaris, var. Black Velentine) leaves, one (protochlorophyllide650) which is directly photoconvertible to chlorophyllide and another (protochlorophyllide632) which is not. Dark-grown leaves placed in solutions of δ-aminolevulinic acid accumulate protochlorophyllide632. Protochlorophyllide650 and protochlorophyllide632 can be partially separated on sucrose density gradients. A nitrogen atmosphere blocks chlorophyll synthesis in light or the regeneration of protochlorophyllide650 in the dark, even in the presence of excess δ-aminolevulinic acid, except when a stockpile of protochlorophyllide632 is present in the leaf. Under the latter conditions chlorophyll synthesis or protochlorophyllide650 regeneration is accompanied by a decrease in protochlorophyllide632. These experiments suggest that protochlorophyllide632 may be converted to protochlorophyllide650.
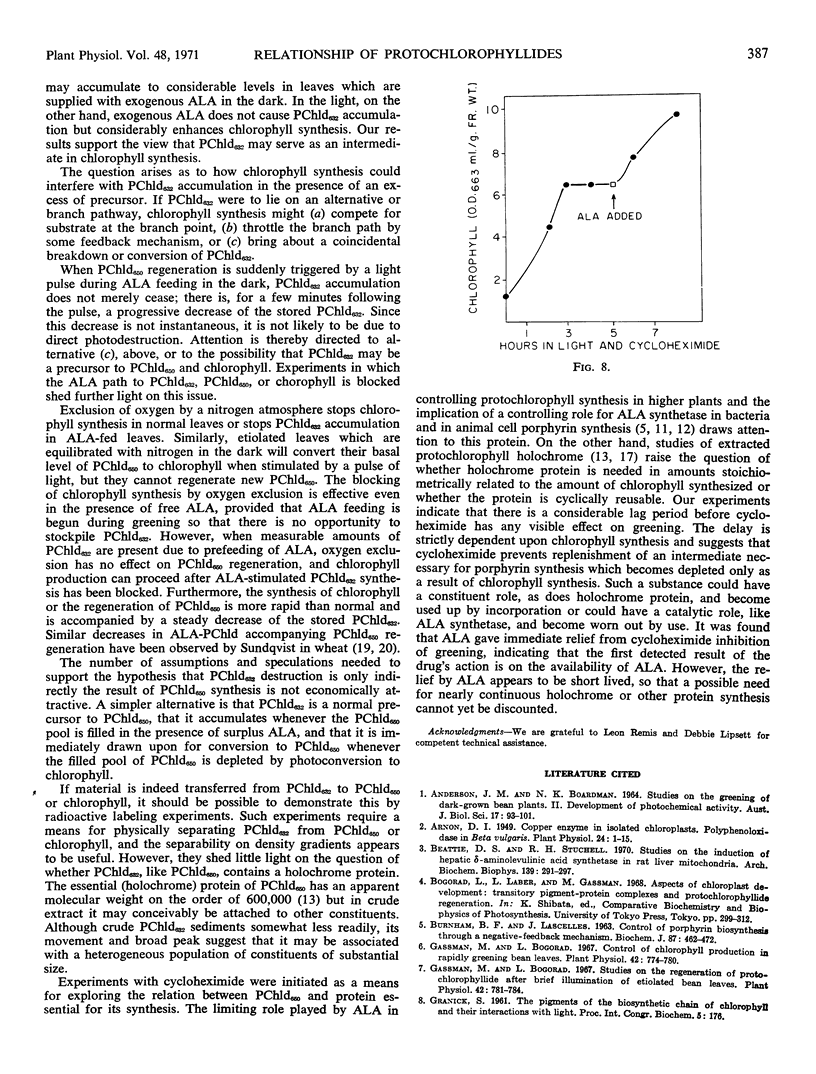
Cycloheximide inhibited greening only after an “action-dependent” delay, requiring a predictable minimal period of illumination. This inhibition could be relieved for a time by feeding δ-aminolevulinic acid.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beattie D. S., Stuchell R. N. Studies on the induction of hepatic delta-aminolevulinic acid synthetase in rat liver mitochondria. Arch Biochem Biophys. 1970 Aug;139(2):291–297. doi: 10.1016/0003-9861(70)90480-7. [DOI] [PubMed] [Google Scholar]
- Gassman M., Bogorad L. Control of chlorophyll production in rapidly greening bean leaves. Plant Physiol. 1967 Jun;42(6):774–780. doi: 10.1104/pp.42.6.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassman M., Bogorad L. Studies on the regeneration of protochlorophyllide after brief illumination of etiolated bean leaves. Plant Physiol. 1967 Jun;42(6):781–784. doi: 10.1104/pp.42.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granick S., Gassman M. Rapid regeneration of protochlorophyllide(650). Plant Physiol. 1970 Feb;45(2):201–205. doi: 10.1104/pp.45.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk J. T., Allen R. L. Dependence of chloroplast pigment synthesis on protein synthesis: effect of actidione. Biochem Biophys Res Commun. 1965 Dec 21;21(6):523–530. doi: 10.1016/0006-291x(65)90516-4. [DOI] [PubMed] [Google Scholar]
- Nadler K., Granick S. Controls on chlorophyll synthesis in barley. Plant Physiol. 1970 Aug;46(2):240–246. doi: 10.1104/pp.46.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer P., Siegelman H. W. Purification of protochlorophyllide holochrome. Plant Physiol. 1968 Jun;43(6):990–996. doi: 10.1104/pp.43.6.990. [DOI] [PMC free article] [PubMed] [Google Scholar]



