Abstract
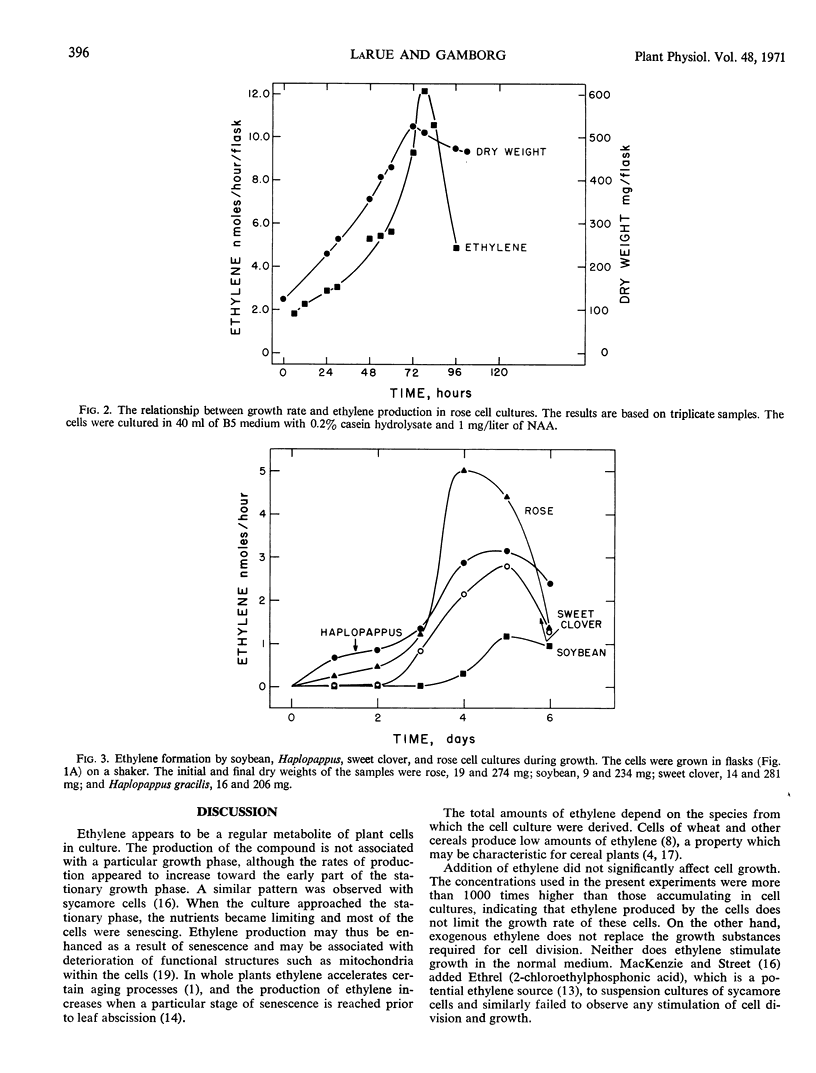
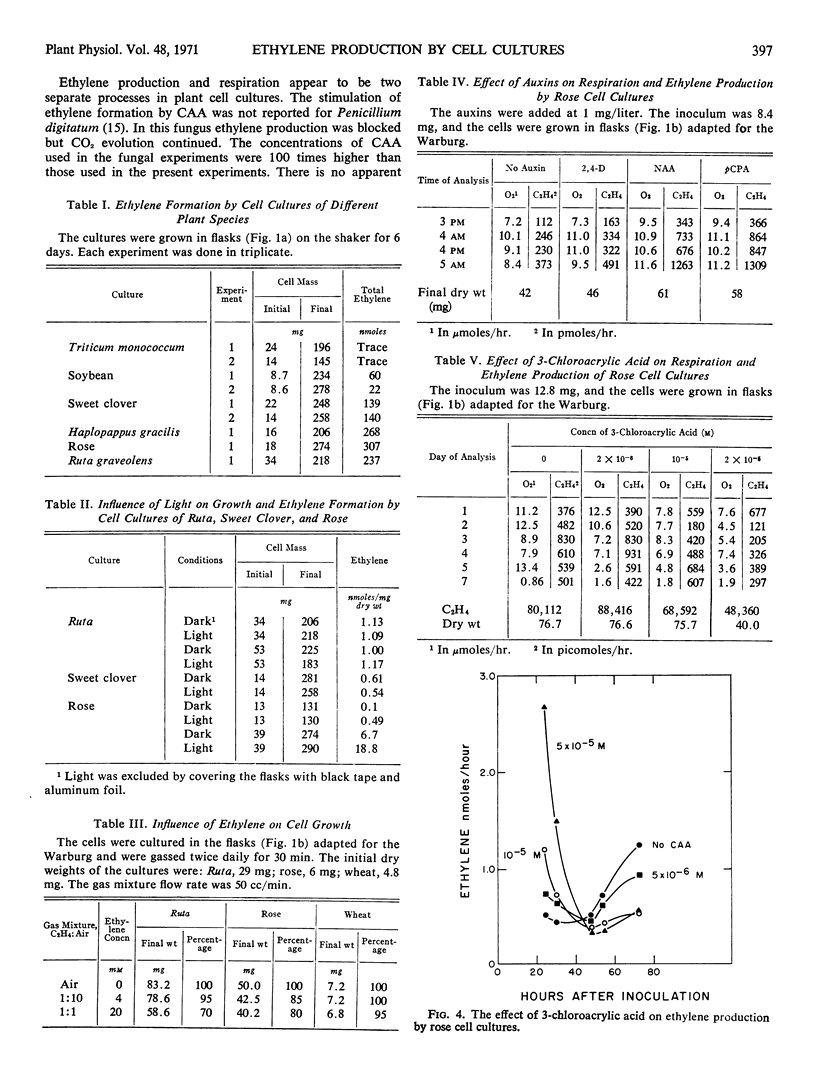
Suspension cultures of Rosa sp., soybean (Glycine max L.), wheat (Triticum monococcum L.), sweet clover (Melilotus alba Desc.), Haplopappus gracilis Nutt., and rue (Ruta graveolens) produced ethylene. The amount varied with the species. The rate of formation in rose and Haplopappus cells paralleled growth but accelerated when the stationary phase was reached, after which the rate declined sharply. Light was not required for ethylene production. Exogenous ethylene could not replace 2,4-dichlorophenoxyacetic acid or naphthalineacetic acid in the cell cultures, and there was no stimulation of growth in the normal medium. Ethylene at 20 mm reduced growth of Ruta and rose cells by 30 and 20%, respectively. The amounts of ethylene produced by the cultures do not affect growth.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burg S. P., Burg E. A. Auxin-induced ethylene formation: its relation to flowering in the pineapple. Science. 1966 May 27;152(3726):1269–1269. doi: 10.1126/science.152.3726.1269. [DOI] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. The interaction between auxin and ethylene and its role in plant growth. Proc Natl Acad Sci U S A. 1966 Feb;55(2):262–269. doi: 10.1073/pnas.55.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P. Ethylene, plant senescence and abscission. Plant Physiol. 1968 Sep;43(9 Pt B):1503–1511. [PMC free article] [PubMed] [Google Scholar]
- Fuchs Y., Lieberman M. Effects of Kinetin, IAA, and Gibberellin on Ethylene Production, and Their Interactions in Growth of Seedlings. Plant Physiol. 1968 Dec;43(12):2029–2036. doi: 10.1104/pp.43.12.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg O. L. Aromatic metabolism in plants. II. Enzymes of the shikimate pathway in suspension cultures of plant cells. Can J Biochem. 1966 Jun;44(6):791–799. doi: 10.1139/o66-097. [DOI] [PubMed] [Google Scholar]
- Gamborg O. L., Eveleigh D. E. Culture methods and detection of glucanases in suspension cultures of wheat and barley. Can J Biochem. 1968 May;46(5):417–421. doi: 10.1139/o68-063. [DOI] [PubMed] [Google Scholar]
- Gamborg O. L., LaRue T. A. Ethylene produced by plant cells in suspension cultures. Nature. 1968 Nov 9;220(5167):604–605. doi: 10.1038/220604a0. [DOI] [PubMed] [Google Scholar]
- Gamborg O. L., Larue T. A. Ethylene production by plant cell cultures: the effect of auxins, abscisic Acid, and kinetin on ethylene production in suspension cultures of rose and ruta cells. Plant Physiol. 1971 Oct;48(4):399–401. doi: 10.1104/pp.48.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Gamborg O. L. The effects of amino acids and ammonium on the growth of plant cells in suspension culture. Plant Physiol. 1970 Apr;45(4):372–375. doi: 10.1104/pp.45.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrett R. A., Kurtz A. N. cis-3-Chloroacrylic Acid: A New Cotton Defoliant and Crop Desiccant. Science. 1963 Sep 20;141(3586):1192–1193. doi: 10.1126/science.141.3586.1192. [DOI] [PubMed] [Google Scholar]
- Iwahori S., Lyons J. M., Sims W. L. Induced femaleness in cucumber by 2-chloroethanephosphonic acid. Nature. 1969 Apr 19;222(5190):271–272. doi: 10.1038/222271a0. [DOI] [PubMed] [Google Scholar]